Effect of cationic polyelectrolytes addition in cement cohesion
DOI:
https://doi.org/10.15446/dyna.v81n188.41834Palabras clave:
cement cohesion, molecular simulation, cationic polyelectrolytes, osmotic pressure, primitive model. (es)Descargas
https://doi.org/10.15446/dyna.v81n188.41834
Effect of cationic polyelectrolytes addition in cement cohesion
Efecto de la adición de polielectrólitos catiónicos en la cohesión del cemento
Edison Albert Zuluaga-Hernández a & Bibian A. Hoyos b
a Facultad de Minas, Universidad Nacional de Colombia, Medellín, Colombia. eazuluagh@unal.edu.co
b Facultad de Minas, Universidad Nacional de Colombia, Medellín, Colombia. bahoyos@unal.edu.co
Received: January 30th, 2014. Received in revised form: July 30th, 2014. Accepted: August 19th, 2014
Abstract
Here is studied the variation in cohesion of cement main
phase (C-S-H) as a result of cationic polyelectrolytes addition (quaternary
amines spermine and norspermidine). Cohesion study was carried out by molecular
simulation techniques (Monte Carlo) using a primitive model in a canonical
ensemble (NVT). The proposed model takes into account the influence of ionic
size of each particle and the addition of polyelectrolytes with different
charge number and separation. The results obtained show that electrostatic interactions
are responsible for the cohesion of the hardened cement. It was found that in
absence of cationic polyelectrolytes, cohesion is lost when the C-S-H lamellae
are at separations larger than 1 nm. Adding cationic polyelectrolytes generates
a distribution of hydroxide ions around the polyelectrolyte charges,
facilitates the distribution of calcium and sodium ions in the entire space
between C-S-H surfaces; this allows the cohesive forces exist at greater
distances of separation between the surfaces.
Keywords: cement cohesion, molecular simulation, cationic polyelectrolytes, osmotic pressure, primitive model.
Resumen
Se estudia la variación en la cohesión de la fase
principal del cemento (C-S-H) por efecto de la adición de polielectrolitos catiónicos
(aminas cuaternarias espermina y norespermidina). El estudio de la cohesión se
realiza mediante técnicas de simulación molecular (Monte Carlo) usando un
modelo primitivo en el ensamble Canónico (NVT). El modelo propuesto tiene en
cuenta la influencia del tamaño iónico de cada partícula y la adición diferentes
polielectrólitos. Los resultados muestran que las interacciones electroestáticas
son las responsables de la cohesión del cemento. Sin polielectrolitos la
cohesión se pierde cuando las láminas de C-S-H tienen separaciones mayores de 1
nm. Los polielectrolitos generan que los iones hidróxido se distribuyan
alrededor de las cargas de estos, facilitando la distribución de los iones de
calcio y de sodio en el espacio de separación entre las superficies del C-S-H,
esto permite que las fuerzas de cohesión existan a mayores distancias de separación.
Palabras clave: cohesión del cemento, simulación molecular, polielectrolitos catiónicos, presión osmótica, modelo primitivo.
1. Introduction
Cement is considered an artificial mineral, whose raw materials for elaboration are limestone, clay, sandstone and gypsum. These materials are put through a series of processes to complete the elaboration of the final product, which include grinding, homogenization (wet or dry), calcination and clinker trituration [1]. The lamellae of calcium silicate hydrate (C-S-H) constitute between 50 to 67% of the final volume of hydration products, becoming the main component of hardened cement. C-S-H is therefore responsible of the main macroscopic mechanic properties of cement, specially the resistance to compression stress and durability.
To give an appropriate description of factors which influence the cement of cohesion, it must consider the interactions that can occur among C-S-H lamellae, ions in interstitial solution and the interaction between these two factors. A detailed description of cohesion, it should contemplate in its model the attractive forces which generate surfaces of C-S-H charged negatively and confined ions that are located among C-S-H lamellae.
Up to now it has postulated two interactions that give explanation to the cement of cohesion phenomenon: the attraction between the ions of interstitial solution with lamellae, due to electrostatic attractions generated by the high ionic correlation [2], this occurs with high pH values; and the interaction between ions that are embedded in the C-S-H lamellae which generate attractions closer to a covalent bond character [3]. Hence the system presents characteristics of ionic interactions and covalent bonds, which allow the lamellae remain linked together, these interactions are mainly responsible for the cohesion of hardened cement.
The debate that has taken place among researchers to elucidate the interactions is more representative, it has been continuous in recent years [4-5]. However, the character of covalent bond is not clearly defined. The results obtained in a previous study [4] show that about 60% are interactions of ionic character, which implies that the covalent character is around 40%, this could give clue that calcium ions are embedded in C-S-H structure which have a less interchangeable character and they have a limited movement. While interstitial ions (among lamellae) have a mobile more character.
In this article, we wish to present a model that describes the most representative interactions which generate cement cohesion in C-S-H lamellae with separations of up to 2 nm and the cations presence between the lamellae. For this model only considered electrostatic interactions (ionic character), with the understanding that such interactions are the most representative. This has been widely studied, both theoretically and experimentally [6-11].
The Poisson-Boltzmann and the DLVO theories were the first two theoretical explanations to the cohesion phenomenon. These theories do not consider the high ionic correlation generated between divalent ions of Ca2+ and the C-S-H lamellae, and inaccurately predict the cohesion of hardened cement paste [6,8,11-14].
The primitive models have allowed explaining the cohesion of cement in an adequate manner, and allowing calculating the attraction forces generated between the ions present in solution and the C-S-H lamellae, using Coulomb's potential. Results show that these kinds of models are able to represent the strong ionic correlation in cement at a molecular scale, due to the coupling between co-ions and counterions in the electrolytic solution and the lamellae of C-S-H (with high density of negative superficial charges). The strength of attraction forces is associated with the cement's capability of resisting compression stress, but is also associated with a weak response to traction stress [4-5,11].
Recent studies propose a possible way to modify the molecular interactions in order to modulate the ductility of cement by implementing changes in the interactions between the electrolytic solution and the C-S-H lamellae [5]. This consists in adding polyelectrolytes (to the clinker) with the goal of creating cement with higher resistance to traction stress due to higher interaction range between C-S-H lamellae and the electrolytic solution.
Literature shows important breakthroughs in this matter, both theoretical and experimental [5,15-18]. However, there is still work left in the way to improve the macroscopic properties of cement, modifying the interactions solution-lamella by the inclusion of new compounds in the solution.
In this work, Monte Carlo molecular simulation is used to determine the changes in cohesion forces due to the addition of cationic polyelectrolytes to the clinker and to establish the variation of this cohesion force with the separation between C-S-H lamellae. Thus, an important step is made towards accurately modeling and understanding cement in a nanometric scale, which allow modulating and ultimately improving the macroscopic properties of this important material.
2. Model
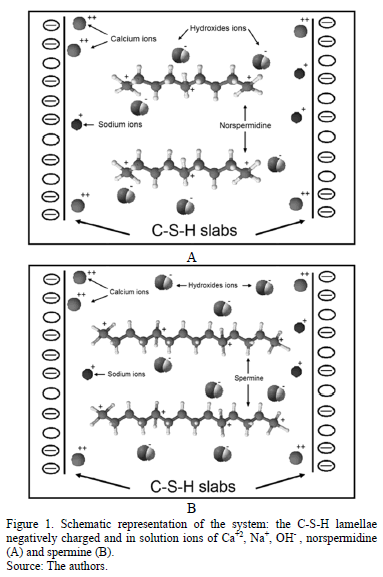
For the development of this model it was considered that the interactions between nanoparticles of C-S-H can be represented as the interaction of two flat walls negatively charged, with a charge density correspondent to the natural mineral tobermorite. All the ions in the interstitial solution were represented explicitly, and water was modeled as a continuum dielectric medium, characterized only by its relative permittivity. The confined solution was represented as a solution of electrolytes and polyelectrolytes with positively and negatively charged ions in order to guarantee neutral charge of the system as a whole, and whose mean concentration varies with the separation between the C-S-H lamellae.
For the simulations without polyelectrolytes, the interstitial solution was represented with calcium, sodium and hydroxide ions. In order to study the effect of the addition of cationic polyelectrolytes, this compounds were represented as quaternary amines, as they are the only stable polyelectrolytes at the pH conditions that characterize the cement paste (pH>12.5). The two polyelectrolytes chosen for this study were norspermidine (H3N+-(CH2)3-N+H2-(CH2)3-N+H3) (Fig. 1 A) and spermine (H3N+-(CH2)3-N+H2-(CH2)4-N+H2-(CH2)3-N+H3) (Fig. 1 B).
In order to represent the attractive and repulsive interactions generated at a molecular scale in cement cohesion, a primitive model was used, with Lennard-Jones and Coulomb's potential.
The model here proposed presents two novel aspects respect to previous studies [5-6,8]. The first aspect is the consideration of different atomic sizes of the species involved in the electrolytic solution. The second aspect is the addition of a cationic polyelectrolyte with different separation distance between the positively charged amine groups.
The primitive model represents the ions in the
electrolytic solutions as soft spheres with a positive or negative electric
charge, depending on the nature of the ion. These ions are separated a distance
(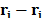 ).
The solvent in which ions are submerged is modeled as a continuum medium with a
constant relative permittivity (
).
The solvent in which ions are submerged is modeled as a continuum medium with a
constant relative permittivity (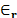 ).
).
Coulomb's potential is used to describe the electrostatic interactions between all ions in the solution, and between the ions and the negatively charged sites at the surfaces of C-S-H:

Where 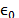 and d are the vacuum permittivity and the
characteristic diameter of each ion present in the system, respectively.
and d are the vacuum permittivity and the
characteristic diameter of each ion present in the system, respectively.
Short range interactions are described using Lennard-Jones potential:
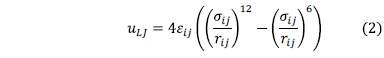
The values of parameters e and s were taken from the general force field CLAYFF [19], and are listed in Table 1.
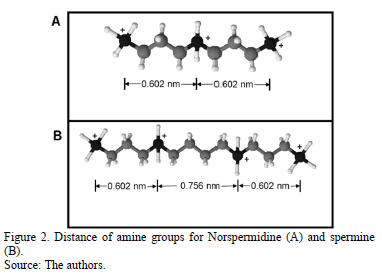
Cationic polyelectrolytes were added in the center of the simulation box and were distributed equally and perpendicular to the surfaces of C-S-H. The potentials for bonds between carbons and hydrogens in the polyelectrolytes were not considered explicitly, as they are not relevant in electrostatic interactions. The polyelectrolyte norspermidine have three positive charges, and the spermine is a quaternary amine that has four positive charges. The distance between each amine group is illustrated in Fig. 2.
The interaction parameters between atoms of different nature are evaluated using the Lorentz-Berthelot mixing rules:
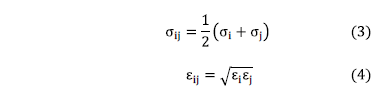
The lamellae of C-S-H were modeled as uniform surfaces of 60 nm long (X axis) and 30 nm wide (Y axis). For the separation between lamellae of C-S-H, four different separations were considered in simulations (0.5, 1, 1.5 and 2 nm).
Assuming C-S-H has a structure similar to tobermorite [13], the surface charge density for the walls was set to 4.8 e-/nm2. Although size and charge density of C-S-H lamellae can vary with concentration of species and pH, these variations are not considered in this work.
For flat surfaces, cohesion force is directly related to the osmotic pressure of the system, and this osmotic pressure is also related to the interactions between particles. To obtain the net osmotic pressure it is necessary to evaluate the confinement osmotic pressure and the bulk osmotic pressure when the system is equilibrated. The confinement osmotic pressure of the interstitial solution can be calculated with the ionic distribution near the C-S-H surface:
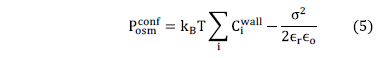
With the concentration of ions
near the charged wall it is possible to evaluate the expression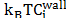 .
The sum of the interactions of a wall with all the ions present in the
simulation box and the other charged wall is obtained by the Maxwell term
.
The sum of the interactions of a wall with all the ions present in the
simulation box and the other charged wall is obtained by the Maxwell term 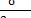 [6].
[6].
The bulk osmotic pressure
considers the effects of the ions fraction volume (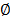 ),
the ion bulk concentration for each ion species (
),
the ion bulk concentration for each ion species (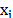 ),
the average energy of the system
),
the average energy of the system 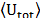 and the correlation function between the ions
and the correlation function between the ions 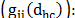
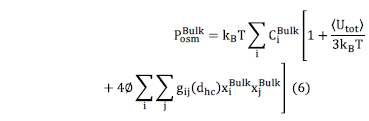
For the calculation of 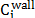 and
and  in eq. (5 and 6), it is considered that the
"wall" region extends from each wall to a distance of 0.6
in eq. (5 and 6), it is considered that the
"wall" region extends from each wall to a distance of 0.6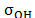 (2.13
Å), and the "bulk" region the remaining space in between them.
(2.13
Å), and the "bulk" region the remaining space in between them.
The correlation function between ions indicates the direct influence of a given particle over another; located at a distance d (the diameter of the ion). This function is calculated as:

Therefore, the net osmotic pressure of the system is obtained by:
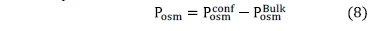
Simulation results are the equilibrium distributions of all ions in the interstitial solution, which allow calculating the osmotic pressure. This osmotic pressure is an indicator of the cohesion force of the composed system of the C-S-H lamellae and the electrolytic interstitial solution.
3. Simulation details
Monte Carlo simulations were performed using a canonical ensemble (NVT) at 25°C, with periodic boundary conditions in two dimensions (X and Y axis). The cut radius for electrostatic interactions was set to 60 nm, which corresponds to the length of the simulation box.
For the analysis of the cohesion between C-S-H lamellae, three different systems were used: with no polyelectrolytes (only ions of Ca+2, Na+ and OH-); with norspermidine: H3N+-(CH2)3-N+H2-(CH2)3-N+H3, and lastly with spermine: H3N+-(CH2)3-N+H2-(CH2)4-N+H2-(CH2)3-N+H3. For each system four simulations were conducted, at four different separation distances, for a total of twelve simulations.
Table 2 summarizes the amount of ions used in each simulation. The polyelectrolytes were added in the Z axis, perpendicular to the surfaces of C-S-H.
The number of ions was determined considering that the concentration of the solution was 20mM for Ca2+ ions, 100 mM for Na+ ions and 15 mM for polyelectrolytes (Norspermidine and spermine) [5,11]. OH- ions were added as necessary in order to guarantee the electric neutrality of the system. Thus, it is possible to obtain a similar pH to that observed in real cement paste (pH>13).
For all simulations, 3x106 equilibration cycles and 1x106 production cycles were conducted, with a random initial configuration for ions in the interstitial solution and the charges distributed in the surface of C-S-H.
In order to determine density profiles (dimensionless) of ionic species, the number of particles contained in thin layers, of thickness DZ, parallel to the C-S-H lamellae was determined:
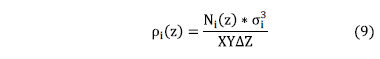
4. Results
The results of different simulations conducted in the canonical ensemble (NVT) were divided in three groups as follows: in absence of polyelectrolytes, with norspermidine and with spermine. For each case, four different simulations were conducted, at four different separation distances (0.5, 1, 1.5 y 2 nm). As some of the results show similar patterns, only the most representative are shown.
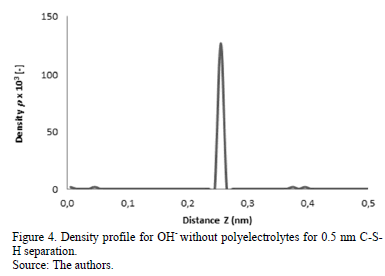
From density profile of the system in absence of polyelectrolytes (Fig. 3) it can be seen that the Ca2+ ions are distributed along the surface of the two lamellae of C-S-H. This favors the cohesion between the walls, given that the OH- ions (Fig. 4) are mostly located in the middle and attract the Ca2+ ions that are distributed evenly at the two walls.
It can be seen from Fig. 3 that the sodium ions (gray line) are mostly located near the two surfaces of C-S-H, but unlike the calcium ions, some of these ions are located near the middle plane, aiding to stabilize the region with high concentration of OH- ions. Up until a separation distance of 1 nm a strong attraction between C-S-H lamellae was observed.
Unlike the separations of 0.5 and 1 nm, when the separation between the walls is 1.5 and 2 nm, the calcium and sodium ions are located near only one wall (Fig. 5) at a distance of approximately 0.5 nm, and the OH- ions (Fig. 6) are located in a layer at a further distance. This means that all the species are adhered at just one surface, which is the main reason why the cohesion in cement is lost.
The results obtained for the system at separation distances of 1.5 and 2 nm are similar. For greater separation distances, a rupture in the system is observed, generating the adhesion of the species at only one lamella causing loss of cohesion.
These results are associated with the macroscopic property of cement causing great resistance to compression stress but very little to traction stress.
The results obtained with the addition of cationic polyelectrolytes show that attraction forces that guarantee cohesion can be achieved between the lamellae of C-S-H at separations of 1.5 and 2 nm, which are not observed in the absence of these polyelectrolytes in the electrolytic solution.
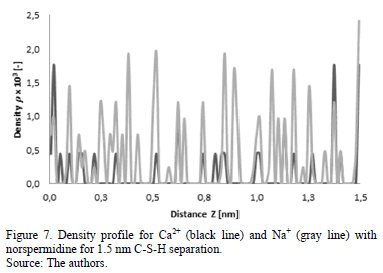
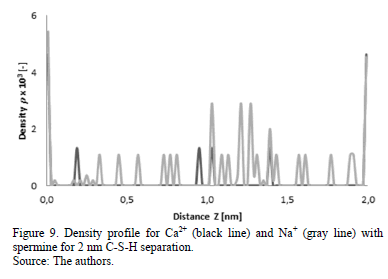
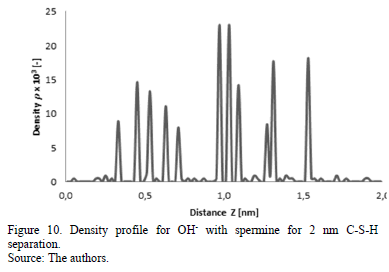
This behavior can be corroborated with the density profiles of calcium, sodium and hydroxide ions (Fig. 7-10) obtained for simulations at separations of 1.5 and 2 nm with the presence of norspermidine and spermine, respectively.
Fig. 7 and Fig. 8 show that the addition of norspermidine generates a distribution of hydroxide ions around the positive charges. This in turn, by means of electrostatic attraction, enables the calcium and sodium distribution in the entire separation space between C-S-H lamellae.
The addition of norspermidine generates attraction forces between the two negatively charged surfaces of C-S-H, at separations of 1.5 and 2 nm. These results indicate that the presence of norspermidine indirectly enables the distribution of the calcium and sodium ions throughout the separation space between the C-S-H lamellae, which allows cohesion forces to exist at greater separation distances between the surfaces. The same phenomenon is observed with the addition of spermine (Fig. 9 and Fig. 10). This polyelectrolyte has four different positive charges at different bond distances between charged amine groups.
From the analysis of the previous results, it can be established that the systems with the addition of cationic polyelectrolytes (norspermidine and spermine) in presence of sodium, calcium and hydroxide ions evidence attraction forces strong enough to accomplish cohesion at separations greater than 1 nm.
The attraction present in cement is possible due to electrostatic interactions between the highly charged C-S-H walls and the ions present in the interstitial solution. This interaction was evaluated by the calculation of the net osmotic pressure (Fig. 11). For the system in absence of electrolytes it is observed that for the separations of 1.5 and 2 nm there is no evidence of cohesive forces, given that the ions distribute along a single surface of C-S-H. The opposite is observed when cationic polyelectrolytes are added to the system, where an attractive force between C-S-H lamellae is evident at separations greater than 1nm.
From Fig. 11 it can be seen that the results with spermine are better than for norspermidine (greater net cohesion force and longer range effect). This behavior is due to the additional charge on the spermine structure, and a greater chain length, which allow the spermine to generate attractive forces at a greater distance between negative charges of the C-S-H lamellae. This is congruent with studies previously conducted [5,13], which explain the cohesion of the cement paste due to the strong Coulombic interactions between the charged surfaces of C-S-H and the confined ions between this surfaces, showing that the two main factors that govern cohesion are the superficial charge density of C-S-H lamellae and the valence of positively charged ions present in the interstitial solution.
The attraction forces between the C-S-H lamellae can be modified with the addition of quaternary amines, since these generate a distribution of the sodium and calcium ion throughout the entire separation space between C-S-H lamellae. This causes a net osmotic pressure that guarantee cohesion force at greater separation distances than in the absence of amines.
5. Conclusions
Results obtained in this work allow concluding that the primitive model here developed is an adequate representation of the electrostatic interactions between the C-S-H lamellae and the electrolytic solution. This corroborates that the electrostatic interactions are responsible for the cohesion of hardened cement, and are crucial to widen the understanding of the phenomenon that take place at a nanometric scale. In this manner, it is possible to design strategies to modify and improve the macroscopic mechanic properties of cement.
In the absence of polyelectrolytes, cohesion is lost at separation distances greater than 1 nm. Adding polyelectrolytes, such as quaternary amines, causes the hydroxide ions to distribute around the positive amine charges, and these hydroxide ions, by means of electrostatic interactions, also enable the distribution of calcium and sodium ions throughout the entire space of separation between the C-S-H lamellae. This allows the cohesion forces to exist at greater separation distances between surfaces.
The results here obtained show that the spermine produces a greater net cohesion force, and an effect of longer range than norspermidine.
With the obtained results it is expected that systems with a higher charge density and polyelectrolytes with a higher number of positive charges would produce greater ranges of separation between the C-S-H lamellae.
References
[1] Neville, A.M. and Brooks, J.J., Concrete technology. London: Prentice Hall, 2010.
[2] Bellotto, M. and Bozzetto, G., On the origin of cement setting, and how to control the properties of the fresh and hardened material, Special Issue for International Congress on Materials & Structural Stability, pp. 74-79, 2013.
[3] Thomas, J., Scherer, W., Biernacki, G., Luttge, A., Bullard, J., Bishnoi, S., and Dolado, J., Modeling and simulation of cement hydration kinetics and microstructure development, Cement and Concrete Research, 41, pp. 1257-1278, 2011. https://doi.org/10.1016/j.cemconres.2010.10.004
[4] Pellenq, R., Lequeux, N. and Damme, H., Engineering the bonding scheme in C-S-H: The iono-covalent framework, Cement and Concrete Research, 38, pp. 159-174, 2008. https://doi.org/10.1016/j.cemconres.2007.09.026
[5] Pochard, I., Labbez, C., Nonat, A., Vija, H. and Jönsson, B., The effect of polycations on early cement paste, Cement and Concrete Research, 40, pp. 1488-1494, 2010. https://doi.org/10.1016/j.cemconres.2010.06.002
[6] Kjellander, R., Marcelja, S. and Quirk, J., Attractive double-layer interactions between calcium clay particles. Journal of Colloid and Interface Science, 126, pp. 194-211, 1988. https://doi.org/10.1016/0021-9797(88)90113-0
[7] Manzano, H., Dolado, J.S., and Ayuela, A., Elastic properties of the main species present in portland cement pastes, Acta Materialia, 57, pp. 1666-1674, 2009. https://doi.org/10.1016/j.actamat.2008.12.007
[8] Jonsson, B., Wennerstrom, H., Nonat, A. and Cabane, B., Onset of cohesion in cement paste, Langmuir, 20, pp. 6702-6709, 2004. https://doi.org/10.1021/la0498760
[9] Labbez, C., Nonat, A., Pochard, I. and Jönsson, B., Experimental and theoretical evidence of overcharging of calcium silicate hydrate, Journal of Colloid and Interface Science, 309, pp. 303-307, 2007. https://doi.org/10.1016/j.jcis.2007.02.048
[10] Pellenq, R., Caillol, J. and Delville, A., Electrostatic attraction between two charged surfaces: A (N,V,T) Monte Carlo simulation, The Journal of Physical Chemestry, 101, pp. 8584-8594, 1997. https://doi.org/10.1021/jp971273s
[11] Jonsson, B., Nonat, C., Labbez, B., Cabane, B. and Wennerstrom, H., Controlling the cohesion of cement paste, Langmuir, 21, pp. 9211-9221, 2005. https://doi.org/10.1021/la051048z
[12] Lesko, S., Lesniewskaa, E., Nonat, A., Mutinb, J. and Goudonneta, J., Investigation by atomic force microscopy of forces at the origin of cement cohesion, Ultramicroscopy, 86, pp. 11-21, 2001. https://doi.org/10.1016/S0304-3991(00)00091-7
[13] Labbez, C., Jonsson, B., Pochard, I., Nonat, A. and Cabane, B., Surface charge density and electrokinetic potential of highly charged minerals: Experiments and Monte Carlo simulations on calcium silicate hydrate, The Journal of Physical Chemistry B, 110, pp. 9219-9230, 2006. https://doi.org/10.1021/jp057096
[14] Kjellander, R., Marcelja, S., Pashley, R. and Quirk, J., Double-layer ion correlation forces restrict calcium-clay swelling, The Journal of Physical Chemestry, 92, pp. 6489-6492, 1988. https://doi.org/10.1021/j100334a005
[15] Delville, A., Influence of interionic correlations on the free energy of charged interfaces: a direct derivation from (N,V,T) Monte Carlo simulations, The Journal of Physical Chemistry, 108, pp. 9984-9988, 2004. https://doi.org/10.1021/jp049189h
[16] Labbez, C. and Jonsson, B., New Monte Carlo method for the titration of molecules and minerals, Computer Science, pp. 4699, 66-72, 2007.
[17] Labbez, C., Jonsson, B., Skarba, M. and Borkovec, M., Ion-ion correlation and charge reversal at titrating solid interfaces, Langmuir, 25, pp. 7209-7213, 2009. https://doi.org/10.1021/la900853e
[18] Delville, A., Gasmi, N., Pellenq, R., Caillol, J. and Damme, H., Correlations between the stability of charged interfaces and ionic exchange capacity: A Monte Carlo study, Langmuir, 14, pp. 5077-5082, 1998. https://doi.org/10.1021/la9802872
[19] Randall, T., Jian-jie, L. and Kalinichev, A., Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field, The Journal of Physical Chemestry, 108, pp. 1255-1266, 2004. https://doi.org/10.1021/jp0363287
E.A. Zuluaga-Hernández, received the BSc in 2010 and MSc in 2014 degrees both in Chemical Engineering; all of them from the Universidad Nacional de Colombia, Medellín, Colombia. Currently He is a student of the PhD in Universidad Nacional de Colombia, Medellín, Colombia.
B.A. Hoyos, received the BSc. in 1994 and MSc in 2003 degrees both in Chemical Engineering; and the PhD degree in Energy Systems in 2010, all of them from the Universidad Nacional de Colombia, Medellín, Colombia. Currently he is a Full Professor in the Departamento de Procesos y Energía, Facultad de Minas, Universidad Nacional de Colombia, Medellín, Colombia. His research interests include molecular simulation, modeling asphaltene aggregation and viscosity, modelling wettability alteration by surfactants and specific adsorption.
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2014 DYNA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
El autor o autores de un artículo aceptado para publicación en cualquiera de las revistas editadas por la facultad de Minas cederán la totalidad de los derechos patrimoniales a la Universidad Nacional de Colombia de manera gratuita, dentro de los cuáles se incluyen: el derecho a editar, publicar, reproducir y distribuir tanto en medios impresos como digitales, además de incluir en artículo en índices internacionales y/o bases de datos, de igual manera, se faculta a la editorial para utilizar las imágenes, tablas y/o cualquier material gráfico presentado en el artículo para el diseño de carátulas o posters de la misma revista.