Caracterización Fisiológica y Físico-Química del Fruto de la Guanábana (Annona muricata L. cv. Elita)
Physiological and Physico-Chemical Characterization of the Soursop Fruit (Annona muricata L. cv. Elita)
Keywords:
Respiration, ethylene, transpiration, postharvest physiology. / Respiración, etileno, transpiración, fisiología poscosecha. (es)Downloads
Physiological and Physico-Chemical Characterization of the Soursop Fruit (Annona muricata L. cv. Elita)
Caracterización Fisiológica y Físico-Química del Fruto de la Guanábana (Annona muricata L. cv. Elita)
Carlos Julio Márquez Cardozo1; Verónica Villacorta Lozano2; Diana Paola Yepes Betancur3; Héctor José Ciro Velásquez4 and José Régulo Cartagena Valenzuela5
1 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Faculty of Agricultural Sciences - Department of Agriculture Engineering and Foods. A.A. 1779, Medellín, Colombia. <cjmarque@unal.edu.co>
2 Agricultural Technical Engineer. Universidad Nacional de Colombia - Sede Medellín - Faculty of Agricultural Sciences - Department of Agriculture Engineering and Foods. A.A. 1779, Medellín, Colombia. <vvillacortal@unal.edu.co>
3 Food Engineer. Agroindustry Area, Innovation Center, Agribusiness and Tourism, National Apprenticeship Service - SENA. Rionegro, Colombia. <diayepesb@misena.edu.co>
4 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Faculty of Agricultural Sciences - Department of Agriculture Engineering and Foods. A.A. 1779, Medellín, Colombia. <hjciro@unal.edu.co>
5 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Faculty of Agricultural Sciences - Department ol Agronomy Sciences. A.A. 1779, Medellín, Colombia. Medellín, Colombia. <jrcartag@unal.edu.co>
Recibido: Diciembre 10 de 2010; aceptado: Abril 9 de 2012.
Abstract. Fruit consumption is increasing around the world, just as its population. The World Health Organization recommends a minimum consumption of fruit 120 kg/person - year. Fruits such as soursop provide nutrients, phytochemicals and antioxidants which are vital to human health, as well as bioactive substances such as vitamin C, flavonoids, anthocyanins and carotenoids, among others. In this research, soursop (Annona muricata L. cv. Elita) fruits were collected at physiological maturity in two production seasons for their physiological (respiration rate, ethylene production and physiological loss of weight) and physico-chemical characterization (pulp, seeds and skin yield, total soluble solids (TSS), total acidity, pH and firmness). We found that ethylene production peaked at day 6 after-harvest, scoring 133.2 mL kg-1 h-1. This parameter was found to increase during postharvest, with peaks on days 4 and 6, coinciding with the climacteric peaks of biphasic respiration, the largest of which reached a value of 186.9 mg CO2 kg-1 h-1. This is probably the starter for the cascade of events that feature the ripening process, among which changes in TSS, acidity and fruit firmness were outstandingly visible.
Key words: Respiration, ethylene, transpiration, postharvest physiology.
Resumen. En el mundo, el consumo de frutas es creciente, al igual que la población. La Organización Mundial de la Salud recomienda un consumo mínimo de 120 kg/persona al año. Frutas como la guanábana aportan nutrientes, fitoquímicos y antioxidantes de vital importancia para la salud humana, además de sustancias bioactivas como vitamina C, flavonoides, antocianinas y carotenoides, entre otros. En la presente investigación, frutos de guanábana (Annona muricata L. cv. Elita), fueron recolectados en estado de madurez fisiológica o de cosecha, en dos épocas de producción, para determinar sus características fisiológicas (tasa de respiración, producción de etileno y pérdida fisiológica de peso), y físico-químicas (rendimiento en pulpa, semillas y piel, sólidos solubles totales (SST), acidez total, pH y firmeza). Se pudo constatar que la producción de etileno alcanzó un máximo en el día 6 de la poscosecha, con un valor de 133,2 mL kg-1 h-1. Este parámetro presentó un comportamiento creciente durante la poscosecha, con picos en los días 4 y 6, coincidentes con los picos climatéricos bifásicos de la respiración, el mayor de los cuales alcanzó un valor de 186,9 mg de CO2 kg-1 h-1. Esto, probablemente es el activador de la cascada de eventos propios de la maduración, donde sobresalen los cambios los niveles de sólidos solubles totales, acidez y la firmeza de los frutos.
Palabras clave: Respiración, etileno, transpiración, fisiología poscosecha.
Soursop (Annona muricata L.) is grown in the Caribbean and the equatorial belt of the Americas, primarily in Bermuda, Bahamas, Cuba, Dominican Republic, St. Vincent, Grenada, southern Mexico, Costa Rica, Puerto Rico, Colombia, Brazil and Ecuador. In addition, there are some commercial crops in Vietnam, Australia, New Zealand, Africa, Southeast China and Southern United States (Rami et al., 1995; Orellana and Martínez, 2002; Cuadros, 2008).
Colombia has 114,174,800 million hectares of total area, out of which 54% corresponds to sparsely studied, mostly forested lands (Arango and Bacanumenth, 1999). Of the remaining area, only 0.23% (equivalent to 259,409 ha) is devoted to fruit crops, out of which about 1,300 ha correspond to soursop plantations, with a production of 10,010 t year-1, and 7.7 t ha-1 year-1 average yield. The departments of Valle, Tolima, Cauca and Cundinamarca are the most productive ones (Ramírez et al., 1998).
In climacteric fruits such as soursop, ripening is a complex, genetically controlled process marked by the synthesis and degradation of new compounds (Salisbury and Ross, 1994; Taiz and Zeiger, 2006; Azcón, 2008). Some of the changes that occur during ripening and postharvest directly affect the shelf life and sensorial and nutritional quality of fruits (Hosakote et al., 2005; Wu et al., 2005; Ornelas et al., 2008).
The annonaceae exhibit important metabolic changes, manifested as color and brightness variations, as well as decrease of green hue; all of them resulting from the degradation of chlorophyll to pheophorbide and clorofilida (Castro et al., 2008). Another aspect that reveals intense metabolic activity is related to the hydrolysis of high molecular weight carbohydrates (starch type) to simpler compounds such as disaccharides and monosaccharides (mainly sucrose, glucose and fructose), usually due to high enzymatic activity of a and b amylases (DeMan, 1999; Kader, 2002).
Postharvest handling and processing of these fruits is clearly deficient. This can be largely attributed to lack of knowledge on many of the features that typify their ripening, among which physiological (Trinchero et al., 1999) and physicochemical (Mercado et al., 1998) ones certainly stand out.
In this context, the purpose of the present study was the assessment of the physiological and physico-chemical features that express the most significant metabolic changes taking place in the soursop fruit (A. muricata L. cv. Elita) during postharvest, by means of a study conducted on fruits from the Cauca valley, in Colombia.
MATERIALS AND METHODS
The present research was carried out on soursop fruits obtained from the orchards of "La Española" farm (1,070 m.a.s.l., 23 °C average temperature, 1,225 mm annual precipitation, mean solar radiation of 4.8 W m2 - d, and relative humidity of 83%), which belongs to the firm "Agrícola Varahonda", and is located in the municipality of Pradera, in the agroindustrial area of the Cauca Valley.
The fruits were collected at physiological maturity, which corresponds to 16 weeks after fruit set, featured by the proper harvest color and firmness of this cultivar. On the same day, they were transported in styrofoam containers to the Fruit and Vegetable Laboratory of the Faculty of Agricultural Science at Universidad Nacional de Colombia, Medellin campus, where all the analyses were conducted.
For the analysis of the physiological traits, we used 12 experimental units (EU) on which, for a period of 10 d, we recorded the following parameters. In the first place, the respiration rate was determined by adapting the chemical method of Petenkoffer, which consisted in weighing and placing the sample in an airtight container. CO2resulting from respiration was driven through a basic solution (NaOH 0.1 N), which it partially neutralized. The amount of carbonic acid (H2CO3) produced by this reaction was calculated as the difference between the initial and final concentrations. The amount of CO2 released by the fruit in 1 h of experiment at 23 °C was calculated by stoichiometry and expressed in milligrams of CO2 kg-1 h-1 (Angueira et al., 2003). In the second place, the production of ethylene was analyzed in a VarianT 3800 gas chromatographer (GC) equipped with a 60 m long DB capillary column of internal diameter 53 mm (J & W ScientificT). On a Flame Ionization Detector (FID), we used Helium (He) carrier gas at a flow rate of 3 mL min-1. The temperature conditions were 150 °C for the injection port, 280 °C for the detector, and 40 °C for the oven, which was then increased at a rate of 40 °C min-1, to finally reach 300 ºC. Each run took a total of 20 min. The areas under the curve obtained from the analysis were compared to a calibration curve based on the performance of a standard gas (Oxígenos de ColombiaT, 20 ppm). Ethylene production was expressed in L of ethylene/ kilogram/hour (adapted from Cruz et al., 2007). Assessed on a daily basis, the physiological loss of weight was calculated by gravimetry as the difference with the initial record using an Ohaus™ Navigator 1121 analytical balance (Bernal, 1993).
Studied at a rate of 12 per day, 120 fruits were available for the physico-chemical assessment, covering a period of 10 days, during which they started senescence. The parameters in question were external and internal color (i.e., epidermal and endocarpic, respectively), assessed with an X-Rite™ SP-60 sphere spectrophotometer, measurement aperture size 4 mm, and illuminant D-65 with a 10° observer angle. From the reflection spectra we obtained the color coordinates CIE L*, a* and b*; where L* is a measure of brightness, a* represents green (-) to red (+) chromaticity, and b* stands for blue (-) to yellow (+) chromaticity. Three equatorial measures expressed as arithmetic average were taken on the epidermis of each EU. The same was done for the endocarp or pulp (Almela et al., 2000). Pulp, seeds and skin yield values were obtained by gravimetry, the results expressed as percentage (Camacho and Romero, 1996). The concentration of total soluble solids (TSS) was determined at 23 °C using a Leica auto ABBE™ refractometer (0-32% measuring range), and the results were expressed in Brix degrees. Total acidity was obtained by potentiometric acid-base titration and expressed as percentage of malic acid using a CG-840B Schott™ potentiometer, which was also used to determine pH (Bernal, 1993). Unidirectional compression tests were applied to measure fruit firmness using a cylindrical stainless probe, diameter 8 mm, used at a penetration rate of 2 mm s-1, to reach a deformation depth of 40 mm. The experiments were conducted under average laboratory conditions of 23 °C and 65% RH. The equipment was a TA-XT2™ texture analyzer operated through Texture Expert Excced 2.64 (Stable Micro Systems, London, UK 2002™) (Ciro et al., 2005).
The results were statistically processed through standard deviation and arithmetic average (on STATGRAPHIC plus 5.1.), which were then plotted against time after harvest.
RESULTS AND DISCUSSION
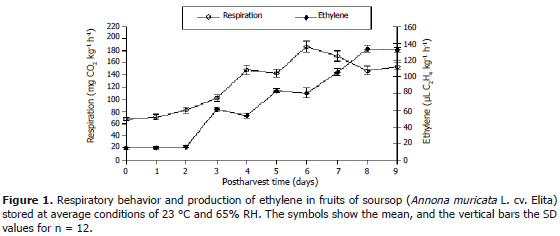
Figure 1 shows the first respiratory peak on day 4 after harvest, with a production rate of 148.1 mg CO2 kg-1 h-1± 9.43. On day 5, there was a slight decrease, followed by a sharp increase, reaching its maximum intensity (highest climacteric peak) on day 6, with a value of 186.1 mg CO2 kg-1 h-1 ± 9.56 SD. This allows classifying this fruit as climacteric of the biphasic type, as reported by Worrel et al. (1994). This feaature contrasts partially with Cherimoya (A. cherimola Mill), whose respiratory behavior has shown only one climacteric peak (Paull, 1996). These respiratory peaks are probably reflecting an shift in mitochondrial activity, which in turn results from increased availability of carboxylate substrates. The later are likely to be induced by starch enzymatic degradation resulting in the formation of low molecular weight carbohydrates due to very intense activity of specific tissues and structures (Bruinsma and Paull, 1984). Increase in CO2 production during climacteric respiration is associated to the availability of large amounts of ATP, which is required for the biochemical and physiological processes that are in turn associated to ripening, and allow the fruit to reach both the desirable organoleptic features required for consumption and its later senescence. This feature is confirmed by the short shelf life and high perishability of this product (Kader, 2002).
As for the production of C2H4, the lowest concentration records remained steady for the first 3 days after harvest. On day 4, there was an increase that reached a value of 44.9 mL of C2H4 kg-1 h-1, followed by a slight decrease on day 5. Again on day 6, there was a raise, and then a new decrease on day 7, after which we observed a steady increase until day 9, when ethylene production reached a maximum of 133.2 mL kg-1 h-1. This reveals an intense synthesis of this product during the studied period, accompanied by rapid respiratory activity.
As for the production of C2H4, the lowest concentration records remained steady for the first 3 days after harvest. On day 4, there was an increase that reached a value of 44.9 mL of C2H4 kg-1 h-1, followed by a slight decrease on day 5. Again on day 6, there was a raise, and then a new decrease on day 7, after which we observed a steady increase until day 9, when ethylene production reached a maximum of 133.2 mL kg-1 h-1. This reveals an intense synthesis of this product during the studied period, accompanied by rapid respiratory activity.
Marking the end of the ripening stage and the onset of senescence, this process occurs without any external influence. It is characterized by a series of changes in the chemical composition of the pulp, involving the action of several enzymes such as polygalacturonase (PG), pectin methyl esterase (PME), endo-1-4 b-glucanase, a-arabinosidase and b galactosidase (b-GAL), all of which exhibit activity changes as part of the metabolic dynamics that induce ripening (Bruinsma and Paul, 1984; Brovelli et al., 1999; Armida et al. 2011).
Physiological loss of weight. According to Figure 2, the physiological loss of weight showed a linear increase throughout the postharvest stage (represented as Y = 2.15X + 1.19; R2 = 0.98), although a slight slow down could be observed since day 6, marked by a weight loss of 14.71%. Throughout the post-harvest stage (including ripening and senescence), the studied soursop fruits lost a total of 21.72% of their weight. In fruits of custard apple (Annona cherimola Mill) stored at 22 °C and 60% RH, this parameter has has been observed to reach 14.2% on day 8 after harvest (Paull, 1996); while fruits of sugar apple (Annona squamosa L.) stored for 7 d at 4 °C and 95% RH have shown records of 6.7% (Poot, 2003). Remarkable physiological losses in fruit weight in the Annonaceae lead to their rapid physical deterioration as compared to other climacteric fruits such as sapote (Pouteria sapota Jacq.), which have been found to lose 10.8% of their weight in 6 d of storage at 25 °C (Díaz et al., 2000); or mango (Mangifera indica L. cv. Namdok Mai), registering losses up to 16% after 17 d of storage at 30 °C. Physiological loss of weight is defined as the result of transpiration and respiration, which deteriorate the fruit by reducing tissue turgor (Sothornvit and Rodsamran, 2008).
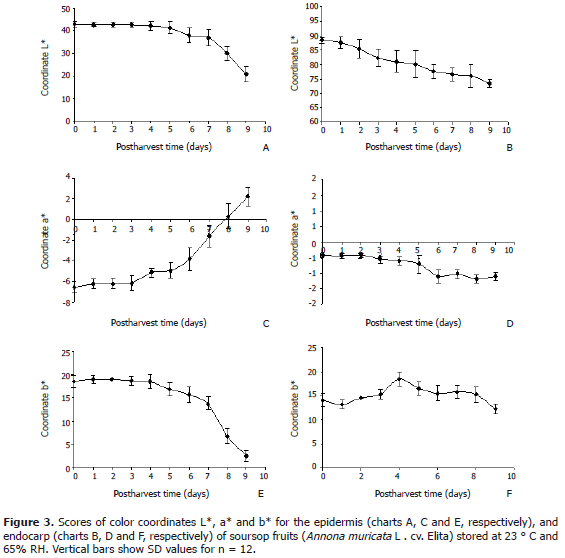
Skin and endocarp color. Figure 3 presents the results of epidermis and endocarp tristimulus color assessment regarding coordinates L*, a* and b*. Epidermal brightness (L*) showed a slightly decreasing behavior until day 5 of postharvest, after which the process sped up, to be further intensifyied on days 7, 8 and 9, which correspond to overipening and the onset of senescence. In the pulp and endocarp, brightness decreased steadily throughout postharvest. For both epidermis and endocarp, this parameter showed less variation in the stage corresponding to unripe fruit (days 0, 1 and 2 after harvest). The results obtained here for fruit brightness are consistent with those reported by Islam et al. (1996) and Umme et al. (1997).
As for the epidermis, coordinate a* scored the most negative values in the immature fruit (day 0 after harvest). Then it started to increase, a tendency that was later intensified during ripening and overipening, to finally show positive values in the last stage of post-harvest. Indicating a loss of green hue, this process is associated to decreased levels of chlorophyll b through chlorophyllase mediated hydrolysis to clorofilida and phytol (Yang et al., 2009). For the endocarp this coordinate is not considered as important as for the epidermis. However, it shows a decreasing behavior throughout post-harvest, coinciding with reports by Umme et al. (1997).
As for color coordinate b*, the epidermis presents a slight and continuous decrease until day 4 of post-harvest, after which it drops quickly, and even quicker during ripeness. This results in a color intensity decrease, probably due to reduced levels of chlorophyll a, whose structure is similar to that of Chlorophyll b; the difference consisting in the replacement of group 3-formyl of the latter by group 3-methyl of the former. This small difference leads to changes in their visible absorption spectra, thus conferring to chlorophyll b solutions their characteristic green color, and blue to those of chlorophyll a. Thus, color coordinate b * is likely to be associated to chlorophyll a, whose concentration reduces with maturation, bringing about a concomitant decrease of coordinate b *. Regarding this same coordinate, the endocarp showed a slightly increasing behavior until day 4, and then a continuous decrement until ripening, when said color drop was more accentuated, as also reported by Islam et al. (1996).
Color coordinates L *, a * and b * are indicators of soursop ripeness. Therefore, according to the reproducibility of the procedure used for their determination, its ease of application and generally low result variability, they could be used to determine harvest maturity in the field in a rapid, non destructive and especially useful manner.
Fruit yield. The main product obtained from a fruit is its pulp, a basic raw material for different agro-industrial products or for direct consumption. In the case of soursop, the fractions corresponding to skin and seeds are of great importance as well (Camacho, 1995).
Table 1 presents the different fractions obtained from the studied soursop fruits, assessed on a daily basis during post-harvest. Pulp yield for day 6 (ripe fruit) was 60%, which is higher than a previous report by Camacho (1995), who reported a score of 55%, but lower than the Colombian Technical Standard (NTC, 5208), set at 74%. The average number of seeds was 129, ranging from 58 to 250. Average seed weight was 0.87 ± 0.11 g. The remaining fraction corresponds to the central axis of the fruit, and to losses due to processing. A previous study on other soursop cultivars reports values ranging from 90 to 266 seeds, with an average of 175. Endocarp reduction is probably due to the biochemical demand that is proper of the metabolic processes of ripening and overipening (Laboren, 1994). Total soluble solids (TSS). Figure 4 shows a rapid increase in the level of TSS from day 1 to day 6 after harvest, when it reached a concentration of 12.8 °Brix. During this period, a typical climacteric fruit reaches peak production of CO2, probably revealing the great metabolic dynamics involved in the activity of a and b amylases. During ripening, these enzymes hydrolyze starch to simpler carbohydrates such as mono and disaccharides (mainly sucrose, glucose and fructose). According to our results, TSS decrease as observed since day 6, may be the result of the consumption of these sugars as respiratory substrates (DeMan, 1999; Kader, 2002). The concentration found in the present research coincides with that reported by Chaparro et al. (1993), who found concentrations of 13°Brix for ripe soursop fruits. The Colombian Technical Standard (NTC[1], 5208) establishes minimum values of 13.5 °Brix for this parameter at consumption ripeness; which is slightly higher than our findings. Just as well, our results were lower than those reported by Ojeda de Rodríguezet al. (2007) for different soursop cultivars at consumption ripeness, which ranged between 14 and 17 °Brix. Total acidity and pH.
Figure 5 shows total acidity behavior in the studied soursop fruits. This parameter increased throughout the climacteric stage to reach a maximum of 0.74% on day 6 after harvest, coinciding with ripeness. pH, whose minimum score was obtained on day 6 after harvest, naturally showed an inverse behavior to that of acidity. According to the results, soursop can be classified as a fruit of intermediate acidity (Camacho, 1995). During the climacteric period, the metabolic activity of this fruit coincided with the production of organic acids, which confer it its characteristic sensory contrast, probably due to the specific genetic load of this cultivar. In the post-climacteric stage, lower acidity may be due to the metabolic consumption of these organic molecules to provide the energy required by the fruit. In addition, many organic acids are involved as precursors of volatile substances, which are more abundant during this period (Park et al., 2006).
Total acidity as assessed in the present work was slightly lower than a previous report by De Lima et al. (2003), and is consistent (in terms of acidity and pH) with values reported by Hernández et al. (1988). Our acidity and pH values are consistent with those recommended by the Colombian Technical Standard (NTC, 5208).
Firmness. This parameter showed great variability in the present study. Figure 6 indicates how immature fruits (day 0) were found to have an average strength of 80 N, a value that is considered to be very high, which persisted until day 2 after harvest. The maximum acceleration in the decline of firmness was observed between days 2 and 4. Since day 4, the curve decreases with a gentle slope, exhibiting respective values of 7.48 and 4.72 N on days 5 and 7 (ripe fruits). Overipe fruits (day 9) showed an average firmness of 3.62 N, which characterizes the tissue as extremely soft. The behavior of this parameter was consistent with that reported by Ciro et al. (2007). Other soursop cultivars have shown values of 60 N on day 0 after harvest, and a similar behavior to that found in the current research during the remaining period (De Lima et al., 2003). In this regard, changes in firmness are the result of the gradual degradation of cell wall polysaccharides, which are solubilized and depolymerized by the enzymatic action of PG, PME, b-GAL, xilogluconasa and xylanase (Silveira, 2007; Sañudo et al., 2008).
Furthermore, the decrease in firmness also affects the turgor loss underwent by the fruit due to transpiration as ripening progresses. It is important to note that - due to hydraulic pressure on the cell wall - swelling is responsible for cell firmness and rigidity (Muy et al., 2004).
CONCLUSIONS
Ethylene production in the studied soursop fruits was observed to grow during post-harvest, with outstanding peaks on days 4 and 6, the latter being the largest one, with a production of 186.9 mg CO2 kg-1 h-1.
The fruits reached their maximum ethylene production on day 8 after harvest, by scoring 133.2 mL kg-1 h-1, when they were already ripening and close to senescence. In turn, the physiological loss of weight reached 14.7% on day 6, and 21.7% at ripeness.
During ripening and until day 6, the concentration of total soluble solids was found to increase; whereas during the overipening period it underwent a slight decline. However, the values found for the physico-chemical parameters, namely TSS, pH and total acidity expressed as malic acid are among those recommended by the Colombian Technical Standard for fruit soursop (NTC, 5208).
Attending color, coordinates L*, a* and b* allowed classifying fruit ripeness. Consumption ripeness was reached on day 6 after harvest, coinciding with the major climacteric peak, with a pulp yield of 60%.
ACKNOWLEDGEMENTS
We want to thank the Research Direction of Universidad Nacional de Colombia, Sede Medellin (DIME), for their support through Quipu project 20201006039. Likewise, we acknowledge the Fruit and Vegetable Laboratory of the same institution through its technician, Food Engineering Fernando Arenas Gil, for his special collaboration.
BIBLIOGRAPHY
Almela, L., C. Sánchez, J. Fernández and F. Romojaro. 2000. Non destructive appraisal of the ripennness in cantaloupe melons. Food Science Technology 6(1): 47-51.
Angueira, M., A.J. Sandoval and J.A. Barreiro. 2003. Tasas de respiración en cuatro híbridos de pimentón (Capsicum annum L.). Interciencia 28(10): 1-9.
Arango, T.J. y P.A. Bacanumenth. 1999. La adecuación de tierras en el departamento de Antioquia. Revista Facultad Nacional de Agronomía Medellín 52(1): 395-424.
Armida, F., J. Fortiz y M. Villegas. 2011. Cambios en enzimas pectolíticas durante la maduración del duraznero ´Flordaprince´. Interciencia 36(1): 65-70.
Azcón, J. 2008. Fundamentos de fisiología vegetal. Second edition. McGraw Hill Zaragoza, España. 656 p.
Bernal, I. 1993. Análisis de alimentos. Guadalupe, Bogotá. 313 p.
Brovelli, A.E., K.J. Brecht and B.W. Sherman. 1999. Nonmelting flesh trait in peaches is not related to low ethylene production rates. Hortscience 34(2): 313-315.
Bruinsma, J. and P. Paull. 1984. Respiration during postharvest development of soursop fruit (Annona muricataL.). Plant Physiology 76(1): 131-138.
Camacho, G. y G. Romero. 1996. Obtención y conservación de pulpas: mora, guanábana, lulo y mango. ICTA, Universidad Nacional de Colombia, Bogotá. 130 p.
Camacho Olarte, G. 1995. Obtención y conservación de pulpas. En: Conferencia de Ciencia y Tecnología de Vegetales. Universidad Nacional de Colombia, Bogotá.
Castro, B.M., G. Jerz, P. Winterhalter y P. Restrepo. 2008. Degradación de la clorofila en la corteza del baby banano (Musa acuminata) durante diferentes estados de maduración. pp. 35-36. En: Memorias. RED-ALFA LAGROTECH Comunidad Europea, Cartagena, Colombia. 202 p.
Chaparro, M., R. Guzmán, F. Gallo y G. Moreno. 1993. Manejo poscosecha de la guanábana (Annona muricata L.). Agricultura Tropical 30(2): 63-70.
Ciro, H., D. Vahos y C. Márquez. 2005. Estudio experimental de la fuerza de fractura en frutas tropicales: El tomate de árbol (Cyphomandra betacea Sendt). Dyna 72(146): 55-64.
Ciro, H., G.O. Buitrago and A.S. Pérez. 2007. Estudio preliminar de la resistencia mecánica a la fractura y fuerza de firmeza para fruta de uchuva (Physalis peruviana L.). Revista Facultad Nacional de Agronomía Medellín 60(1): 3785-3796.
Cruz, J., A. Hernández y H. García. 2007. Efecto de la aplicación de metil jasmonato sobre la fisiología postcosecha de piña (Ananas comosus Cv. MD-2) y su relación con el daño por frío. En: Memorias. V Congreso Iberoamericano de Tecnología Postcosecha y Agroexportaciones. Universidad de Cartagena, Cartagena-España. 12 p.
Cuadros, V. 2008. Guanábana: Manejo del cultivo y poscosecha. Proexant, Quito. 25 p.
De Lima, M.A., R.E. Alves, H.A. Cunha e J. Enéas-Filho. 2003. Comportamento respiratorio e qualidade pós - colheita de graviola (Annona muricata L.) "morada" sob temperatura ambiente. Revista Brasileira de Fruticultura 25(1): 49-52.
DeMan, M.J. 1999. Principles of food chemistry. Third edition. Aspen Publishers, Guelph, Ontario. 520 p.
Díaz, J.C., S. Bautista and R. Villanueva. 2000. Quality changes in sapote mamey fruit during ripening and storage. Postharvest Biology and Technology 18(1): 67-73.
Hernández, A., A. Chaverri y J.J. Arrieta. 1988. Estudio de madurez de la guanábana. Asbana 12(30): 7-10.
Hosakote, M., N. Yashoda Tyakal and N. Rudrapatnam. 2005. Mango ripening chemical and structural characterization of pectic and hemicellulosic polysaccharides. Carbohydrate Research 340(1): 1335-1342.
Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC). 2003. NTC 5208. Frutas frescas guanábana. Especificaciones. Icontec, Bogotá. 17 p.
Islam, S., T. Matsui and Y. Yoshida. 1996. Effect of carbon dioxide enrichment on physico-chemical and enzymatic changes in tomato fruits at various stages of maturity. Scientia Horticulturae 65(2-3): 137-149.
Kader, A. 2002. Postharvest technology of horticultural crops. Agriculture and Natural Resources. Third edition. University of California, Davis, CA. 535 p.
Laboren, G. 1994. Resultados preliminares en el estudio de la calidad del fruto del guanábano. FONAIAP 45(1): 1-7.
Mercado, E., B.P. Bautista and M.A. García. 1998. Fruit development, harvest index and ripening changes of guavas produced in central Mexico. Postharvest Biology and Techology 13(1): 143-150.
Muy, D., J. Siller, J. Díaz y B. Valdez. 2004. Efecto de las condiciones de almacenamiento y el encerado en el estatus hídrico y la calidad poscosecha de pepino de mesa. Revista Fitotecnia Mexicana 27(2): 157-165.
Ojeda de Rodríguez, G., J. Coronado, R. Nava, B. Sulbarán, D. Araujo y L. Cabrera. 2007. Caracterización físicoquímica de la pulpa de la guanábana (Annona muricata) cultivada en el occidente de Venezuela. Universidad del Zulia. Boletín del Centro de Investigaciones Biológicas 41(2): 151-160.
Orellana, A.D. y E. Martínez. 2002. Distribución geográfica de anonaceas en Guatemala. ICTA; FAUSAC, Guatemala. 23 p.
Ornelas, J.J., M.E. Yahia and A.A. Gardea. 2008. Changes in external and internal color during postharvest ripening of "Manila" and "Ataulfo" mango fruit and relationship with carotenoid content determined by liquid chromatography APcl+ time of flight mass spectrometry. Postharvest Biology and Technology 50(2-3): 145-152.
Park, Y.S., S.T. Jung and S. Gorinstein. 2006. Ethylene treatment of "Hayward" kiwifruits (Actinidia deliciosa) during ripening and its influence on ethylene biosynthesis and antioxidant activity. Scientia Horticulturae 108(1): 22-28.
Paull, R.E. 1996. Postharvest atemoya fruit splitting during ripening. Postharvest Biology and Technology 8(4): 329-334.
Poot, H.R. 2003. El fruto del saramuyo (Annona squamosa) y su refrigeración a 4°C, Facultad de Ciencias Químico Biológicas. Universidad Autónoma de Campeche, México. 3 p.
Rami, S., A.P. Georgeb, and A.S. Kantharajah. 1995. Tissue culture of Annona spp. (cherimoya, atemoya, sugar apple and soursop). A review. Scientia Horticultarae 62(1): 1-14.
Ramírez, J. 2008. Central Mayorista de Antioquia. Boletín informativo. Medellín. 25 p.
Ramírez, M., M. López y A. Gutiérrez. 1998. Manejo poscosecha y comercialización de la guanábana (Annona muricata L.). SENA. Neiva, Colombia. 332 p.
Salisbury, F. y C. Ross. 1994. Fisiología vegetal. Grupo Editorial Iberoamérica, México. 759 p.
Sañudo, J.A., J. Siller, T. Osuna, D. Muy, G. López, J. Osuna, C. Greve y J. Labavitch. 2008. Solubilización y despolimerización de pectinas durante el ablandamiento de frutos de papaya. Revista Fitotecnia Mexicana 31(2): 149-155.
Silveira, A.C. 2007. Fisiología y bioquímica de los productos MPF. En: Memorias. V Congreso Iberoamericano de Tecnología Postcosecha y Agroexportaciones. Universidad de Cartagena, Cartagena, 12 p.
Sothornvit, R. and P. Rodsamran. 2008. Effect of a mango film on quality of whole and minimally processed mangoes. Postharvest Biology and Technology 47(3): 407-415.
Taiz, L. and E. Zeiger. 2010. Plant physiology. Sinauer Associates, Sunderland, Massachusetts. 700 p.
Trinchero, D.G., G.O. Sozzi, A.M. Cerri and A. Franschina. 1999. Ripening-related changes in ethylene production, respiration rate and cell-wall enzyme activity in goldenberry (Physalis peruviana L.), a solanaceous species. Postharvest Biology and Technology 16(2): 139-145.
Umme, A., B.A. Asbi, Y. Salmah, A.H. Junainah and B. Jamilah. 1997. Characteristics of soursop natural puree and determination of optimum conditions for pasteurization. Food Chemistry 58(1-2): 119-124.
Worrell, B.D., C.C. Sean and D.J. Huber. 1994. Growth, maturation and ripening of soursop (Annona muricata L.) fruit. Scientia Horticulturae 57(1-2): 7-15.
Wu, B.H., B. Quilot, M. Génard, J. Kervella and S. Li. 2005. Changes in sugar and organic acid concentrations during fruit maturation in peaches (Prunus davidiana) and hybrids as analyzed by principal component analysis. Scientia Horticulturae 103(4): 429-439.
Yang, D.S., R. Balandrán, C. Ruiz, R. Toledo and S. Kays. 2009. Effect of hyperbaric, controlled atmosphere and UV treatments on peach volatiles. Postharvest Biology and Technology 51(3): 334-341.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
Article abstract page views
Downloads
License
Copyright (c) 2015 Revista Facultad Nacional de Agronomía

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.