Perfil vasodilatador de compuestos flavonoides y fenilbutanoides aislados de Croton Schiedeanus Schlecht
Vasodilator profile of flavonoid and phenylbutanoid compounds isolated from Croton schiedeanus Schlecht
Palabras clave:
Euphorbiaceae, Rododendrol, 3-O-metil-quer-cetina, 3 7-di-O-metilquercetina, óxido nítrico, agentes vasodilatadores. (es)Euphorbiaceae, Rhododendrol, 3-O-Methylquercetin, 3 7-Di-O-methylquer-cetin, Nitric Oxide, vasodilator Agents. (en)
Objetivo. Evaluar el efecto vasodilatador de los flavonoides: 3-O-metilquercetina, 3,7-di-O-metilquercetina, y 3,3',4',7-tetra-O-metilquercetina; y los fenilbutanoides: (2S)-7,9-dime--to--xiro---doden-drol, (2S)-2-acetato de 7,9-dime-toxirododendrol y (2S)- 2,8-diacetato de 7,9-dimetoxirodo-dendrol en anillos de aorta de ratas Wistar.
Material y métodos. Estos compuestos se evaluaron en anillos de aorta precontraídos con fenilefrina (1 µM) o KCl (80 mM). Para examinar posibles interacciones con endotelio, óxido nítrico, guanilato ciclasa, prostanoides o canales de K+ATP, aquéllos con mayores efectos vasodilatadores: 3-O-metilquercetina y 3,7-di-O-metilquercetina, se evaluaron en anillos estimulados con fenilefrina en presencia o ausencia de: endotelio, L-NAME (G -nitro-L-Arginina-Metil Ester, 100 µM), ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-ona, 1 µM), meclofenamato sódico (10 µM) o glibenclamida (1 µM).
Resultados. En contraste con los compuestos fenilbutanoides que no arrojaron actividad relajante significativa, 3-O-metilquercetina y 3,7-di-O-metilquercetina mostraron una respuesta importante con concentraciones inhibitorias 50 (CI50) de 2,5 y 4,9 µM respectivamente frente a fenilefrina. ODQ y L-NAME desplazaron efectivamente a la derecha la curva dosis-respuesta, en particular la 3-O-metilquercetina (razón de IC50: 7,4 y 3,8).
Conclusión. 3-O-metilquercetina y 3,7-di-O-metilquer-cetina, flavonoides aislados de Croton schiedeanus, ejercen importantes efectos vasodilatadores vinculados con la vía de NO/GMPc. Estos resultados soportan al uso etnobotánico de esta especie.
Objective. To assess the vasorelaxant effect of the flavonoid compounds: 3-O-methyl-quercetin, 3,7-di-O-methylquercetin, and 3,3',4',7-tetra-O-methylquercetin, and the phenylbutanoids compounds: (2S)-7,9-dimethoxyrhododendrol, (2S)-2-acetoxy-7,9-dimetho-xyrho-dodendrol, (2S)-2,8-diacetatoxy-7,9-dimethoxyrho-dodendrol in isolated aortic rings of Wistar rats.
Materials and methods. These compounds were evaluated in phenylephrine (PE, 1 µM) and KCl (80 mM) precontracted aortic rings. In order to examine possible interactions related with: endothelium, nitric oxide (NO), guanylyl ciclasa, prostanoid or ATP dependent potassium (K+ATP) channels, the compounds with greater relaxant effect: 3-O-methylquercetin, and 3,7-di-O-methylquer-cetin, were assessed in phenylephrine precontracted rings in presence or absence of: endothelium, L-NAME (G -nitro-L-Arginine-Methyl Ester, 100 µM), ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, 1 µM), sodium meclofenamate (10 µM) or glibenclamide (1 µM).
Results. Whereas phenylbutanoid compounds did not shown significant relaxant properties, 3-O-methylquercetin and 3,7-di-O-methyl-quercetin displayed important vasodilator responses with IC50 of 2.5 and 4.9 µM against phenylephrine respectively. ODQ and L-NAME effectively displaced to the right the dose-response curves of these compounds, especially in the case of 3-O-methylquercetin (IC50 ratio: 7.4 and 3.8) whereas glibenclamide did not and meclofenamate only slightly.
Conclusion. 3-O-methylquercetin and 3,7-di-O-methylquercetin, flavonoid compounds isolated from Croton schiedeanus play important vasorelaxant effects related to the NO/cyclic GMP pathway. These results support the etnobotanical use of Croton schiedeanus.
INVESTIGACIÓN ORIGINAL
PERFIL VASODILATADOR DE COMPUESTOS FLAVONOIDES Y FENILBUTANOIDES AISLADOS DE CROTON SCHIEDEANUS SCHLECHT
Vasodilator profile of flavonoid and phenylbutanoid compounds isolated from Croton schiedeanus Schlecht
Sofía Ximena Correa-Hernández1, Pilar Puebla-Ibánez2, Rosalía Carrón de la Calle3, María Luisa Martín-Calvo3, Luis San Román del Barrio4, Mario Francisco Guerrero Pabón5.
1. QF. Departamento de Farmacia. Facultad de Ciencias. Universidad Nacional de Colombia, Bogotá, Colombia.
2. QF, D Sc, Profesora Titular. Departamento de Química Farmacéutica. Facultad de Farmacia. Universidad de Salamanca. Salamanca, España.
3. QF, D Sc, Profesora Titular. Departamento de Fisiología y Farmacología. Facultad de Farmacia. Universidad de Salamanca. Salamanca, España.
4. QF, D Sc, Profesor Catedrático. Departamento de Fisiología y Farmacología. Facultad de Farmacia. Universidad de Salamanca. Salamanca, España.
5. MD. D Sc. Profesor Asociado. Departamento de Farmacia. Facultad de Ciencias. Universidad Nacional de Colombia, Bogotá, Colombia.
Correspondencia: mfguerrerop@unal.edu.co
Resumen
Antecedentes. El Croton schiedeanus Schelecht (N.V: "almizclillo" Euporbiaceae), es una especie popularmente utilizada en Colombia para el tratamiento de la hipertensión arterial.
Objetivo. Evaluar el efecto vasodilatador de los flavonoides: 3-O-metilquercetina, 3,7-di-O-metilquercetina, y 3,3',4',7-tetra-O-metilquercetina; y los fenilbutanoides: (2S)-7,9-dime--to--xiro---doden-drol, (2S)-2-acetato de 7,9-dime-toxirododendrol y (2S)- 2,8-diacetato de 7,9-dimetoxirodo-dendrol en anillos de aorta de ratas Wistar.
Material y métodos. Estos compuestos se evaluaron en anillos de aorta precontraídos con fenilefrina (1 µM) o KCl (80 mM). Para examinar posibles interacciones con endotelio, óxido nítrico, guanilato ciclasa, prostanoides o canales de K+ATP, aquéllos con mayores efectos vasodilatadores: 3-O-metilquercetina y 3,7-di-O-metilquercetina, se evaluaron en anillos estimulados con fenilefrina en presencia o ausencia de: endotelio, L-NAME (G -nitro-L-Arginina-Metil Ester, 100 µM), ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-ona, 1 µM), meclofenamato sódico (10 µM) o glibenclamida (1 µM).
Resultados. En contraste con los compuestos fenilbutanoides que no arrojaron actividad relajante significativa, 3-O-metilquercetina y 3,7-di-O-metilquercetina mostraron una respuesta importante con concentraciones inhibitorias 50 (CI50) de 2,5 y 4,9 µM respectivamente frente a fenilefrina. ODQ y L-NAME desplazaron efectivamente a la derecha la curva dosis-respuesta, en particular la 3-O-metilquercetina (razón de IC50: 7,4 y 3,8).
Conclusión. 3-O-metilquercetina y 3,7-di-O-metilquer-cetina, flavonoides aislados de Croton schiedeanus, ejercen importantes efectos vasodilatadores vinculados con la vía de NO/GMPc. Estos resultados soportan al uso etnobotánico de esta especie.
Palabras clave: Euphorbiaceae, Rododendrol, 3-O-metil-quer-cetina, 3,7-di-O-metilquercetina, óxido nítrico, agentes vasodilatadores.
Summary
Background. Croton schiedeanus Schlecht (Euphorbiaceae), specie is used in Colombian folk medicine in hypertension treatment.
Objective. To assess the vasorelaxant effect of the flavonoid compounds: 3-O-methyl-quercetin, 3,7-di-O-methylquercetin, and 3,3',4',7-tetra-O-methylquercetin, and the phenylbutanoids compounds: (2S)-7,9-dimethoxyrhododendrol, (2S)-2-acetoxy-7,9-dimetho-xyrho-dodendrol, (2S)-2,8-diacetatoxy-7,9-dimethoxyrho-dodendrol in isolated aortic rings of Wistar rats.
Materials and methods. These compounds were evaluated in phenylephrine (PE, 1 µM) and KCl (80 mM) precontracted aortic rings. In order to examine possible interactions related with: endothelium, nitric oxide (NO), guanylyl ciclasa, prostanoid or ATP dependent potassium (K+ATP) channels, the compounds with greater relaxant effect: 3-O-methylquercetin, and 3,7-di-O-methylquer-cetin, were assessed in phenylephrine precontracted rings in presence or absence of: endothelium, L-NAME (G -nitro-L-Arginine-Methyl Ester, 100 µM), ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, 1 µM), sodium meclofenamate (10 µM) or glibenclamide (1 µM).
Results. Whereas phenylbutanoid compounds did not shown significant relaxant properties, 3-O-methylquercetin and 3,7-di-O-methyl-quercetin displayed important vasodilator responses with IC50 of 2.5 and 4.9 µM against phenylephrine respectively. ODQ and L-NAME effectively displaced to the right the dose-response curves of these compounds, especially in the case of 3-O-methylquercetin (IC50 ratio: 7.4 and 3.8) whereas glibenclamide did not and meclofenamate only slightly.
Conclusion. 3-O-methylquercetin and 3,7-di-O-methylquercetin, flavonoid compounds isolated from Croton schiedeanus play important vasorelaxant effects related to the NO/cyclic GMP pathway. These results support the etnobotanical use of Croton schiedeanus.
Key words: Euphorbiaceae, Rhododendrol, 3-O-Methylquercetin, 3,7-Di-O-methylquer-cetin, Nitric Oxide, vasodilator Agents.
Introduction
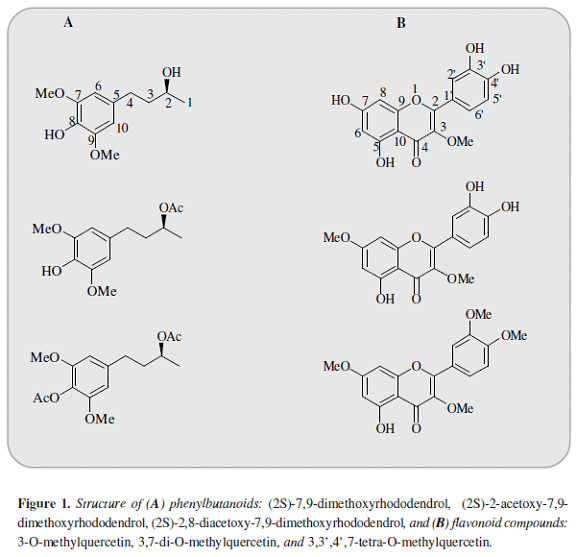
Croton schiedeanus Schlecht (N.V.: "almizclillo". Euphorbiaceae) is among the medicinal plants used in Colombian folk medicine for the treatment of arterial hypertension (1, 2). The extract of this specie decreases the blood pressure in Wistar and spontaneous hypertensive rats (SHR) as well as the vascular tone in isolated aortic rings (3). Among its main constituents are the neo-clerodane diterpenoids: (12R)-12-hydroxycascarillone, 5-hydroxy-cis-dehydro-crotonin, cis-dehydrocrotonin and trans-dehydrocrotonin in addition to the flavonoids: 3,4',7-tri-O-methylquercetin (ayanin) and 3,7-di-O-methylquercetin (4, 5). The most abundant compound in this specie is ayanin, which possess vasorelaxant properties linked with NO/cGMP cyclic pathway (6). However its effect is rather weak compared with another flavonoid isolated from C. schiedeanus: 3,7-di-O-methyl-quercetin(7). In addition, more than one compound seems to play a role in the response induced by this specie (8). Another kind of molecules isolated from this species are the phenylbutanoids (2S)-7,9-dimethoxyrhodo-dendrol and (2S)-2-acetoxy-7,9-dimethoxyro-dodendrol(9) besides to the flavonoid 3-O-methylquercetin, closely related to the previously described 3,7-di-O-methylquercetin (Figure 1). Given the interest elicited by Croton schiedeanus as a natural source of compounds with potential cardiovascular utility, this study assessed the vasorelaxant effect of these molecules and their possible interactions with the NO/cGMP cyclic pathway.
Materials and methods
Rat aortic rings experiments
Previous ether anesthesia, the descending thoracic aorta of each male Wistar rat (320-400 g) was cleaned, dissected and placed in a oxygenated Krebs solution that contained the following composition (in mM): NaCl, 118.0; KCl, 4.75; CaCl2, 1.8; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 25; glucose, 11 and ascorbic acid 0.1. Then the aorta was cut in rings of 4-6 mm in length that were carefully excised and submerged in Allhin organ chambers that contained 5 ml of Krebs solution at 37°C, bubbled with 95% O2 and 5% CO2 gas mixture (pH=7.4). The basal tension applied to each ring was 2 g, and the isometric contractile force was recorded by mean of a transducer (Harvard UF1) connected to a MacLab/8-computer system (A.D. InstruMents Ltd, London, U.K.). The tension of each preparation required a period of 60-90 min to attain stabilization, changing the incubation media every 15 min. When indicated, endothelium was gently removed trough the tip of small forceps, fact confirmed by lacking of relaxation to acetylcholine (1 µM).
After the stabilization period, phenylephrine (PE, 1 µM) or high KCl (80 mM) were added to the bath. Once the contractile response reached a steady tension, the compounds: (2S)-7,9-dimethoxyrhododendrol, (2S)-2-acetoxy-7,9-dimethoxyrhododendrol, (2S)-2,8-diace-tatoxy-7,9-dimethoxyrhododendrol, 3-O-methylquer-cetin, 3,7-di-O-methylquercetin, and 3,3',4',7-tetra-O-methylquercetin (Figure 1) were added in a cumulative way in 15 min interval (10-6-10-3 M), using dimethylsulfoxide (DMSO, <0.01%) in some rings as control.
To asses the roll of endothelium dependent relaxation a similar protocol was followed in rings without endothelium contracted with PE (1 µM) or KCl (80 mM) exposed to the compounds with the greater responses in the previous experi-ments: 3-O-methylquercetin and 3,7-di-O-methylquercetin. In order to asses the possible participation of the NO/cyclic GMP pathway these compounds were added to intact rings contracted with PE (1 µM) previously incubated during 20 minutes with the NO synthase inhibitor L-NAME (100 µM) or the selective nitric oxide-sensitive guanylyl cyclase inhibitor ODQ (1 µM). In the same way, PE contracted rings were incubated with the cyclooxygenase inhibitor sodium meclofenamate (10 µM) and the ATP dependent potassium channels inhibitor glibenclamide (1 µM) to asses possible prostanoid and K+ATP channels participation.
All off these experiments fulfilled the provisions concerning the protection of animals used for experiments stipulated by Spanish law and European Community specifications (EEC 1986) and the Ethics Committee of Universidad Nacional de Colombia.
Data analysis and statistics
The response of aortic rings was expressed as a percentage of the initial contraction to PE (1 µM) or KCl (80 mM). Dose-response curves were analysed to give the concentration that produces a 50 percent inhibition of the maximal contractile response (IC50) by sigmoid curve-fitting analysis.
All the results are expressed as means standard error of the mean (SEM) of n over 5 experiments. Differences in concentration-response curves were analyzed by one way analysis of variance (ANOVA) followed by Dunett post hoc test with a criterion set for statistical significance at p<0.05. Excel® and SPSS® software were used for data analysis.
Compound and solutions
The aerial plant material was collected from the region of Tocaima, Cundinamarca and classified at Instituto de Ciencias Naturales (COL432164, Universidad Nacional de Colombia) as Croton schiedeanus Schlecht (Euphorbiaceae). From this specie 3-O-methylquercetin; 3,7-di-O-methylquercetin; (2S)-7,9-dimethoxyrhodo-dendrol and (2S)-2-acetoxy-7,9-dimethoxyrodo-dendrol were isolated and identified by comparison of m-p., 1H-NMR, 13C-NMR values with reported data according to the extraction and isolation procedures previously described (9-12) (Figure 1). In addition 3,3',4',7-tetra-O-methylquer-cetin and (2S)-2,8-diacetoxy-7,9-dimethoxiro-dodendrol were obtained by synthesis.
The following salts and drugs were used: NaCl, CaCl2, NaHPO4, NaHCO3, glucose; KCl, MgCl2, KH2PO4 and MgSO4; ascorbic acid; phenylephrine hydrochloride, acetylcholine chloride, L-NAME (Nw-nitro-L-arginine methyl ester hydrochloride), ODQ (1H-[1,2,4]-oxadiazolo[4,3]-aquinoxalin-1-one), glibenclamide, dimethylsulfoxide (Sigma-Aldrich). Glibenclamide, flavonoid and phenylbutanoid compounds were dissolved in DMSO. The final DMSO concentrations in the bathing media always was less than 0.1 percent. All other substances were dissolved in physiological saline solution.
Results
The maximal contractile response obtained with PE (1 mM) and KCl (80 mM) on intact aortic rings were 2855±110 mg (n=54) and 3278±78 mg (n=40) respectively. In de-endothelized rings the contraction magnitudes were 2865±152 (n=41) and 2336±133 (n=26) respectively. 3-O-methylquercetin in first place and 3,7-di-O-methylquercetin in the second, induced greater relaxant response against PE than KCl (IC50 values of 2.5 [2.4-2.7] and 4.9 [4.0 - 6.1] µM against PE and 28 [21-38]) and 14 [13 - 17] µM against KCl).
The relaxant response was greater in intact than in de-endothelized rings (5.9 [5.7-6.2] and 9.9 [9.7-10] µM against PE denuded rings). Instead, 3,3',4',7-tetra-O-methylquercetin induced only a slight response. Furthermore 3- O-methylquercetin and 3,7-di-O-methylquercetin reached >99% of efficacy relaxation (Emax) whereas 3,3',4',7-tetra-O-methylquercetin only 44% (Figures 2 y 3).
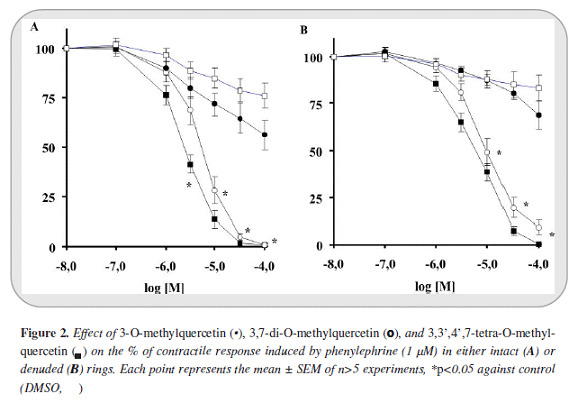
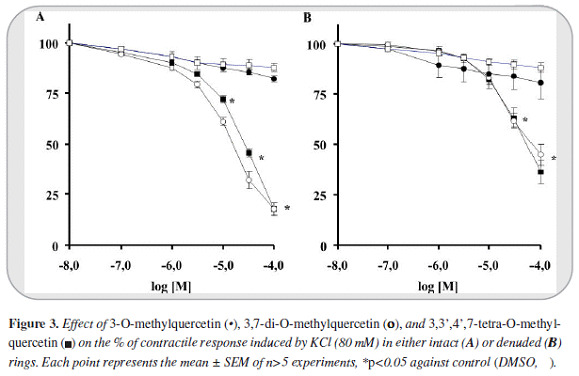
On the other hand, in all cases the relaxant response induced by the phenylbutanoid compounds (2S)-7,9-dimethoxyrho-doden-drol, (2S)-2-acetoxy-7,9-dimetho-xyrho-dodendrol, and (2S)-2,8-diacetoxy-7,9-dimethoxyrho-dodendrol was notably lower than that induced by 3-O-methylquercetin and 3,7-di-O-methylquer-cetin. Only (2S)-2,8-diacetatoxy-7,9-dimethoxyrhodo-dendrol displayed a moderate relaxant effect against intact rings contracted with PE (IC50: 56 [48 - 65] µM). In addition, the relaxant effect of all of these compounds was less than 59 percent (Figure 4).

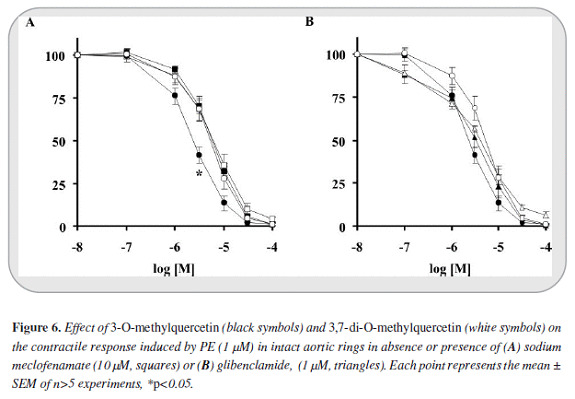
ODQ displaced most to the right the relaxant curves of 3-O-methylquercetin and 3,7-di-O-methylquercetin (IC50 ratios: 7.42 and 3.69 respectively). L-NAME also significantly modified the relaxant curves of these compounds (IC50 ratios: 3.8 and 1.8, Figure 5). Meclofenamate affected significantly only the 3-O-methylquercetin relaxant curve (IC50 ratio of 2.42) whereas the effect of glibenclamide was not significant in any case (Figure 6). None of these compounds altered the maximum effectiveness of relaxation shown by 3-O-methylquerce-tin and 3,7-di-O-methylquercetin (> 99%, Figures 5 y 6).

Discussion
This study shows that the flavonoids 3-O-methylquercetin and 3,7-di-O-methylquercetin are probably the main active principles isolated from C. schiedeanus and that the new phenylbutanoid compounds (2S)-7,9-dimetho-xyrho-dodendrol, and (2S)-2-acetoxy-7,9-dimethoxyrhododendrol do not play an important role as vasorelaxant agents.
Interestingly, according to their IC50 values, less than 0.01 mM, 3-O-methylquercetin and 3,7-di-O-methylquercetin are among the flavonoid compounds with highest relaxation potency. In fact, although differences in the protocols followed can affect the results, the relaxant profile of flavonols (fisetin, rutin, quercetin), flavanones (chrysin, flavone, baicalein) and isoflavones (diadzein), are reported about the mM order of IC50 (13 - 15).
The structure-activity relationship studies of flavonoids for vascular relaxation show that flavone is the subgroup with the major potency. So, the C2=C3 bond and C=O are functionality necessary. However, there are additional criteria to fulfill. Xu et al., concluded that 5-OH, 7-OH and 4'-OH are also necessary (16). These premises are applicable to the results obtained with 3-O-methylquercetin, 3,7-di-O-methyl-quer-cetin and 3,3',4',7-tetra-O-methylquer-cetin. The last one differs structurally only in the -OCH3 substitutions at 3'and 4' decreasing considerably the relaxation of the flavone. In addition, given the better profile of 3-O-methylquercetin, the 3-OCH3 seems to add for a good relaxant response.
On the other hand, the better response induced by 3-O-methylquercetin and 3,7-di-O-methylquercetinagainst PE than KCl are in concordance with previous works that shown that 5-OH confers selectivity for the former agonist (15). This antiadrenergic response would be linked to the NO/cyclic GMP pathway because the NO synthase inhibitor L-NAME, and the inhibitor of guanilyl cyclase, ODQ displaced to the right the relaxant curve of 3-O-methylquercetin and 3,7-di-O-methyl-quercetin. The fact that this response is higher in presence of ODQ suggests that mechanisms beyond the NO synthesis, like enhancement in the bioactivity of nitric oxide, would be more important (17). Dioclein and flavone are flavonoid compounds that could elicit a mechanism like this (18).
Little could be the participation of mechanisms that led to activation of prostacyclin, another important endothelium derived relaxing factor, because sodium meclofenamate, a COX inhibitor, does not affect the dose response curve of 3,7-di-O-methylquercetin while only slightly displaced the curve of 3-O-methylquercetin. Therefore, in spite of the results reported by others, the 5-OH does not seem to shift the relaxant response to the release of prostaglandins (15).
At the same time, non endothelium dependent mechanisms seems to play a role in the vasorelaxant response obtained with 3-O-methylquercetin and 3,7-di-O-methylquercetin because de-endothelization reduces but not abolishes the relaxation induced by them. In addition, they elicit important vasorelaxant responses against intact rings stimulated with high K+, and glibenclamide, a K+ATP channels antagonist, does not affect the relaxant response. Therefore, mechanism that led to inhibition of voltage dependent calcium channels could participate. Although flavones as 5-hydroxy-flavone and luteolin exert some non-endothelium dependent relaxant response linked to opening of K+ATP channels (19), at higher concentrations they can inhibit the Ca2+ release from the sarcoplasmic reticulum stores (20).
The vasorelaxant effects of flavonoid compounds are linked to their antioxidant properties (21, 22). Although there is controversy about the structure-activity relationship of them, lines of evidence signals the importance of the 3',4'-dihydroxyl (catechol group), the C2=C3 double bond and the C=O carbonyl function to attenuate processes involving reactive oxygen species (23 - 25). However, there are non free radicals scavenging mechanism that also participate in the vasorelaxant mechanisms of some flavonoid. That is the case of flavone, whose ring structure although lacks of hydroxyl substitutions, seems to improve the bioactivity of available nitric oxide, probably through increase of guanylil ciclasa/cGMP activity, rather than scavenging superoxide anions (17). 3-O-methylquercetin and 3,7-di-O-methylquercetin accomplish criteria for a good antioxidant activity but non antioxidant vasorelaxant mechanism could also be considered, given the profile of their vasorelaxant response in presence of L-NAME and ODQ, as previously mentioned.
Prospective studies show the association between flavonoid-rich diets and protective effects against coronary artery disease and myocardial infarction (26, 27). This could be due to their capacity to inhibit LDL oxidation and platelet aggregation (28, 29). In addition, increase consumption of flavonoid-rich foods may decrease rates of arterial hypertension (30, 31). All of these beneficial properties had led to the searching for a development of agents useful in treatment of cardiovascular disease based on the identification of the best structural characteristics that promote vascular and antioxidant activity of flavonoids. However, as to plausible vascular effect refers, when the pIC50 (-log CI50) values of the flavonoid compounds are compared with á antagonist drugs and calcium channels blockers in isolated aorta, the relaxant potency of these natural products, is about 1000 times lower (32, 33).
Therefore, there is low probability to get a drug based on a flavonoid structure to treat cardiovascular disorders like coronary artery disease or high blood pressure. This does not reduce their pharmacological interest, however. It is known that many medicinal plants exert their effects throw the synergistic interaction of several of their compounds (34). This seems to be the case of C. schiedeanus whose flavone compounds 3-O-methylquercetin and 3,7-di-O-methylquercetin, in addition to previously described ayanin, would be the most important (6-8).
In conclusion, the vasorelaxant profile of phenylbutanoid and flavonoid compounds isolated from Croton schiedeanus shows that 3-O-methylquercetin and 3,7-di-O-methylquercetinhave significant antiadrenergic vasorelaxant properties linked specially to the NO/cGMP pathway. These results give support to the ethnobotanical use of this specie.
Acknowledgments and financial sources
The authors are grateful for the support and financiation from: COLCIENCIAS; Vicerrec-toría de Investigación, Universidad Nacional de Colombia; Grupo Principios Bioactivos en Plantas and Facultad de Farmacia, Universidad de Salamanca.
Conflict of interest
The authors state that there is no conflict of interest in connection with this research.
References
1. García H. Flora Medicinal de Colombia. Santafe de Bogotá, Colombia. 1975;2:87-91.
2. Correa J, Bernal H. Especies Vegetales Promisorias de los Países del Convenio Andrés Bello. Santafe de Bogotá, Colombia. 1992;7:314-322.
3. Guerrero MF, Puebla P, Carrón R, Martín ML, Arteaga L, San Román L. Assessment of the antihypertensive and vasodilator effects of ethanolic extracts of some colombian medicinal plants. J Ethnopharmacol. 2002;80:37-42.
4. Puebla P, López JL, Guerrero MF, Carrón R, Martín ML, San Román L, San Feliciano A. Neo-clerodane diterpenoids from Croton schiedeanus. Phytochemistry. 2003;62:551-555.
5. Puebla P, Correa SX, Guerrero M, Carron R, San Feliciano A. New cis-clerodane diterpenoids from Croton schiedeanus. Chem Pharm Bull (Tokyo). 2005;53:328-9.
6. Guerrero MF, Puebla P, Martin ML, Carron R, San Roman L, Reguero MT, Arteaga L. Inhibitory effect of N(G)-nitro-l-arginine methyl ester on the anti-adrenergic response elicited by ayanin in the pithed rat. Planta Med. 2002;68: 322-325.
7. Guerrero MF, Puebla P, Carrón R, Martín ML, San Román L. Quercetin 3,7-dimethyl ether, a vasorelaxant flavonoid isolated from Croton schiedeanus Schlecht. J Pharm Pharmacol. 2002;54:1373-1378.
8. Guerrero MF, Puebla P, Carrón R, Martín ML, San Román L. Vasorelaxant effect of new neo-clerodane diterpenoids isolated from Croton schiedeanus. J Ethnopharmacol. 2004;94:185-189.
9. Puebla P, Correa SX, Guerrero MF, San Feliciano A. Phenylbutanoid derivatives from Croton schiedeanus. Biochem Syst Ecol. 2005;33:849-854.
10. Agraval PK. Carbon-13 NMR of flavonoids. Elsevier, Amsterdam. 1989.
11. Malan E, Roux DG. Flavonoids from distemonanathus benthamianus baillon. Methoxylated flavones and inter-relationships of benthamianin, [2]benzopyrano[4,3-b][1]benzopyran. J Chem Soc [Perkin 1]. 1979;1:2696-2703.
12. Bacon JD, Urbatsch LE, Bragg LH, Mabry TJ, Newman P, Jackson DW. The flavonoids of tetragonotheca (compositae). Phytochemistry. 1978;17:1939-1943.
13. Duarte J, Vizcaíno FP, Utrilla P, Jiménez J, Tamargo J, Zarzuelo A. Vasodilatatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen Pharmacol. 1993;24:857-862.
14. Herrera MD, Zarzuelo A, Jiménez J, Marhuenda E, Duarte J. Effects of flavonoids on rat aortic smooth muscle contractility: strucutre-activity relationship. Gen Pharmacol. 1996;27:273-277.
15. Ajaya M, Gilanib AH, Mustafa MR. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003;74:603-612.
16. Xu YC, Leung SWS, Yeung DKY, Hu LH, Chen GH, Che CM, Man RYK. Structure-activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry. 2007;68:1179-1188.
17. Ajay M, Achike FI, Mustafa MR. Modulation of vascular reactivity in normal, hypertensive and diabetic rat aortae by a non-antioxidant flavonoid. Pharmacol Res. 2007;55:385-391.
18. Lemos VS, Freitas MR, Muller B, Lino YD, Queiroga CEG, Côrtes SF. Dioclein, a new nitric oxide- and endothelium-dependent vasodilator flavonoid. Eur J Pharmacol. 1999;386:41-46.
19. Calderone V, Chericoni S, Martinelli C, Testai L, Nardi A, Morelli I, et al. Vasorelaxing effects of flavonoids: investigation on the possible involvement of potassium channels. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:290-298.
20. Chan E, Pannangpetch P, Woodman O. Relaxation to flavones and flavonols in rat isolated thoracic aorta: mechanism of action and structure-activity relationships. J Cardiovasc Pharmacol. 2000;35:326-333.
21. Woodman OL, Meeker WF, Boujaoude M. Vasorelaxant and antioxidant activity of flavonols and flavones: structure-activity relationships. J Cardiovasc Pharmacol. 2005;46:302-9.
22. Woodman OL, Chan ECh. Vascular and anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol Physiol. 2004;31:786-90.
23. Calgarotto AK, Miotto S, Honorio KM, da Silva ABF, Marangoni S, Silva JL, et al. A multivariate study on flavonoid compounds scavenging the peroxynitrite free radical. Theochem. 2007;808:25-33.
24. Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572-584.
25. Matsuda H, Morikawa T, Ando S, Toguchida I, Yoshikawa M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg Med Chem. 2003;11:1995-2000.
26. Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen elderly study. Lancet. 1993;342:1007-1011.
27. Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. 1999;149:943-949.
28. Mazur A, Bayle D, Lab C, Rock E, Rayssiguier Y. Inhibitory effect of procyanidin-rich extracts on LDL oxidation in vitro. Atherosclerosis. 1999;145:421-422.
29. Middleton EJr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673-751.
30. Moline J, Bukharovich IF, Wolff MS. Phillips R. Dietary flavonoids and hypertension: is there a link? Med Hypotheses. 2000;55:306-309.
31. Ginter E. Cardiovascular Disease Prevention in Eastern Europe. Nutrition. 1998; 14:452-457.
32. Minarini A, Budriesi R, Chiarini A, Leonardi A, Melchiorre C. Search for a1-adrenoceptor subtypes selective antagonists: design, synthesis and biological activity of cystazosin, an a1d-adrenoceptor antagonist. Bioorg Med Chem Lett. 1998;8:1353-1358.
33. Mirkhani H, Dirin M, Youssef-Zayeh I. Mechanism of vasoselective action of mebudipine, a new calcium channel blocker. Vascul Pharmacol. 2005;42:23-29.
34. Gilani AH, Rahmanb A. Trends in ethnopharmacology. J Ethnopharmacol. 2005;100:43-49.
Referencias
García H. Flora Medicinal de Colombia. Santafe de Bogotá, Colombia. 1975;2:87-91.
Correa J, Bernal H. Especies Vegetales Promisorias de los Países del Convenio Andrés Bello. Santafe de Bogotá, Colombia. 1992;7:314-322.
Guerrero MF, Puebla P, Carrón R, Martín ML, Arteaga L, San Román L. Assessment of the antihypertensive and vasodilator effects of ethanolic extracts of some colombian medicinal plants. J Ethnopharmacol. 2002;80:37-42.
Puebla P, López JL, Guerrero MF, Carrón R, Martín ML, San Román L, San Feliciano A. Neo-clerodane diterpenoids from Croton schiedeanus. Phytochemistry. 2003;62:551-555.
Puebla P, Correa SX, Guerrero M, Carron R, San Feliciano A. New cis-clerodane diterpenoids from Croton schiedeanus. Chem Pharm Bull (Tokyo). 2005;53:328-9.
Guerrero MF, Puebla P, Martin ML, Carron R, San Roman L, Reguero MT, Arteaga L. Inhibitory effect of N(G)-nitro-l-arginine methyl ester on the anti-adrenergic response elicited by ayanin in the pithed rat. Planta Med. 2002;68: 322-325.
Guerrero MF, Puebla P, Carrón R, Martín ML, San Román L. Quercetin 3,7-dimethyl ether, a vasorelaxant flavonoid isolated from Croton schiedeanus Schlecht. J Pharm Pharmacol. 2002;54:1373-1378.
Guerrero MF, Puebla P, Carrón R, Martín ML, San Román L. Vasorelaxant effect of new neo-clerodane diterpenoids isolated from Croton schiedeanus. J Ethnopharmacol. 2004;94:185-189.
Puebla P, Correa SX, Guerrero MF, San Feliciano A. Phenylbutanoid derivatives from Croton schiedeanus. Biochem Syst Ecol. 2005;33:849-854.
Agraval PK. Carbon-13 NMR of flavonoids. Elsevier, Amsterdam. 1989.
Malan E, Roux DG. Flavonoids from distemonanathus benthamianus baillon. Methoxylated flavones and inter-relationships of benthamianin, [2]benzopyrano[4,3-b][1]benzopyran. J Chem Soc [Perkin 1]. 1979;1:2696-2703.
Bacon JD, Urbatsch LE, Bragg LH, Mabry TJ, Newman P, Jackson DW. The flavonoids of tetragonotheca (compositae). Phytochemistry. 1978;17:1939-1943.
Duarte J, Vizcaíno FP, Utrilla P, Jiménez J, Tamargo J, Zarzuelo A. Vasodilatatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen Pharmacol. 1993;24:857-862.
Herrera MD, Zarzuelo A, Jiménez J, Marhuenda E, Duarte J. Effects of flavonoids on rat aortic smooth muscle contractility: strucutre-activity relationship. Gen Pharmacol. 1996;27:273-277.
Ajaya M, Gilanib AH, Mustafa MR. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003;74:603-612.
Xu YC, Leung SWS, Yeung DKY, Hu LH, Chen GH, Che CM, Man RYK. Structure-activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry. 2007;68:1179-1188.
Ajay M, Achike FI, Mustafa MR. Modulation of vascular reactivity in normal, hypertensive and diabetic rat aortae by a non-antioxidant flavonoid. Pharmacol Res. 2007;55:385-391.
Lemos VS, Freitas MR, Muller B, Lino YD, Queiroga CEG, Côrtes SF. Dioclein, a new nitric oxide- and endothelium-dependent vasodilator flavonoid. Eur J Pharmacol. 1999;386:41-46.
Calderone V, Chericoni S, Martinelli C, Testai L, Nardi A, Morelli I, et al. Vasorelaxing effects of flavonoids: investigation on the possible involvement of potassium channels. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:290-298.
Chan E, Pannangpetch P, Woodman O. Relaxation to flavones and flavonols in rat isolated thoracic aorta: mechanism of action and structure-activity relationships. J Cardiovasc Pharmacol. 2000;35:326-333.
Woodman OL, Meeker WF, Boujaoude M. Vasorelaxant and antioxidant activity of flavonols and flavones: structure-activity relationships. J Cardiovasc Pharmacol. 2005;46:302-9.
Woodman OL, Chan ECh. Vascular and anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol Physiol. 2004;31:786-90.
Calgarotto AK, Miotto S, Honorio KM, da Silva ABF, Marangoni S, Silva JL, et al. A multivariate study on flavonoid compounds scavenging the peroxynitrite free radical. Theochem. 2007;808:25-33.
Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572-584.
Matsuda H, Morikawa T, Ando S, Toguchida I, Yoshikawa M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg Med Chem. 2003;11:1995-2000.
Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen elderly study. Lancet. 1993;342:1007-1011.
Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. 1999;149:943-949.
Mazur A, Bayle D, Lab C, Rock E, Rayssiguier Y. Inhibitory effect of procyanidin-rich extracts on LDL oxidation in vitro. Atherosclerosis. 1999;145:421-422.
Middleton EJr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673-751.
Moline J, Bukharovich IF, Wolff MS. Phillips R. Dietary flavonoids and hypertension: is there a link? Med Hypotheses. 2000;55:306-309.
Ginter E. Cardiovascular Disease Prevention in Eastern Europe. Nutrition. 1998; 14:452-457.
Minarini A, Budriesi R, Chiarini A, Leonardi A, Melchiorre C. Search for a1-adrenoceptor subtypes selective antagonists: design, synthesis and biological activity of cystazosin, an a1d-adrenoceptor antagonist. Bioorg Med Chem Lett. 1998;8:1353-1358.
Mirkhani H, Dirin M, Youssef-Zayeh I. Mechanism of vasoselective action of mebudipine, a new calcium channel blocker. Vascul Pharmacol. 2005;42:23-29.
Gilani AH, Rahmanb A. Trends in ethnopharmacology. J Ethnopharmacol. 2005;100:43-49.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2008 Revista de la Facultad de Medicina

Esta obra está bajo una licencia Creative Commons Reconocimiento 3.0 Unported.
Derechos de autor
Los autores deben aceptar transferir a la Revista de la Facultad de Medicina los derechos de autor de los artículos publicados. La editorial tiene el derecho del uso, reproducción, transmisión, distribución y publicación en cualquier forma o medio. Los autores no podrán permitir o autorizar el uso de la contribución sin el consentimiento escrito de la revista. Estos archivos están disponibles en https://goo.gl/EfWPdX y https://goo.gl/6zztk4 y deben cargarse en el paso 4 del envío OJS (archivos complementarios).
La carta de cesión de derechos de autor y la de responsabilidad de autoría deben ser entregadas junto con el original.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).





















