Diets, food and Idiopathic Parkinson´s disease
Dietas, alimentos y Enfermedad de Parkinson Idiopática
DOI:
https://doi.org/10.15446/revfacmed.v62n1.43783Palabras clave:
Parkinson Disease, Diet, Vegetarian, Diseases Prevention, Methionine (en)Enfermedad de Parkinson, Dieta, Dieta Vegetariana, Metionina, Prevención de Enfermedades (es)
A partir de dos casos individuales, se presenta un esbozo personal sobre el conocimiento de la etiopatogenia en la Enfermedad de Parkinson Idiopática (EPI), con un énfasis en las dietas, la comida y las sustancias comestibles probadas y reportadas. En el primer caso (adherencia a una dieta prácticamente vegana, con una séptima parte de la proteína suplida de productos de origen animal), tuvo un impresionante efecto benéfico en sus quince años de desarrollo clínico, mientras que en el segundo caso (que siguió una dieta casi ovo-lacto-vegetariana) no se modificó sustancialmente el desarrollo clínico. La relevancia de la dieta en la EPI se concentra en la redistribución diaria de la ingesta de proteínas cuando se ha prescrito levodopa. La dieta por sí misma es fundamental para el manejo de cada persona predispuesta o que sufre de EPI. Las dietas veganas, ricas en proteínas debidamente balanceadas con cereales y suplementadas con vitamina B12, reducen la ingesta de metionina y mantiene la cuenta de aminoácidos aromáticos a un nivel moderado.
CASOS CLÍNICOS
Diets, food and Idiopathic Parkinson´s disease
Dietas, alimentos y Enfermedad de Parkinson Idiopática
José Perea-Sasiaín DMC ¹ • Roberta Hanfling Schwartz MJS2
¹ Facultad de Medicina. Universidad Nacional de Colombia. Bogotá, Colombia.
2 Independent scholar and lecturer, Chicago, USA.
José Perea-Sasiaín. Correo electrónico: josepesa@gmail.com
Recibido: 20/01/2014 / Aceptado:5/3/2014
Summary
Based on two individual cases, this is a personal depict on knowledge of Idiopathic Parkinson´s Disease (IPD) etiopathogeny emphasizing diets, food and edible substances tested and reported. In the first case (adherence to an almost vegan diet, with one seventh of the protein supplied from animal origin), had an impressive beneficial effect on his fifteen years clinical course, while in the second case (continuation of a quasi ovo-lacto-vegetarian diet), did not modify substantially the clinical course. The relevance of diet on IPD focused in redistribution of protein daily intake when levodopa prescribed. Diet by itself is fundamental for the management of each individual person prone to or suffering from IPD. Vegan diets with pulses rich in protein duly balanced with cereals and supplemented with vitamin B12, reduce the intake of methionine and keep the amount of aromatic amino acids at a moderate level.
Key words: Parkinson Disease, Diet, Vegetarian, Diseases Prevention, Methionine (MeSH).
Resumen
A partir de dos casos individuales, se presenta un esbozo personal sobre el conocimiento de la etiopatogenia en la Enfermedad de Parkinson Idiopática (EPI), con un énfasis en las dietas, la comida y las sustancias comestibles probadas y reportadas. En el primer caso (adherencia a una dieta prácticamente vegana, con una séptima parte de la proteína suplida de productos de origen animal), tuvo un impresionante efecto benéfico en sus quince años de desarrollo clínico, mientras que en el segundo caso (que siguió una dieta casi ovo-lacto-vegetariana) no se modificó sustancialmente el desarrollo clínico. La relevancia de la dieta en la EPI se concentra en la redistribución diaria de la ingesta de proteínas cuando se ha prescrito levodopa. La dieta por sí misma es fundamental para el manejo de cada persona predispuesta o que sufre de EPI. Las dietas veganas, ricas en proteínas debidamente balanceadas con cereales y suplementadas con vitamina B12, reducen la ingesta de metionina y mantiene la cuenta de aminoácidos aromáticos a un nivel moderado.
Palabras clave: Enfermedad de Parkinson, Dieta, Dieta Vegetariana, Metionina, Prevención de Enfermedades (DeCS).
Introduction
The six patients described by James Parkinson (1) as afflicted by Shaking Palsy, motivated Jean Marie Charcot to label them as suffering Parkinson´s disease (2), yet this syndrome would have been a better choice with its classic triad: tremor, stiffness and slowness. A large number of other non-motor afflictions add to the protracted clinical course of each patient.
Idiopathic Parkinson´s Disease (IPD) is more prevalent in countries with high industrial development and high economic standards. However, there have been studies linking higher incidence of Parkinson's among rural inhabitants as compared to urban residents. Nowadays it is apparent that there are several distinct types of parkinsonian disorders (3) and each patient individually endures his lot.
Discussing its "origin", Parkinson stated, "we are led to seek for it in some slow morbid change in the structure of the medulla" (1). It is well established that damage of the substantia nigra (SN) melanic neurons, more severe in its pars compacta, is responsible for the motor signs of IPD and in such cases cell loss is substantial and done over several years before overt clinical signs appear (3,4).
Among causal agents of Parkinsonism, the lethargic encephalitis virus was prominent after the influenza pandemic of 1919 with many cases of its postencephalitic form, mostly with rigidity and bradikynesia. Several exogenous toxic agents (reserpine, manganese, MPTP, substances in the leaves of the sour sop tree Annona muricata L) cause Parkinsonism or closely related syndromes. The endogenous toxins causing the development of IPD signs and symptoms are methylated abnormal intermediates akin to those of dopamine secondary metabolites (4): in persons prone to IPD their very low tyrosine-hydroxylase (TH) activity leaves a local abundance of both tyrosine and tyramine (its decarboxylation product) available for abnormal reactions amongst them methylation.
Tyramine is produced in excess and is excreted in the urine, though in other cases is further metabolized and is excreted in lower amount than by normal persons (5). The resulting abnormal metabolites metabolize in an unknown way. The reason why the extention of those metabolites is responsible for the SN damage is also unknown. Matsubara (6), Naoi (7) and Williams (8) propose several abnormal methylated substances as SN neurotoxins. The last author considers IPD as an autointoxication related to N-methylnicotinamide. People susceptible to IPD submitted in addition to exogenous neurotoxins will develop their IPD earlier and more severely.
During the last decades, the genetics for Parkinsonism have been studied and many different genes have been located (3). Some early onset (or young onset) of Parkinsonism have a direct genetic component, while several genes have been scrutinized in idiopathic cases (nowadays called "sporadic"), by far more frequent. Two different mutations in LRRK2 dardarin (the euskera name for tremor), R1441G in Basques and some of them also carry mutation G2019S present in Ashkenazis (9). The study of these remarkable ethnic groups should give many answers for the prevention of the Parkinson´s syndrome.
Materials and methods
The study counted with two cases:
Case 1
An Ashkenazi man developed his Parkinsonism at the age of 67 (10). He was able to function for about 42 months from the time of diagnosis, without drugs for his Parkinson´s disease. Constant loss of weight in the fourth year necessitated turning to levodopa, and his weight brought up (15 lbs over 3 months). Thereafter, sinemet (levodopa/carbidopa) (100/25 3/day) was taken and increased annually.
Roughly three years after being on drugs for IPD, he found one morning there was no response after taking the usual doses of levodopa/carbidopa and entacapone. Recalling some readings about interference of animal protein with absorption of Parkinson´s disease medicaments and checking two texts on PD that corroborated this fact, he had a vegan lunch and was overwhelmed at the significant difference in his body's response. This was a powerful motivator. Further reading led to understand that a meal with 87% whole grains and vegetables/fruit with the remainder animal protein, works well. This was also the first time in four years that there was no need to increase the PD drugs after one year on the semi-vegan diet.
In his 75th year, on his eighth year of clinical course, the patient completed 2 years on quasi-vegan diet. In that period, clinical signs of the disease did not appear to have advanced, levodopa dosage remaining the same, and incremental improvement benefited quality of life. He passed away on January 2011 at the age of 82.
Case 2
A basque woman, mother of three children, two of them afflicted with essential tremor, and grandmother of eleven grandchildren, started her IPD clinical signs at the age of 84 (11). She had a mild tremor of her left hand fingers. Along her life she had been very moderate at table, so her siblings called her "chupa huesos" (bone sucker) because when young she asked for bones with very little meat. On the other side she indulged in milk and eggs (thrice a day plus those entering in making her most delicious "flan", a gel prepared by heating milk, eggs and sugar, readily consumed) so she could be considered a not too strict ovo-lacto-vegetarian. For years, she took a vitamin supplement with a substantial amount of iron. Her clinical course lasted ten years and was steadily downhill in spite of her very good response during the last six years to low doses of sinemet or madopar. While shifting proteins on the last meal has also been proposed (3), in her case little protein in the last meal plus minimal doses of sinemet (levodopa/carbidopa) or madopar (levodopa/benserazide) in the evening induced a restful sleep overnight.
Pubmed database was consulted to identify investigations about diets, food and natural substances in relation with Parkinson´s disease.
Results
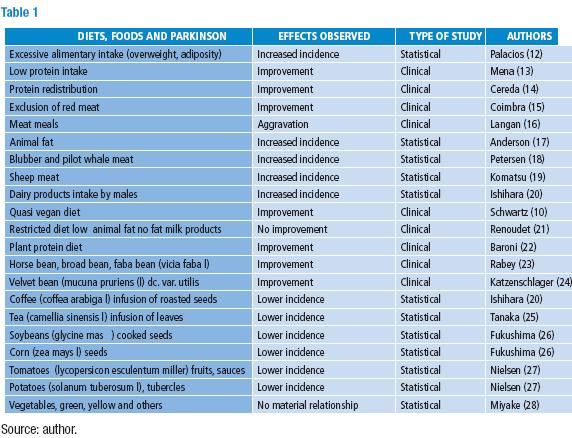
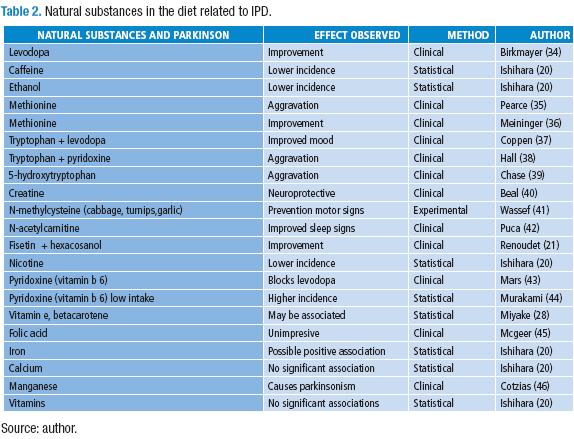
Table 1 lists diets and food reported along with IPD. A reference was given whenever a thorough review has been published. Table 2 lists the natural substances in the diet related to IPD.
Palacios (12) reported that IPD patients were heavier and had more fat in their bodies: this can be assume as the result of overeating and/or not enough physical exercise. On the other hand, several studies show that IPD patients can lose weight, as in case 1 disease (10), and many like Perpe (11) have problems to swallow their meals.
Dairy products are correlated with higher incidence of IPD (20). Chen (29) concluded that: "Our results suggest that higher intake of dairy products may increase the risk of PD in men; however, this finding needs further evaluation, and the underlying active components need to be identified". On the contrary, a paper from Japan (28) reported no difference; other dietary factors in the usual Japanese diet (rice, soy beans) should be considered. A most recent article from Greece correlates statistically milk consumption with higher incidence but neither yoghurt nor cheese (30) showed this correlation.
Mixing two different experimental variables make uncertain the evaluation of the results observed, as in the study by Coimbra and Junqueira (15) who suppressed red meat from the diet of their IPD patients and overloaded them with riboflavin. They observed clinical improvement but the statistical design was later severely disputed; yet evaluating two variables would have required additional control groups for each variable. Animal fat has been taken in consideration (17) disregarding the amount of meat ingested along with it.
Cotzias and cols., found in their studies with levodopa that animal protein had a clear effect on their patients: "hamburgers" caused worsening of the signs in some of them (16,31). They proposed that "neutral" amino acids compete with levodopa in transport systems, but hypothesized "levodopa is perhaps doing something more permanent than merely supplying readily consumable materials to the brain" (33). Since then, all the attention directs to distribute protein intake in such a way as to avoid interference with the absorption of levodopa and its entrance into the central nervous system. An individual case (10) reported the good effect of restricting animal protein to around one seventh of the protein provided in the diet. The case reported by Renoudet (21) did not show an improvement by the decrease of "animal fat" consumption and non-fat milk products, but an amelioration resulted when eating fisetin (a tetrahydroxyflavone present in strawberries and many plants: CAS 528-48-3) and hexacosanol (a long chain aliphatic alcohol CAS 506-52-5 present in wheat germ and many other food items).
Meat in addition to high levels of methionine (MET) and aromatic amino acids (AAA) contains several methylated substances (carnitine, creatine, phosphocreatine, betaine, choline, acetylcholine, sarcosine) that can contribute with methyl groups for the recycling of homocysteine; this affects the MET accounting even up to the half of its daily requirement in the diet.
Very little attention has been paid (3) to Langan and Cotzias warning (16): "The foods to limit include milk, meat, fish, poultry, cheese, eggs, whole grains and soy bean products" and they illustrated their paper with a drawing of a man running away from them. A report by Cotzias team clarify however that, "the fact that some patients and their relatives have demanded low-protein diets on a long-term basis does not change the experimental status of those regimens" (31).
A number of plant food and substances found in them match with lower incidence or to improve the course of IPD patients. Only recent replacement of animal protein by plant protein, as achieved by following a vegetarian diet, showed a clear-cut clinical improvement of IPD patients: Baroni (22) performed an experiment shifting from omnivorous to vegetarian diets a group of twelve patients with Parkinson´s disease who improved on the latter regimen. Plants like the broad bean (23) and the velvet bean (24) contain levodopa. Nielsen ascribes (27) the effect of solanaceous plants in diets to their nicotine content (32) disregarding other substances contained, mostly in tomatoes, and other components in those diets.
The decrease in MET and AAA ingestion by vegetarians that exclude dairy products explains these findings. The overload of MET and AAAs can also explains the coincidence of high dairy products consumption with increased number of IPD persons.
The impressive motor improvement of most IPD patients when taking levodopa (34) and the relation of this action with the timing of eating protein has been clearly established and diet instructions have been imparted to IPD patients basically as an adjunct to levodopa treatment. This is accepted as the result of better absorption of levodopa when not competed by other amino acids both at the intestinal absorption sites and at the blood brain transport systems. The redistribution of protein ingestion during the day increases the absorption of levodopa both in the gastrointestinal tract and by neurones, by less competed transport through the blood brain barrier (BBB).
Each substance listed, starting with levodopa, deserves additional studies to determine their mechanism of action. Methionine is implicated in the dopamine secondary metabolism to synthesize endogenous morphine (4) and has been shown to be damaging to IPD patients receiving levodopa (35), though a preliminary report of a trial (36) claimed that it was as effective as levodopa. No subsequent reports have been founded on this matter by these authors, though Zhu (47), based on some concepts, proposes to supplement a diet rich in fruits and vegetables with S-adenosylmethionine. Beal (40) states that: "A phase 2 futility trial in PD showed approximately a 50% improvement in Unified Parkinson's Disease Rating Scale at one year, and the compound [carnitine] was judged to be non futile."
Discussion
The pharmacologic treatment of Parkinsonism is considered paramount; because of that, physiotherapy and diet have little consideration on the attention of the disease. Both of them are fundamental for the wellbeing of IPD patients. Indeed, Singer (48) does not mention the latter and gives a forty words mention to the former. Sääksjärvi and Knekt (49) suggest that "...since most of the single food groups or the quality of diet did not predict PD occurrence, the role of diet is apparently rather modest". However, they found that low meat and high dairy eating by women positively correlated with Parkinson´s disease, and do not give a global evaluation of animal protein ingested. No report compares a pulse rich diet with catechol orthomethyl transferase (COMT) inhibitors on levodopa metabolism and/or clinical response.
Alcalay paper (50) points out that "greater Mediterranean diet adherence was associated with later PD age-at-onset" and starts the discussion of results stating that: "lower adherence to Mediterranean diet is associated with PD status...the fact that among PD participants lower adherence was associated with earlier PD age-at-onset further suggests a possible dose-response effect".
Conclusion
Based in the limited, but in-depth, personal experiences, diet has a primary importance and must be the first step in prevention and treatment of IPD: it is fundamental for people (1-2%) enduring, or susceptible to, IPD invalidating conditions.
The "do not" (15) items (animal protein, including milk and cheeses), which are usually high in MET and AAA, should be very few in the diet of IPD patients (and of those prone to this ailment, whenever a preclinical diagnostic method becomes available). On the positive side their diet must be abundant in fruits, vegetables and, on a calculated proportion, pulses (common beans, soya bean, lentils, chickpeas, etc.), all of them low in methionine, combined with cereals (as done traditionally in Mexico), which level the relative amount of methionine with that of lysine, deficient particularly in maize (indian corn=Zea mays L).
A diet like that is healthier than a diet that depends on animal products with an excess of essential aminoacids. The sufficient amount of calories must be provided as well as vitamin B 12 in adequate dosage. It is urgent to carry on studies on the prevalence of IiPD in vegetarians, comparing them with omnivorous and carnivorous persons. These studies should be performed in Basques and Ashkenazi, carriers of mutation at LRRK2, correlating their diet to age of IPD signs onset. Those patients should start vegetarian diets, with minimal amounts of dairy products and eggs, as soon as possible and feasible.
Conflict of interest
None declared by the author.
Funding
None declared by the author.
Acknowledgements
This study is dedicated in loving memory to Rabbi Frederick Schwartz (Fred), Perpetua Sasiaín Aberasturi (Perpe) and with perennial recognition to Aaron Bunsen Lerner, outstanding head and soul of the team that discovered melatonin, who entered immortality suffering Parkinson´s disease (51). We thank Jhon Rios and Juan Carlos Buitrago, of the Hemeroteca Nacional Universitaria, Universidad Nacional de Colombia, for their most efficient recovery of bibliographic material. We are indebted to Ruth Myriam Molina Lizcano for the final setting of the excel tables and the manuscript.
Referencias
1. Parkinson J. An essay on the shaking palsy. London: Sherwood, Neely, Jones; 1817. Reprinted in J Neuropsychiatry Clin Neurosci. 2002;14:223-36.
2. Goetz CG. Charcot on Parkinson´s disease. Mov Disord. 1986;1:27-32.
3. Micheli F. Enfermedad de Parkinson y trastornos relacionados. 2ª ed. Buenos Aires: Ed. Médica Panamericana; 2006.
4. Perea-Sasiain J. The Biosynthesis of morphine: its importance in Parkinson´s disease. Rev. Fac. Med. 2008;56:161-89.
5. Perea-Sasiain J. Tiramina en la orina de los parkinsonianos idiopáticos. Rev. Fac. Med. 2010;58:157-60.
6. Matsubara K, Aoyama K, Suno M, Awaya T. N-methylation underlying Parkinson's disease. Neurotoxicol Teratol. 2002;24:593-8.
7. Naoi M, Maruyama W, Niwa T, Nagatsu T. Novel toxins and Parkinson's disease: N-methylation and oxidation as metabolic bioactivation of neurotoxin. J Neural Transm. 1994;41:197-205.
8. Williams AC, Hill LJ, Ramsden DB. Nicotinamide NAD(P)(H), and Methyl-Group Homeostasis Evolved and Became a Determinant of Ageing Diseases: Hypotheses and Lessons from Pellagra. Curr Gerontol Geriatr Res. 2012;2012:302875.
9. Gorostidi A, Ruiz-Martínez J, Lopez de Munain A, Alzualde A, Martí Massó JF. LRRK2 G2019S and R1441G mutations associated with Parkinson's disease are common in the Basque Country, but relative prevalence is determined by ethnicity. Neurogenetics. 2009;10:157-9.
10. Schwartz RH. Letter to the editor on VEGAN diet. Med Hypothes. 2004;63:178.
11. Perea-Sasiaín J. Reflexiones sobre la biosíntesis de la morfina y el Parkinson idiopático. Revista de Salud Pública. 2007;9:308-14.
12. Palacios N, Gao X, McCullough ML, Jacobs EJ, Patel AV, Mayo T et al. Obesity, diabetes, and risk of Parkinson disease. Mov Disord. 2011;26:2253-9.
13. Mena I, Cotzias GC. Protein intake and treatment of Parkinson's disease with levodopa. N Engl J Med. 1975;292:181-4.
14. Cereda E, Barichella M, Pedrolli C, Pezzoli G. Low-protein and protein-redistribution diets for Parkinson's disease patients with motor fluctuations: a systematic review. Mov Disord. 2010;25:2021-34.
15. Coimbra CG, Junqueira VB. High doses of riboflavin and the elimination of dietary red meat promote the recovery of some motor functions in Parkinson's disease patients. Braz J Med Biol Res. 2003;36:1409-17.
16. Langan RJ, Cotzias GC. Do´s and don´ts for the patient on levodopa therapy. Am J Nurs. 1976;76:917-8.
17. Anderson C, Checkoway H, Franklin GM, Beresford S, Smith-Weller T, Swanson PD. Dietary factors in Parkinson's disease: the role of food groups and specific foods. Mov Disord. 1999;14:21-7.
18. Petersen MS, Halling J, Bech S, Wermuth L, Weihe P, Nielsen F, et al. Impact of dietary exposure to food contaminants on the risk of Parkinson´s disease. Neurotoxicology. 2008;29:584-90.
19. Komatsu F, Kaagawa Y, Kawabata T, Kaneko Y, Chimedregzen U, Purvee B, Otgon J. A high accumulation of hair minerals in Mongolian people: 2(nd) report; influence of manganese, iron, lead, cadmium and aluminum to oxidative stress, Parkinsonism and arthritis. Curr Aging Sci. 2011;4:42-56.
20. Ishihara LS, Brayne C. A systematic review of nutritional risk factors of Parkinson´s disease. Nutr Res Rev. 2005;18:259-82.
21. Renoudet VV, Costa-Mallen P, Hopkins E. RA diet low in animal fat and rich in N-hexacosanol and fisetin is effective in reducing symptoms of Parkinson's disease. J Med Food. 2012;15:758-61.
22. Baroni L, Bonetto C, Tessan F, Goldin D, Cenci L, Magnanini P, et al. Pilot dietary study with normoproteic protein-redistributed plant-food diet and motor performance in patients with Parkinson's disease. Nutr Neurosci. 2011;14:1-9.
23. Rabey JM, Vered Y, Shabtai H, Graff E, Korczyn AD. Improvement of parkinsonian features correlate with high plasma levodopa values after broad bean (Vicia faba) consumption. J Neurol Neurosurg Psychiatry. 1992;55:725-7.
24. Katzenschlager R, Evans A, Manson A, Patsalos PN, Ratnaraj N, Watt H, et al. Mucuna pruriens in Parkinson's disease: a double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry. 2004;75:1672-7.
25. Tanaka K, Miyake Y, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y, et al. Fukuoka Kinki Parkinson's Disease Study Group. Intake of Japanese and Chinese teas reduces risk of Parkinson's disease. Parkinsonism Relat Disord. 2011;17:446-50.
26. Fukushima T, Tanaka K, Ushijima K, Moriyama M. Retrospective study of preventive effect of maize on mortality from Parkinson's disease in Japan. Asia Pac J Clin Nutr. 2003;12:447-50.
27. Nielsen SS, Franklin GM, Longstreth WT, Swanson PD, Checkoway H. Nicotine from edible Solanaceae and risk of Parkinson disease. Ann Neurol. 2013 May 9. doi: 10.1002/ana.23884.
28. Miyake Y, Tanaka K, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y, et al. Fukuoka Kinki Parkinson's Disease Study Group. Lack of association of dairy food, calcium, and vitamin D intake with the risk of Parkinson's disease: a case-control study in Japan. Parkinsonism Relat Disord. 2011;17:112-6.
29. Chen H, O´Reilly E, McCullough ML, Rodríguez C, Schwarzschild MA, Calle EE, et al. Consumption of dairy products and risk of Parkinson's disease. Am J Epidemiol. 2007;165:998-1006.
30. Kyrozis A, Ghika A, Stathopoulos P, Vassilopoulos D, Trichopoulos D, Trichopoulou A. Dietary and lifestyle variables in relation to incidence of Parkinson's disease in Greece. Eur J Epidemiol. 2013;28:67-77.
31. Gillespie NG, Mena I, Cotzias GC, Bell MA. Diets affecting treatment of parkinsonism with levodopa. J Am Diet Assoc. 1973;62:525-8.
32. Siegmund B, Leitner E, Pfannhauser W. Determination of the nicotine content of various edible nightshades (Solanaceae) and their products and estimation of the associated dietary nicotine intake. J Agric Food Chem. 1999;47:3113-20.
33. Cotzias GC, Papavasiliou PS, Steck A, Düby S. Parkinsonism and levodopa. Clin Pharmacol Therap. 1971;12:319-22.
34. Birkmayer W, Hornykiewicz O. Der L-3,4-dioxyphenylalanin (DOPA) -Effect bei der Parkinson-Akinese. Wien Klin Wochenschr. 1961;73:787-8.
35. Pearce LA, Waterbury LD. L-methionine: a possible levodopa antagonist. Neurology. 1974;24:840-1.
36. Meininger V, Flamier A, Phan T, Ferris O, Uzan A, Lefur G. Traitement de la maladie de Parkinson par la L-méthionine: résultats préliminaires. Rev Neurol (Paris). 1982;138:297-303.
37. Coppen A, Metcalfe M, Carroll JD, Morris JGL. Levodopa and L-tryptophan therapy in parkinsonism. Lancet. 1972;i:654-8.
38. Hall CD, Weiss EA, Morris CE, Prange AJ. Jr. Rapid deterioration in patients with parkinsonism following tryptophan-pyridoxine administration. Neurology. 1972;22:231-7.
39. Chase TN, Larry KY, Watanabe AM. Parkinson´s disease. Modification by 5-hydroxytryptophan. Neurology. 1972;22:479-84.
40. Beal MF. Neuroprotective effects of creatine. Amino Acids. 2011;40:1305-13.
41. Wassef R, Haenold R, Hansel A, Brot N, Heinemann SH, Hoshi T. Methionine sulfoxide reductase A and a dietary supplement S-methyl-L-cysteine prevent Parkinson's-like symptoms. J Neurosci. 2007;27:12808-16.
42. Puca FM, Genco S, Specchio LM, Brancasi B, D'Ursi R, Prudenzano A, et al. Clinical pharmacodynamics of acetyl-L-carnitine in patients with Parkinson's disease. Int J Clin Pharmacol Res. 1990;10:139-43.
43. Mars H. Levodopa, carbidopa and pyridoxine in Parkinson disease. Arch Neurol. 1974;30:444-7.
44. Murakami K, Miyake Y, Sasaki S, Tanaka K, Fukushima W, Kiyohara C et al. Parkinson's Disease Study Group, Dietary intake of folate, vitamin B6, vitamin B12 and riboflavin and risk of Parkinson's disease: a case-control study in Japan. Br J Nutr. 2010;104:757-64.
45. McGeer PL, Zeldowicz L, McGeer EG. A clinical trial of folic acid in Parkinson's disease. Can Med Assoc J. 1972;106:145-6.
46. Cotzias GC, Papavasiliou PS, Ginos J, Steck A, Düby S. Metabolic modification of Parkinson's disease and of chronic manganese poisoning. Annu Rev Med. 1971;22:305-26.
47. Zhu BT. CNS dopamine oxidation and catechol-O-methyltransferase: importance in the etiology, pharmacotherapy, and dietary prevention of Parkinson´s disease. Int J Mol Med. 2004;13:343-53.
48. Singer C. Managing the patient with newly diagnosed Parkinson disease. Cleve Clin J Med. 2012;79(Suppl 2):S3-7.
49. Sääksjärvi K, Knekt P, Lundqvist A, Männistö S, Heliövaara M, Rissanen H, Järvinen R. A cohort study on diet and the risk of Parkinson's disease: the role of food groups and diet quality. Br J Nutr. 2013;109:329-37.
50. Alcalay RN, Gu Y, Mejia-Santana H, Cote L, Marder KS, Scarmeas N. The association between Mediterranean diet adherence and Parkinson's disease. Mov Disord. 2012;27:771-4.
51. Lerner E. Aaron B. Lerner, in memoriam. J Invest Dermatol. 2007;127:2077-9.
*This report is not indexed in pubmed, and we were very fortunate to locate it directly.
Referencias
Parkinson J. An essay on the shaking palsy. London: Sherwood, Neely, Jones; 1817. Reprinted in J Neuropsychiatry Clin Neurosci. 2002;14:223-36.
Goetz CG. Charcot on Parkinson´s disease. Mov Disord. 1986;1:27-32.
Micheli F. Enfermedad de Parkinson y trastornos relacionados. 2ª ed. Buenos Aires: Ed. Médica Panamericana; 2006.
Perea-Sasiain J. The Biosynthesis of morphine: its importance in Parkinson´s disease. rev.fac.med. 2008;56:161-89.
Perea-Sasiain J. Tiramina en la orina de los parkinsonianos idiopáticos. rev.fac.med. 2010;58:157-60.
Matsubara K, Aoyama K, Suno M, Awaya T. N-methylation underlying Parkinson's disease. Neurotoxicol Teratol. 2002;24:593-8.
Naoi M, Maruyama W, Niwa T, Nagatsu T. Novel toxins and Parkinson's disease: N-methylation and oxidation as metabolic bioactivation of neurotoxin. J Neural Transm. 1994;41:197-205.
Williams AC, Hill LJ, Ramsden DB. Nicotinamide NAD(P)(H), and Methyl-Group Homeostasis Evolved and Became a Determinant of Ageing Diseases: Hypotheses and Lessons from Pellagra. Curr Gerontol Geriatr Res. 2012;2012:302875.
Gorostidi A, Ruiz-Martínez J, Lopez de Munain A, Alzualde A, Martí Massó JF. LRRK2 G2019S and R1441G mutations associated with Parkinson's disease are common in the Basque Country, but relative prevalence is determined by ethnicity. Neurogenetics. 2009;10:157-9.
Schwartz RH. Letter to the editor on VEGAN diet. Med Hypothes. 2004;63:178.
Perea-Sasiaín J. Reflexiones sobre la biosíntesis de la morfina y el Parkinson idiopático. Revista de Salud Pública. 2007;9:308-14.
Palacios N, Gao X, McCullough ML, Jacobs EJ, Patel AV, Mayo T et al. Obesity, diabetes, and risk of Parkinson disease. Mov Disord. 2011;26:2253-9.
Mena I, Cotzias GC. Protein intake and treatment of Parkinson's disease with levodopa. N Engl J Med. 1975;292:181-4.
Cereda E, Barichella M, Pedrolli C, Pezzoli G. Low-protein and protein-redistribution diets for Parkinson's disease patients with motor fluctuations: a systematic review. Mov Disord. 2010;25:2021-34.
Coimbra CG, Junqueira VB. High doses of riboflavin and the elimination of dietary red meat promote the recovery of some motor functions in Parkinson's disease patients. Braz J Med Biol Res. 2003;36:1409-17.
Langan RJ, Cotzias GC. Do´s and don´ts for the patient on levodopa therapy. Am J Nurs. 1976;76:917-8.
Anderson C, Checkoway H, Franklin GM, Beresford S, Smith-Weller T, Swanson PD. Dietary factors in Parkinson's disease: the role of food groups and specific foods. Mov Disord. 1999;14:21-7.
Petersen MS, Halling J, Bech S, Wermuth L, Weihe P, Nielsen F, et al. Impact of dietary exposure to food contaminants on the risk of Parkinson´s disease. Neurotoxicology. 2008;29:584-90.
Komatsu F, Kaagawa Y, Kawabata T, Kaneko Y, Chimedregzen U, Purvee B, Otgon J. A high accumulation of hair minerals in Mongolian people: 2(nd) report; influence of manganese, iron, lead, cadmium and aluminum to oxidative stress, Parkinsonism and arthritis. Curr Aging Sci. 2011;4:42-56.
Ishihara LS, Brayne C. A systematic review of nutritional risk factors of Parkinson´s disease. Nutr Res Rev. 2005;18:259-82.
Renoudet VV, Costa-Mallen P, Hopkins E. RA diet low in animal fat and rich in N-hexacosanol and fisetin is effective in reducing symptoms of Parkinson's disease. J Med Food. 2012;15:758-61.
Baroni L, Bonetto C, Tessan F, Goldin D, Cenci L, Magnanini P, et al. Pilot dietary study with normoproteic protein-redistributed plant-food diet and motor performance in patients with Parkinson's disease. Nutr Neurosci. 2011;14:1-9.
Rabey JM, Vered Y, Shabtai H, Graff E, Korczyn AD. Improvement of parkinsonian features correlate with high plasma levodopa values after broad bean (Vicia faba) consumption. J Neurol Neurosurg Psychiatry. 1992;55:725-7.
Katzenschlager R, Evans A, Manson A, Patsalos PN, Ratnaraj N, Watt H, et al. Mucuna pruriens in Parkinson's disease: a double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry. 2004;75:1672-7.
Tanaka K, Miyake Y, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y, et al. Fukuoka Kinki Parkinson's Disease Study Group. Intake of Japanese and Chinese teas reduces risk of Parkinson's disease. Parkinsonism Relat Disord. 2011;17:446-50.
Fukushima T, Tanaka K, Ushijima K, Moriyama M. Retrospective study of preventive effect of maize on mortality from Parkinson's disease in Japan. Asia Pac J Clin Nutr. 2003;12:447-50.
Nielsen SS, Franklin GM, Longstreth WT, Swanson PD, Checkoway H. Nicotine from edible Solanaceae and risk of Parkinson disease. Ann Neurol. 2013 May 9. doi: 10.1002/ana.23884.
Miyake Y, Tanaka K, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y, et al. Fukuoka Kinki Parkinson's Disease Study Group. Lack of association of dairy food, calcium, and vitamin D intake with the risk of Parkinson's disease: a case-control study in Japan. Parkinsonism Relat Disord. 2011;17:112-6.
Chen H, O´Reilly E, McCullough ML, Rodríguez C, Schwarzschild MA, Calle EE, et al. Consumption of dairy products and risk of Parkinson's disease. Am J Epidemiol. 2007;165:998-1006.
Kyrozis A, Ghika A, Stathopoulos P, Vassilopoulos D, Trichopoulos D, Trichopoulou A. Dietary and lifestyle variables in relation to incidence of Parkinson's disease in Greece. Eur J Epidemiol. 2013;28:67-77.
Gillespie NG, Mena I, Cotzias GC, Bell MA. Diets affecting treatment of parkinsonism with levodopa. J Am Diet Assoc. 1973;62:525-8.
Siegmund B, Leitner E, Pfannhauser W. Determination of the nicotine content of various edible nightshades (Solanaceae) and their products and estimation of the associated dietary nicotine intake. J Agric Food Chem. 1999;47:3113-20.
Cotzias GC, Papavasiliou PS, Steck A, Düby S. Parkinsonism and levodopa. Clin Pharmacol Therap. 1971;12:319-22.
Birkmayer W, Hornykiewicz O. Der L-3,4-dioxyphenylalanin (DOPA) -Effect bei der Parkinson-Akinese. Wien Klin Wochenschr. 1961;73:787-8.
Pearce LA, Waterbury LD. L-methionine: a possible levodopa antagonist. Neurology. 1974;24:840-1.
Meininger V, Flamier A, Phan T, Ferris O, Uzan A, Lefur G. Traitement de la maladie de Parkinson par la L-méthionine: résultats préliminaires. Rev Neurol (Paris). 1982;138:297-303.
Coppen A, Metcalfe M, Carroll JD, Morris JGL. Levodopa and L-tryptophan therapy in parkinsonism. Lancet. 1972;i:654-8.
Hall CD, Weiss EA, Morris CE, Prange AJ. Jr. Rapid deterioration in patients with parkinsonism following tryptophan-pyridoxine administration. Neurology. 1972;22:231-7.
Chase TN, Larry KY, Watanabe AM. Parkinson´s disease. Modification by 5-hydroxytryptophan. Neurology. 1972;22:479-84.
Beal MF. Neuroprotective effects of creatine. Amino Acids. 2011;40:1305-13.
Wassef R, Haenold R, Hansel A, Brot N, Heinemann SH, Hoshi T. Methionine sulfoxide reductase A and a dietary supplement S-methyl-L-cysteine prevent Parkinson's-like symptoms. J Neurosci. 2007;27:12808-16.
Puca FM, Genco S, Specchio LM, Brancasi B, D'Ursi R, Prudenzano A, et al. Clinical pharmacodynamics of acetyl-L-carnitine in patients with Parkinson's disease. Int J Clin Pharmacol Res. 1990;10:139-43.
Mars H. Levodopa, carbidopa and pyridoxine in Parkinson disease. Arch Neurol. 1974;30:444-7.
Murakami K, Miyake Y, Sasaki S, Tanaka K, Fukushima W, Kiyohara C et al. Parkinson's Disease Study Group, Dietary intake of folate, vitamin B6, vitamin B12 and riboflavin and risk of Parkinson's disease: a case-control study in Japan. Br J Nutr. 2010;104:757-64.
McGeer PL, Zeldowicz L, McGeer EG. A clinical trial of folic acid in Parkinson's disease. Can Med Assoc J. 1972;106:145-6.
Cotzias GC, Papavasiliou PS, Ginos J, Steck A, Düby S. Metabolic modification of Parkinson's disease and of chronic manganese poisoning. Annu Rev Med. 1971;22:305-26.
Zhu BT. CNS dopamine oxidation and catechol-O-methyltransferase: importance in the etiology, pharmacotherapy, and dietary prevention of Parkinson´s disease. Int J Mol Med. 2004;13:343-53.
Singer C. Managing the patient with newly diagnosed Parkinson disease. Cleve Clin J Med. 2012;79(Suppl 2):S3-7.
Sääksjärvi K, Knekt P, Lundqvist A, Männistö S, Heliövaara M, Rissanen H, Järvinen R. A cohort study on diet and the risk of Parkinson's disease: the role of food groups and diet quality. Br J Nutr. 2013;109:329-37.
Alcalay RN, Gu Y, Mejia-Santana H, Cote L, Marder KS, Scarmeas N. The association between Mediterranean diet adherence and Parkinson's disease. Mov Disord. 2012;27:771-4.
Lerner E. Aaron B. Lerner, in memoriam. J Invest Dermatol. 2007;127:2077-9
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2014 Revista de la Facultad de Medicina

Esta obra está bajo una licencia Creative Commons Reconocimiento 3.0 Unported.
Derechos de autor
Los autores deben aceptar transferir a la Revista de la Facultad de Medicina los derechos de autor de los artículos publicados. La editorial tiene el derecho del uso, reproducción, transmisión, distribución y publicación en cualquier forma o medio. Los autores no podrán permitir o autorizar el uso de la contribución sin el consentimiento escrito de la revista. Estos archivos están disponibles en https://goo.gl/EfWPdX y https://goo.gl/6zztk4 y deben cargarse en el paso 4 del envío OJS (archivos complementarios).
La carta de cesión de derechos de autor y la de responsabilidad de autoría deben ser entregadas junto con el original.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).