Tomato peel (Solanum lycopersicum L.) colonization by the endophyte yeast Candida guilliermondii (Castellani) Langeron et Guerra
Keywords:
postharvest, histology, apoplast, biocontrol. (es)
Downloads
During the postharvest period, fruits are lost due to attack by phytopathogens and incorrect storage and manipulation. The use of yeasts has been presented as one of the most promising strategies to resolve some of these problems. This research standardized a methodology to study, under controlled conditions, the colonization of the epidermis and subepidermal region of tomato fruit by the yeast Candida guilliermondii. In addition, it determined the path of yeast distribution from histological sections in tissues within 70 hours after inoculation. The results showed that C. guilliermondii is an endophytic yeast capable of entering through the healthy cuticle of ripe tomato fruits and colonizing apoplastic spaces without causing damage to plant tissues. The dynamics of colonization were established during the first 70 hours and the speed of yeast migration into the fruit
was estimated at 0.55 μm hour-1.
CROP PROTECTION
Tomato peel (Solanum lycopersicum L.) colonization by the endophyte yeast Candida guilliermondii (Castellani) Langeron et Guerra
Colonización de la corteza del fruto de tomate (Solanum lycopersicum L.) por la levadura endófita Candida guilliermondii (Castellani) Langeron et Guerra
Esperanza del Pilar Infante1, Xavier Marquínez2 and Gerardo Moreno3
1Department of Physics, Faculty of Sciences and Education, Universidad Distrital de Colombia. Bogotá (Colombia); Faculty of Sciences, Pontificia Universidad Javeriana. Bogota (Colombia). epinfantel@udistrital.edu.co2Department of Biology, Faculty of Sciences, Universidad Nacional de Colombia. Bogota (Colombia).
3Department of Microbiology, Faculty of Sciences, Pontificia Universidad Javeriana, Bogota (Colombia).
Received for publication: 11 February, 2011. Accepted for publication: 30 October, 2012.
ABSTRACT
During the postharvest period, fruits are lost due to attack by phytopathogens and incorrect storage and manipulation. The use of yeasts has been presented as one of the most promising strategies to resolve some of these problems. This research standardized a methodology to study, under controlled conditions, the colonization of the epidermis and subepidermal region of tomato fruit by the yeast Candida guilliermondii. In addition, it determined the path of yeast distribution from histological sections in tissues within 70 hours after inoculation. The results showed that C. guilliermondii is an endophytic yeast capable of entering through the healthy cuticle of ripe tomato fruits and colonizing apoplastic spaces without causing damage to plant tissues. The dynamics of colonization were established during the first 70 hours and the speed of yeast migration into the fruit was estimated at 0.55 µm hours-1.
Key words: postharvest, histology, apoplast, biocontrol.
RESUMEN
Durante el período postcosecha de las frutas se producen pérdidas debido tanto al ataque de fitopatógenos, como al almacenamiento incorrecto y a la manipulación indebida de las mismas. El uso de levaduras se ha presentado como una de las estrategias más prometedoras para resolver algunos de estos problemas. Esta investigación estandarizó una metodología con el fin de analizar, bajo condiciones controladas, el proceso de la colonización de la epidermis y de la región subepidérmica de fruto de tomate después de ser inoculados con la levadura Candida guilliermondii. Adicionalmente, mediante cortes histológicos se determinó su ruta de distribución en los tejidos, durante las primeras 70 horas después de la inoculación. Los resultados mostraron que C. guilliermondii es una levadura endófita capaz de entrar a través de la cutícula sana del fruto de tomate maduro y colonizar espacios apoplásticos sin crear daños en los tejidos vegetales. Se estableció la dinámica de la colonización durante las primeras 70 horas, y la magnitud de la velocidad de migración de la levadura en el fruto se estimó en 0,55 µm hora-1.
Palabras clave: postcosecha, histología, apoplasto, biocontrol.
Introduction
Due to the boom of the organic food industry, which is free of toxic agrochemicals, the use of yeasts has presented as one of the most promising strategies to control pathogens that produce postharvest lesions (Yu et al., 2007). Different authors have recommend them because they colonize the surface for long periods of time and produce extracellular polysaccharides, which favor their survival and, simutaneously, slow the growth of pathogens (Sharma et al., 2009). Isaeva et al. (2010) consider that knowledge about distribution patterns and biological properties of these yeasts will enable them to advance, from a practical point of view, in terms of solutions to problems such as fruit storage and phytopathogen control.
Nicot (2011) reviewed microorganisms used as biological controls for phytopathogens, including 14 species of Candida used against Botrytis sp. and Monilia sp. C. guilliermondii (Castellani) Langeron et Guerra is the anamorph (asexual form) of Pichia guilliermondii Wickerham; both forms have been used as biological controls of phytopathogens: C. guilliermondii against Rhizopus sp., Botrytis cinerea Pers.: Fr. and Aspergillus niger Tiegh. in the peach and grape (Zahavi et al., 2000; Fan et al., 2001); P. guilliermondii against Colletotrichum capsici (Syd.) Butler & Bisby in chilli fruit (Nantawanit et al, 2010) and against Botrytis cinerea in apples (Zhang et al., 2011a, b).
Saligkarias et al. (2002a,b) found that C. guilliermondii secretes hydrolytic enzymes (exoglucanases and chitinases) which inhibit the development of B. cinerea hyphae on tomato plant stems. Zhao et al. (2008) detected that when applying P. guilliermondii and Rhyzopus nigricans to artificially injured tomato fruits at the same time, the former colonizes the fruit tissue, reducing the rate of infection and the size of the lesions, and stimulating responses mediated by multiple enzymes. Zhao et al. (2011) found that the exogenous application of P. guilliermondii improved the postharvest life and the quality of stored "cherry" tomato fruit. Zhao et al. (2009) and Zong et al. (2010) determined that the use of thermal shock treatments improved the biocontrolling activity of C. guilliermondii against B. cinerea in tomato fruits.
Some of the above-mentioned papers have studied the growth of yeast on the tissues of the tomato plant and they have even determined that it is able to grow (and antagonize pathogens) in fruit tissues exposed by artificial lesions. However, they have not confirmed the possibility of colonizing tomato fruit with Candida guilliermondii in healthy tissues, nor have the dynamics of this process been studied. This research sought to standardize a methodology to study the colonization of the epidermis and the subepidermal region of tomato fruit under controlled conditions, after being inoculated with the yeast C. guilliermondii. Consequently, it determined the yeast's path of entry and distribution in the tissues, from histological sections, within 70 h after inoculation.
Materials and methods
Plant material and disinfection protocols Tomato fruits, without external damage and in stage six of the ripening process (Wills et al., 1998), were collected from tomato plants (Solanum lycopersicum L.) of the Chonto- Calima variety, grown under greenhouse conditions in the facilities of the "Tibaitata research center - Corpoica" (Mosquera, Colombia).
The fruits were disinfected by the following protocol: the fruits were immersed in sodium hypochlorite 0.2% for 2 min, before rinsing with sterile distilled water and immersing in 70% alcohol for 1 min. Afterwards, the fruits were rinsed with sterile, distilled water and dried with sterile absorbent paper in a laminar flow cabinet (Microzone Corporation, H6-MW-99-C30, Ottawa, Canada).
Pre-inoculation controls were carried out to verify the absence of microorganisms in the tissues. Non-inoculated fruits were cut with a sterile scalpel in a laminar flow cabinet to obtain peel samples, 3 cm² in area and 3 mm thick, which were placed in YGC agar (Yeast Glucose Cloramphenicol agar; Merck and Co., Franklin Lakes, NJ) Petri dishes and incubated at 25°C for 24 h.
Yeast material and inoculum preparation
The yeasts used for inoculation were isolated by the UNIDIA group (Unidad de Investigaciones Agropecuarias) from "Pontificia Universidad Javeriana" and identified as C. guilliermondii through biochemical methods using the API 20C AUX kit (Biomerieux Company, Hazelwood, MO). This result was confirmed using molecular techniques using PCR (polymerase chain reaction) following by RFLP (restriction fragment length polymorphism), (Orberá, 2004).
Inoculum preparation was performed by scraping colonies obtained in the YGC agar and preparing a suspension of 5 mL in saline solution 0.85% (w/v), equalized with McFarland tube 3 (Pedroza et al., 2006). The concentration was verified by counting with a Neubauer chamber; this suspension was added to 45 mL of YGC broth and incubated for 24 h at 27±2°C and 150 rpm, using a New Brunswick Scientific shaker gyrotory model G2 (Artisan Technology Group, Champaign, IL).
Inoculation and sampling procedures
Whole fruits were used in the first series of inoculation tests, taking one hemisphere as a control and the other one for yeast application, using three different procedures: a) by sterile swab, 200 µL of the yeast solution (78·108 cells/ mL), b) immersing the fruit hemisphere in 50 mL of the solution of the same concentration for 3 min and c) spraying with 0.3 mL of the solution. In the three cases, the inoculated fruits were stored at room temperature in sterile containers for 5 d. After that time, fruit peel samples (3 cm² in area and 3 mm thick) were taken of both the inoculated hemisphere and the control hemisphere. They were placed in Petri dishes with YGC agar and incubated for 3 d at a temperature of 25°C. These inoculation trials were discarded due to the frequent presence of contaminating microorganisms.
In the second series of inoculation tests, carried out in a laminar flow cabinet, samples were taken from the peel of the fruit (3 cm² in area and 3 mm thick) and placed individually in Petri dishes with YGC agar. Inoculation in the center of the sample was done in two different ways:
using a micropipette of 10 µL and a swab of 200 µL of yeast solution (78·108 cells/µL). Since the use of a micropipette yielded more repeatable results, we consider this the standard procedure.
The samples inoculated with a micropipette were incubated at 25°C for time periods of 2, 5, 8, 22, 48 and 70 h (all of which had a control sample). Three replicas were made per treatment (all of which included 10 pseudoreplicas, each one a microslide with 10 serial sections; pseudoreplicas were 1,000 µm apart from each other). The controls corresponded to samples of the same fruit used for the treatments and were prepared under the same conditions, but inoculated with sterile, distilled water.
After inoculation, samples were fixed in a FAA solution (5: 90: 5 formalin, 70% ethanol and acetic acid), dehydrated for 4 h in an EtOH series (70, 90, 95% and absolute EtOH), then rinsed for 4 h in ethanol and HistoChoice Clearing Agent (Sigma-Aldrich, St. Louis, MO) mixtures at different concentrations (90:10, 70:30, 50:50, 30:70, 10:90), we ended with 100% HistoChoice®. Finally, HistoChoice® was replaced by paraffin (paraplast liquid added at 63°C, four times over the course of 12 h). Paraplast blocks, with the samples inside, were cut serially at 8 µm with a rotation microtome (820 Spencer, American Optical Company, New York, NY).
Polychromatic staining was performed on cell walls and yeast following the methodology of Kraus et al. (1998). However, it was modified as follows: serial cuts were spread onto microscope slides using Mayer adhesive and were dried on a hotplate with a temperature under 40°C. They were stained with 0.05% toluidine blue for 15 min, washed with distilled water and dried again at a temperature under 40°C, after which they were deparaffinized with 100% xylene, twice for 3 min and were fixed with citoresine and coverslips.
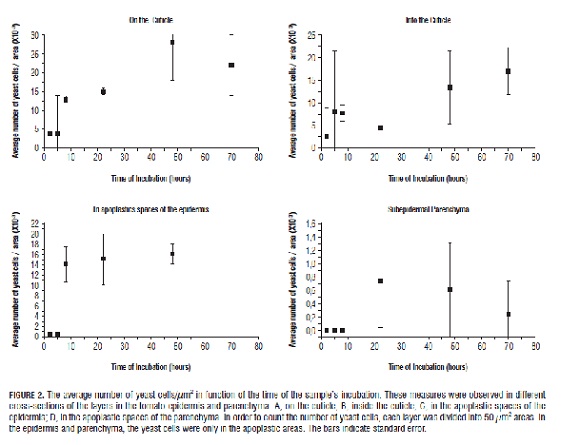
The micropreparations were photographed with a Nikon Coolpix 990 adapted to a Nikon Eclipse E200 microscope (Nikon Corporation, Tokio, Japan). Quantifications of the yeast cells were performed using quadrants of 50 µm², calculating the number of apoplast cells per µm² and discounting the intercellular spaces. All of which were executed in the following regions of interest: on and within the cuticle, in the apoplastic spaces around the epidermis and in those around the subepidermal parenchymatous cells. For quantification we used the free software ImageJ (National Institutes of Health, Bethesda, MD).
Results
The tomato fruit of the Chonto-Calima variety is characterized by an outer cuticular layer which has a thickness that varies between 6.0 and 10.9 µm. A toluidine stain gives the fruit a blue-green color and it may show anticlinal pegs which are deeply extended into the walls of the epidermal cells and have a diameter measuring between 0.8 and 1.86 µm (Fig.1 A).
The cuticle extends between the anticlinal walls of epidermal cells and, in some cases, it penetrates the first layers of subepidermal parenchyma cells, forming cuticular projections. Below are the epidermis cells with a thickness of 11.5 to 20.6 µm and walls that are stained purple. Then there are the parenchyma cells, which increase in size as they move away from the fruit surface, their walls also exhibiting purple stains (Fig.1 A).
Samples inoculated with the yeast C. guilliermondii look violet, oval shaped and with an average size of 3.5 x 1.2 µm (Fig.1 B). The results of the pre-inoculation controls and inoculation with sterile, distilled water indicate the absence of yeast and other endophyte organisms in the studied tissues. In addition, when evaluating the inoculated tissues, the absence of yeast cells was verified in the first 80 µm of the edges of the analyzed samples, ensuring that the yeast entered the tomato tissues through the cuticle.
According to the procedure used for staining the tissue, the yeast cells that are observed on the surface of the cuticle are those able to adhere to the tissue before being fixed with FAA. The results indicate that after 2 h, some yeasts already adhered (Fig.2 A) and some already entered the cuticle (Fig.2 B). At 8-22 h post-inoculation, an increase in the number of yeast cells adhered to the surface was observed. In addition, for storage times equal to or higher than 48 h, "clusters" of cells were formed, probably by cell division (Fig.1 D). For storage times of less than 8 h, inside the cuticle, the number of yeasts present was very low, and randomly distributed. For times higher than 48 h, the increase in the number of yeast cells was correlated with a change in distribution from random to clustered (Fig.2 B and C).
The first yeast cells were observed in apoplastic spaces around the epidermis at 8 h and around the parenchyma at 22 h. The results indicate that the yeast was distributed towards the inside of the fruit (Fig.1 C), moving between the walls of the epidermis and subsequently in the parenchyma apoplast pathway, in a random motion, forming linear groups (Fig.1 E) in the intercellular spaces (apoplast). Cell injury was not observed and nor was invasion into cells by the C. guilliermondii yeast. After crossing the barriers generated by the external cuticle, in the periclinal walls and cuticular projections, the apoplast pathway generated less resistance since the esquizogen spaces, characteristic of parenchyma, facilitated the movement of the yeast cells. The results of Fig.2 show a high standard error for cell densities. This is because the entry of yeast into the tomato fruit occurs randomly, so that there are many quadrants where cells are not observed; whereas once the yeast manages to cross a point, it is distributed along the less resistant routes.
Considering a straight path from the distance traveled by the yeast between the surface and its final position, we estimated an average speed of migration into ripe tomato fruit at 0.55±0.16 µm h-1.
Discussion
According to our microhistological observations, together with the absence of yeast in the border of cut samples (800 µm wide), we conclude that the yeast inoculated in the ripe tomato fruits entered the fruits through the intact cuticle and distributed itself inside the fruits via the apoplastic pathway where the yeast was able to enter the intercellular spaces of the epidermis and parenchyma; conversely, other authors suggest a random entry through microdamage in tissues (Isaeva et al., 2010).
We found that polychromatic staining allowed for the observation of the anticlinal pegs reported by Buda et al. (2009), which established a diameter between 1 and 3 µm. In this research, the pegs' diameters ranged from 0.8 to 1.86 µm and yeast was observed in some of them. As a result, they are proposed as a possible explanation of how the yeast entry is facilitated. This peg structure will soon be studied by atomic force microscopy. In spite of the above, yeast cells were also observed in the cuticle at points far from these structures (Fig.1 C).
Saligkarias et al. (2002b) studied the population dynamics of C. guilliermondii after spraying tomato stems placed in DWA agar. They found that the growth of yeast on the stems increased exponentially during the first two d, from 105 to 106 forming units of colony/portion of stem. Later, this growth slightly increased until it began to stabilize from day 13 onwards. The fact that the yeast persisted in high concentrations on the surface of the stems, between days 2 and 16, was thought by the authors to be a characteristic that makes the yeast an attractive biocontrol for B. cinerea. Saligkarias et al. (2002a, b) considered C. guilliermondii as an epiphytic yeast, and therefore, that measureable yeast populations are only found on the surface (not inside the tissues).
Moreover, Zhao et al. (2008) studied the population dynamics of P. guilliermondii in places of tomato fruit injury and found increases of 105 to 108 forming units of colony/injury in the first 4 d of inoculation. Nevertheless, these values are not directly comparable to Saligkarias et al. (2002b), because they are different forms of yeast and different organs of the plant.
In addition, it is evident that the increase in the amount of yeast is much higher in the injured tissue and this can be related to our observations concerning the ability of C. guilliermondii to grow inside tissues. Zhao et al. (2008) have pointed out that P. guilliermondii may be growing among the injured tissues due to the biocontrol effect it has on R. nigricans, and this may happen due to competition for space and nutrients. On the contrary, these authors did not seem to take into consideration the possibility that yeasts could infect intact tissues of the fruit since, in all treatments, they made lesions before implementation. Even Zhao et al. (2011), who applied P. guilliermondii to tomato fruits and determined its ability to protect them from the effect of fungi affecting postharvest, indicated that the application of antagonistic microorganisms before harvest protects injuries that occur during harvest and transport, therefore, prevents infection with pathogenic fungi, and so P. guilliermondii was considered an epiphytic yeast.
The strain of C. guilliermondii used in this research was originally isolated from internal tissues of tomato fruits and the results of this research indicate that it can colonize the internal tissues of a fruit of this species, passing through the intact epidermis. It can also grow in the apoplastic spaces, distributing itself inside them. This agrees with the definition of endophyte yeast given by Petrini (1991) and Xin et al. (2009). Namely, it states that endophyte yeasts are fungi formed by single cells that reproduce asexually by gemmation without a hyphal phase or a reduced hyphal phase, and can dwell in the living tissues of their host without causing apparent damage. It also complies with the condition indicated by Hallman et al. (1997) because this strain was isolated from vegetable material, which was superficially disinfected, but without apparent damage on the plant. Previously, only Larran et al. (2001) had detected endophytic yeasts in tomato leaves, specifically of the Rhodotorula genus.
Many studies emphasize that the interaction between endophyte microorganisms and plants is associated with the biological effects on the plants, such as biological control of soil pathogens and/or promotion of growth, as in the case of the yeast Cyberlindnera saturnus (Klocker) Minter (Williopsis saturnus), isolated from corn roots, which produces 3-indoleacetic acid and 3-indole butyric acid that promote plant growth (Nassar et al., 2005). In the case of C. guilliermondii, it is clear that it plays a role as a biological control for fruit pathogens, and its mechanisms have been studied by several authors (Saligkarias et al., 2002a, b; Zhao et al., 2011). Endophyte development capacity confers an additional advantage to C. guilliermondii in comparison with other microorganisms that colonize externally. Hence, not surprisingly, it has been selected as one of the top two biocontrols for Botrytis, Aspergillus and Rhizopus in grapes, from 129 different strains of diverse microorganisms all considered epiphytic by Zahavi et al. (2000).
In order to research the nutritional changes in fruits inoculated with the yeast C. guilliermondii, new studies are necessary. Moreover, it is vital to employ other techniques to study possible changes in the fruit and fruit surface before yeast is inoculated. This could contribute to the knowledge of the interaction between fruit and yeast.
Conclusions
The methodology in this research shows a new way of studying the dynamics of colonization of the yeast C. guilliermondii in plant tissues. The results demonstrate that C. guilliermondii is an endophytic yeast, capable of entering through the healthy cuticle of the ripe tomato fruit and colonizing the apoplastic spaces without causing damage to plant tissue cells. This makes an important contribution to the knowledge that we need to use this yeast for bio-control purposes; and improving and increasing the storage time of tomato fruits. Finally, we also calculated the speed magnitude of yeast migration during the first 70 h at 0.55±0.16 µm h-1.
Acknowledgements
The project was supported by the Vicerrectoría Académica - Pontificia Universidad Javeriana. The authors would like to thank the Anatomy and Plant Tissue Laboratory of the Biology Department of the Universidad Nacional de Colombia, Bogota and the researchers from the Mycology and Plant Pathology Laboratory (LAMFU) of the Universidad de Los Andes for technical support in the inoculation procedures.
Literature cited
Buda, G.J., T. Isaacson, A.J. Matas, D.J. Paolillo, and J.K.C. Rose. 2009. Three-dimensional imaging of plant cuticle architecture using confocal scanning laser microscopy. Plant J. 60, 378-385.
Fan, Q., S. Tian, A. Jiang, and Y. Xu. 2001. Isolation and screening of biocontrol antagonists of diseases of postharvest fruits. China Environ. Sci. 21, 313-316.
Hallman, J., A. Quadt-Hallman, W.F. Mahaffee, and J.W. Kloeper. 1997. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 43, 895-914.
Isaeva, O.V., A.M. Glushakova, S.A. Garbuz, A.V. Kachalkin, and I.Y. Chernov. 2010. Endophytic yeast fungi in plant storage tissues. Microbiology 37, 26-34.
Kraus, J., H. de Sousa, M. Rezede, N. Castro, C. Vecchi, and R. Luque. 1998. Astra blue and basic fuchin double staining of plant materials. Biotech. Histochem. 73(5), 235-243.
Larran, S., C. Mónaco, and H.E. Alippi. 2001. Endophytic fungi in leaves of Lycopersicum esculentum Mill. World j. Microbiol. Biotechnol. 17, 181-184.
Matas, A.J., E.D. Cobb, J.A. Bartsch, D.J. Paolillo, and K.J. Niklas. 2004. Biomechanics and anatomy of Lycopersicon esculentum fruit peels and enzyme-treated samples. Amer. J. Bot. 91, 352-360.
Nantawanit, N., A. Chanchaichaovivat, B. Panijpan, and P. Ruenwongsa. 2010. Induction of defense response against Colletotrichum capsici in chili fruit by the yeast Pichia guilliermondii strain R13. Biological Control 52, 145 -152.
Nassar, H.A., A. Khaled, and A. EI-Tarabily. 2005. Promotion of plant growth by an auxin-producing isolate of the endophytic yeast Williopsis saturns in maize (Zea mays L.) roots. Biol. Fert. Soils 42, 97-108.
Nicot, P.C. 2011. Classical and augmentative biological control against diseases and pests: critical status analysis and review of factors influencing their success. In: International Organisation for Biological and Integrated Control of Noxious Animals and Plants: West Palaearctic Regional Section (IOBC), Avignon, France.
Orberá, T. 2004. Métodos moleculares de identificación de levaduras de interés biotecnológico. Rev. Iberoam. Micol. 21, 15-19.
Pedroza, A., B. Quevedo, and A. Matiz. 2006. Manual de laboratorio. Procesos biotecnológicos. Editorial Pontificia Universidad Javeriana, Bogota.
Petrini, O. 1991. Fungal endophytes of tree leaves. pp. 179-197. In: Andrews, J.H. and S.S. Hirane (eds.). Microbial ecology of leaves. Springer, New York, NY.
Saligkarias, I.D., F.T. Gravanis, and H.A.S. Epton. 2002a. Biological control of Botrytis cinerea on pepper and tomato crops by the use of epiphytic yeast Candida guilliermondii strains 101 and US 7 and Candida oleophila strain I-182: II. A study on mode of action. Biological Control 25, 151-161.
Saligkarias, I.D., F.T. Gravanis, and H.A.S. Epton. 2002b. Biological control of Botritys cinerea on tomato plants by the use of epiphytic yeast Candida guilliermondii strains 101 and US 7 and Candida oleophila strain I -1 82: I. In vivo studies. Biological Control 25, 143-150.
Sharma, R.R., S. Dinesh, and S. Rajbir. 2009. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biological Control 50, 205-221.
Wills, R., B. McGlasson, D. Graham, and D. Joyce. 1998. Introducción a la fisiología y manipulación poscosecha de frutas, hortalizas y plantas ornamentales. Ediciones Acribia, Zaragoza, Spain.
Xin, G., D. Glawe, and S. Doty. 2009. Characterization of three endophytic, indole-3-acetic acid producing yeast ocurring in Populus trees. Mycol. Res. 113, 973-980.
Yu, T., J. Chen, R. Chen, B. Huang, D. Liu, and X. Zheng. 2007. Biocontrol of blue and gray mold diseases of pear fruit by integration of antagonistic yeast with salicylic acid. Microbiology 116, 339-345.
Zahavi, T., L. Cohen, B. Weiss, L. Schena, A. Daus, T. Kaplunov, J. Zutkhi, R. Ben-Arie, and S. Drobi. 2000. Biological control of Botrytis, Aspergillus and K rots on table and wine grapes in Israel. Postharv. Biol. Technol. 20, 115-124.
Zhang, D., D. Spadaro, A. Garibaldi, and M.L. Guillino. 2011a. Potential biocontrol activity of a strain of Pichia guilliermondii against grey mold of apples and its possible modes of action. Biological Control 57, 193-201.
Zhang, D., D. Spadaro, S. Valente, A. Garibaldi, and M.L. Guillino. 2011b. Cloning, characterization and expresion of an exo-glucanasa gene from the antagonistic yeast, Pichia guilliermondii against grey mold on apples. Biological Control 59, 284-293.
Zhao, Y., K. Tu, X. Shao, W. Jing, and Z. Su. 2008. Effects of the yeast Pichia guilliermondii against Rhizopus nigricans on tomato fruit. Postharv. Boil. Biotechnol. 49, 113-120.
Zhao, Y., K. Tu, J. Su S. Tu, Y. Hou, F. Liu, and X. Zou. 2009. Heat treatment in combination with antagonistic yeast reduces diseases and elicits the active defense responses in harvested cherry tomato fruit. J. Agric. Food Chem. 57, 7565-7570.
Zhao, Y., R. Wang, T. Tu, and K. Liu. 2011. Efficacy of preharvest spraying with Pichia guilliermondii on postharvest decay and quality of cherry tomato fruit during storage. Afr. J. Biotechnol. 10, 9613-9622.
Zong, Y., J. Liu, B. Li, G. Qin, and G.S. Tian. 2010. Effects of yeast antagonists in combination with hot water treatment on postharvest diseases of tomato fruit. Biological Control 54, 316-321.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
Article abstract page views
Downloads
License
Copyright (c) 2012 Agronomía Colombiana

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
© Centro Editorial de la Facultad de Ciencias Agrarias, Universidad Nacional de Colombia
Reproduction and quotation of material appearing in the journal is authorized provided the following are explicitly indicated: journal name, author(s) name, year, volume, issue and pages of the source. The ideas and observations recorded by the authors are their own and do not necessarily represent the views and policies of the Universidad Nacional de Colombia. Mention of products or commercial firms in the journal does not constitute a recommendation or endorsement on the part of the Universidad Nacional de Colombia; furthermore, the use of such products should comply with the product label recommendations.
The Creative Commons license used by Agronomia Colombiana journal is: Attribution - NonCommercial - ShareAlike (by-nc-sa)

Agronomia Colombiana by Centro Editorial of Facultad de Ciencias Agrarias, Universidad Nacional de Colombia is licensed under a Creative Commons Reconocimiento-NoComercial-CompartirIgual 4.0 Internacional License.
Creado a partir de la obra en http://revistas.unal.edu.co/index.php/agrocol/.