doi: https://dx.doi.org/10.15446/caldasia.v38n2.60921
Taxonomía y Sistemática
On the morphology of Plectrohyla chryses (Anura: Hylida; Hylini), with comments on some controversial characters, phylogenetic relationships, and diagnosis of this species
Sobre la morfología de Pectrohyla chryses (Anura: Hylidae; Hylini), con comentarios sobre algunos caracteres controversiales, sus relaciones filogenéticas, y su diagnosis
Moises Kaplan
1508 Brooklyn Avenue, Ann Arbor, Michigan 48104 USA. moises.kaplan@gmail.com
Peter Heimes
Playa Miramar 441, Colonia Marte 08830, Ciudad de Mexico, Mexico. Estados Unidos heimes@hotmail.com
Eugenia Zarza
Moore Laboratory of Zoology, Occidental College, 1600 Campus Road, Los Angeles, California 90041. Estados Unidos
John Mccormack
Moore Laboratory of Zoology, Occidental College, 1600 Campus Road, Los Angeles, California 90041. Estados Unidos, mccormack@oxy.edu
ABSTRACT
We describe the tadpole and juvenile of Plectrohyla chryses and conduct an analysis of skin thickness and a general phylogenetic analysis of the Hylini to determine the phylogenetic position of P. chryses. We found that P. chryses has thin skin and that is part of the P. bistincta group. We also found that adult P. chryses have an axillary membrane and a thoracic fold, but that the rostral keel is absent. We conclude that only the character "skin thickness" is useful for the diagnosis of P. chryses, and we propose a new diagnosis for this species.
Key words. Mexico, Guerrero, tadpole, phylogeny, skin.
RESUMEN
Describimos el renacuajo y juvenil de Plectrohyla chryses y hacemos análisis del grosor de la piel y un análisis filogenético de los Hylini para determinar las relaciones filogenéticas de P. chryses. Encontramos que P. chryses tiene la piel delgada y es parte del grupo P. bistincta. También encontramos que el adulto de P. chryses tiene membrana axilar y pliegue torácico, pero carece de quilla rostral. Concluimos que solamente el carácter "grosor de la piel" puede ser usado en la diagnosis de P. chryses y proponemos una nueva diagnosis para esta especie.
Palabras clave. México, Guerrero, renacuajos, filogenia, piel.
INTRODUCTION
The genus Plectrohyla contains 42 species of stream-dwelling frogs distributed in the highlands of Mexico, Guatemala, northern El Salvador, central northern Honduras, and northern Nicaragua (Duellman 2001, Frost 2015). There are two monophyletic groups recognized in Plectrohyla, the P. guatemalensis group with 18 species distributed from southern Mexico to northern Nicaragua and the P. bistincta group with 24 species, all distributed in Mexico (Faivovich et al. 2005, Frost 2015).
Duellman et al. (2016) transferred the P. bistincta group to a newly erected genus Sarcohyla, a questionable action given that most of the species of the P. bistincta group (16 out of 24) have not been included in a phylogenetic analysis (see Faivovich et al. 2005:104 for a similar argument).
One of the lesser-known species of the Plectrohyla bistincta group is P. chryses (Adler, 1965). This species is known from 32 individuals. Its tadpole and juvenile are still undescribed. P. chryses inhabits oak-pine-fir forest in the Sierra Madre del Sur west of Chilpancingo, Guerrero at elevations between 2340 and 2600 meters (Duellman 2001, Frost 2015). Adler (1965) described Plectrohyla chryses based on four adult specimens (1 male, 3 females). Mendelson & Toal (1996), after examining the holotype, a paratype (KU 106306), and several newly discovered individuals, concluded that three characters of the diagnostic combination (thoracic fold, axillary membrane, and rostral keel) were erroneous. Presently, it is unclear what the correct condition of these characters are, or whether these characters can be used to diagnose P. chryses from other species of the P. bistincta group.
Adler (1965; see also Duellman 2001 and Duellman et al. 2016) included the character "dorsal skin thickness," among others, in the diagnostic combination of P. chryses (Adler 1965). Toal & Mendelson (1995:13) questioned the utility of this character for the taxonomy and systematics in the P. bistincta group because previous conclusions about whether the species of this group have thin or thick skins were subjective (i.e., based on qualitative assessments, not measurements). Presently, quantitative measurements have only been taken from individuals of three species of the P. bistincta group (i.e., P. bistincta (Cope, 1877), P. chryses, and P. mykter (Adler and Dennis, 1972); see Kaplan et al. 2015), so it remains unknown if the previous conclusions concerning the taxo-nomic distribution of the states "thin skin" and "thick skin" in the P bistincta group are correct.
P chryses is considered part of the P bistincta group because it has long fingers with vestigial webbing, prominent (not projecting) prepollex, and lacks a quadratojugal (Adler 1965, Duellman 2001; but see Duellman & Campbell 1992). However, presently there is no synapomorphy supporting the inclusion of P. chryses in the P. bistincta group or in the genus Plectrohyla (Faivovich et al. 2005). Thus, the phylogenetic position of P. chryses remains in doubt.
We recently obtained tissues for DNA analysis, as well as tadpoles and juveniles of individuals of the P. bistincta group from two localities west of Chilpancingo, Guerrero. Based on the most recent diagnosis (Men-delson and Toal 1996) they appear to be P. chryses. Herein, we conduct a phylogenetic analysis using DNA sequences in order to assess whether P. chryses is part of the P. bistincta group. We describe the tadpole and juvenile, and we reexamine the holotype and two paratypes of P. chryses. Based on our observations, we discuss what are the conditions of the characters thoracic fold, axillary membrane, and rostral keel in this species. We measure the skin thicknesses in fourteen species of the P. bistincta group and discuss whether the thickness of the dorsal skin can be correctly assessed by external examination alone and whether the character skin thickness can still be used in the taxonomy and systematics of this group. Finally, we evaluate whether a diagnosis of P chryses that includes the above characters is still useful.
MATERIALS AND METHODS
We collected tadpoles from two localities near Carrizal de Bravo, on the Sierra Madre del Sur in the state of Guerrero, Mexico (see Examined Specimens). All tadpoles were obtained between February and March of 2004. Four tadpoles were reared through metamorphosis to juvenile stage, and the others were preserved in formalin (10%). Muscle samples were obtained from one juvenile (UMMZ 239651), placed in 95% ethanol, and stored frozen.
To determine the correct conditions of the characters thoracic fold, axillary membrane, and rostral keel in P. chryses, we examined the holotype (UMMZ 125374; male), two paratypes (UMMZ 125373, 125375; both female), and four juveniles (UMMZ 239649-51). For comparison we examined the rostral keels of P. ixil Stuart, 1942 (UMMZ 121518 [8817]) and the axillary membranes of P. charadricola (Duellman, 1964) (UMMZ 118166 [11542]) and Tlalocohyla loquax (Gaige and Stuart, 1934) (UMMZ 115282 [10256]). For osteology examinations we obtained X-rays of one individual of P. chryses (UMMZ 125373) using a Hewlett-Packard, Cabinet X-Ray System Faxitron series, at 24 KV for 15 seconds. To determine whether the thickness of the dorsal skin of individuals of the P. bistincta group can be correctly assessed by external examination and whether the variation of the character "skin thickness" is discrete or continuous, we measured and compared the skins (i.e., dermis plus epidermis) of one individual of the following species: P. ameibothalame (Canseco-Marquez et al., 2002) (UMMZ 239718), P. arborescandens (Taylor, 1939) (UMMZ 239810), P. bistincta (UMMZ 112839 [7105]), P. calthula (Ustach et al., 2000) (KU 289212), P. charadricola (UMMZ 121567 [8738]), P. chryses (CAS 142943), P. crassa (Brocchi, 1877) (KU 148698), P. cyclada (Campbell and Duellman, 2000) (UMMZ 124866[6958]), P. hazelae (Taylor, 1940) (UMMZ 239802), P. mykter (UMMZ 238295), P. pentheter (Adler, 1965) (UMMZ 239773), P. ro-bertsorum (Taylor, 1940) (UMMZ 106401 [5585]), P. sabrina (Caldwell, 1974) (KU 137068), and P. thorectes (Adler, 1965) (UMMZ 125388).
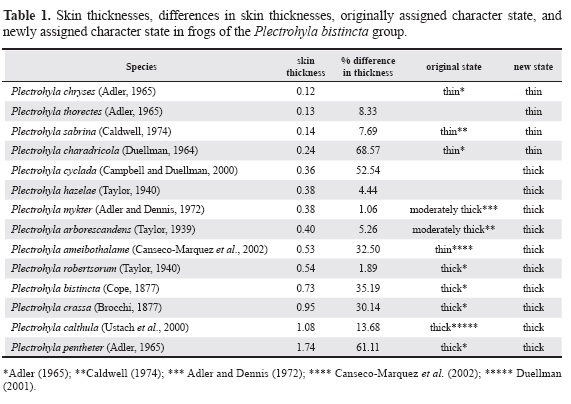
For comparison of the character "thick skin" we sectioned and measured the skins of one individual of P. ixil (UMMZ 124854) and P. matudai Hartweg, 1941 (UMMZ 107738) of the P. guatemalensis group. We removed skin samples from the area above the suprascapula. The skin samples were sectioned histologically (5u), stretched in warm water 30 to 60 seconds prior to placing them on slides, and they were then stained with hematoxilin-eosin (Wessner 1960). Skin thickness was measured as the average of three independent measurements using a compound microscope with a calibrated ocular micrometer. In Table 1 we ranked species according to the skin thicknesses from thin to thick. The difference in skin thickness between adjacent species in column 3 of Table 1 was expressed as the percentage increase in thickness between them.
Tadpoles were staged following Gos-ner (1960). Terminology of tadpole morphology follows that of Altig & McDiarmid (1999). Measurements and terminology used to describe the juvenile specimens follows that used by Duellman (2001). We define axillary membrane as the portion of skin that covers the angle formed at the junction of the humerus and trunk; the axillary membrane must be visible in dorsal and ventral views when the humerus is perpendicular to the trunk. The axillary membrane can be baggy and loose around the axilla (e.g., Tlalocohyla loquax [Figs. 1a, b]) or tight and ventrally flattened (e.g., P. charadricola and P. chryses [Fig. 1c-f]). We define thoracic fold as the most anteriorly-located complete fold, if many are present, traversing the thorax (Fig. 2a). We follow the definitions of Kaplan et al. (2015) for the terms "fold" and "flexible". We define rostral keel as a vertical or nearly vertical, fleshy (i.e., formed by coalesced tubercles or corrugations of the skin) ridge-like elevation that runs along a portion, or the whole, of the midline of the rostrum (e.g., P. ixil. Fig. 2b); more than one rostral keel, and rostral keels of different sizes, may be present in an individual (Fig. 2b). We follow Duellman (2001; but see Adler & Dennis 1972) in recognizing two states of the character "dorsal skin thickness", namely "thin skin" and "thick skin".
To assess whether P. chryses is part of the genus Plectrohyla, specifically of the P bistincta group, we conducted a phy-logenetic analysis that included P. chryses and 62 closely related frog species following Faivovich et al. (2005) using sequences obtained through a BLAST search on GenBank. These sequences included nine other Plectrohyla species and various outgroups (information for all 63 individuals is located in Appendix 1).
We sequenced 421 base pairs of the cytochrome b gene of a juvenile P chryses (UMMZ 239651) using primers MVZ15 (Moritz et al. 1992) and H15149 (Kocher et al. 1989) and standard PCR conditions (Faivovich et al. 2005:44). We aligned the sequences using MAFFT v.7.130b (Katoh & Standley 2013). We conducted several maximum-likelihood analyses in RAxML (Stamatakis 2014) with the GTRGAMMA substitution model and 1000 bootstrap replicates. First, we used all 63 individuals to determine if P. chryses was more closely related to other Plectrohyla or various outgroup taxa. After determining that P. chryses was likely nested within Plectrohyla we analyzed smaller subsets of the taxa to see if we could determine the close relatives of P. chryses.
Museum acronyms: CAS = California Academy of Sciences; CNHM = Chicago Natural History Museum; KU = Natural History Museum University of Kansas; MVZ = Museum of Vertebrate Zoology; MZFC = Museo de Zoologia Facultad de Ciencias, Universidad Nacional Autonoma de Mexico; UIMNH = University of Illinois Museum of Natural History (=Illinois Natural History Survey Herpetology); UMMZ = University of Michigan Museum of Zoology; USNM = United States National Museum; UTA = Amphibian collection of the University of Texas at Arlington.
RESULTS
Phylogenetic analysis.- Including all 63 of the most-closely related DNA sequences in a phylogenetic analysis, P. chryses is nested within the Plectrohyla clade (Figs. 3 and 4). Bootstrap scores are low, but they are generally low throughout the tree. When only Plectrohyla species and close relatives are analyzed, P. chryses falls close to P. mykter, P. calthula, and P. bistincta, i.e., within the P. bistincta group.
Skin thickness. - The skin thicknesses of the examined species, and the differences of skin thicknesses between species, appear in Table 1. P. chryses has the thinnest skin of all the examined species.
Description of the Tadpole of Plectrohyla chryses: A tadpole on stage 32 (UMMZ 239880; units in mm; Fig. 5): total length 43.6; body length 14.4; tail length 29.2; height of caudal musculature, at base of tail, 5.3; width of caudal musculature, at base of tail, 3.7; tail maximum height 8.7; maximum height of dorsal fin 2.5; maximum height of ventral fin 2.0; maximum body width 8.3; maximum body height 7.0; eye diameter 1.7; interorbital distance 3.4; snout-base of spiracle distance 8.2; nostril-eye distance 1.7; oral disc transverse diameter 5.0.
Body ovoid in dorsal view, posterior two-thirds narrower than anterior one-third, widest at level of eyes, wider than high, very depressed in lateral view, slightly higher posteriorly than anteriorly; snout narrow, round in dorsal and lateral views; eyes moderate in size, round, situated dorsolaterally; nostrils round-shaped, directed anterolaterally, closer to eye than tip of snout; spiracle sinistral, angled 45 degrees with respect to longitudinal axis, opening below midline of body, slightly closer to posterior end of body than to tip of snout; inner wall of spiracle free from body; vent tube dextral, with right wall displaced anteriorly; tail very long, making up 67% of total length; caudal musculature robust, highest at a point one-third the distance from tail base to tip of tail, gradually tapering to a narrow tip; posterior tip of tail rounded; dorsal fin slightly higher than ventral fin throughout their lengths.
Oral disc small with an oral disc diameter/ max. body width ratio = 0.6, elliptical, ventrally located, not emarginate, completely bordered by single row of marginal papillae; clumps of 10-15 medium sized submarginal papillae lateral to teeth rows; one row of densely packed medium-sized submarginal papillae between A-1 and anterior marginal papillae, merging with lateral clumps of submarginal papillae; one row of large submarginal papillae between P-4 and posterior marginal papillae; labial teeth row formula 2(2)/4; A-2 gap narrow; A-1 slightly longer than A-2, longer than posterior rows of teeth; lengths of P-2 and P-3 equal, longer than P-1 and P-4; P-4 intermittent; labial teeth on A-1, A-2, P-2, and P-3 longer in height than those of other rows; upper jaw sheath narrow, poorly keratinized, not serrated, medially notched; lateral keratinous flanges of upper jaw sheath absent; lower jaw sheath U-shaped, bearing small, rounded serrations.
One specimen in Stage 32 (UMMZ 239959) lacks rows of submarginal papillae between A-1 and marginal papillae, P-1, P-2, and P-3 equal in length, P-4 is not intermittent, teeth similar in all rows but slightly smaller in P-4, upper jaw sheath finely serrated and wider in the middle, and lower jaw sheath long, thin, not U-shaped.
Description of Juveniles of Plectrohyla chryses: A juvenile (UMMZ 239649; Figs.6a, b): SVL 28.3; head width 9.8; head length 8.6; interorbital distance 3.4; eye diameter 3.4; tympanum diameter 1.2; eyenostril distance 4.2; eyelid width 2.4; disc width of Finger III 1.8; disc width of Toe IV 1.4.
Head broad, 1.1 wider than long, flat; nostrils protuberant, elliptical, angled; loreal region concave; rostral keel absent; snout long, in dorsal view presenting a narrower, short, round elevation, rounded in profile; eyes large, their diameter 40% of head length, round, prominent; tympanum small, its diameter 35% of eye diameter, distinct, slightly higher than wide; tympanic annulus prominent posteriorly; supratympanic fold heavy, extending from the posterior border of eye to above the arm insertion, wider posteriorly than anteriorly, covering upper part of tympanum.
Fingers long, relative lengths I< IV<II<III; webbing vestigial; terminal discs of Fingers II, III, and IV large, elliptical; terminal disc of Finger I slightly expanded; diameter of terminal disc of Finger III larger than that of the tympanum, disc diameter 1.5 larger than tympanum diameter; subarticular tubercles conical, elevated, none bifid; supernumerary tubercles small, conical; elevated ridge of coalesced tubercles between palmar tubercle and first subarticular tubercle of Finger IV; prepollex not projecting; palmar tubercle large, flat; ulnar tubercles large coalesced forming ulnar ridge; few scattered tubercles on ventral surface of ulna near hand.
Toes relative lengths I<II<V<III<IV; webbing I2-21/2II13/4-31/3III2-3IV3-11/2V; inner metatarsal tubercle distinct, small, elliptical; outer metatarsal tubercle absent; subarticular tubercles round, none bifid; supernumerary tubercles scattered on and around proximal ends of toes; terminal disc of toes elliptical, slightly wider than toes; terminal disc of Toes III, IV, and V slightly larger than those of Toes I and II; tarsal fold well-defined and flap-like.
Skin on dorsum of body, head, and limbs smooth; skin on throat, belly and ventromedial part of thighs with tubercles; vent opening at upper level of thighs; vent flap short, wide, thin, not grooved medially; ventral rim of vent flap gently curved; axillary membrane absent; two thoracic folds present; anterior thoracic fold long, flap-like, medially interrupted; posterior thoracic fold short, present on one side of the body.
In preservative, dorsum of body, head, flanks, shanks, tibia, and loreal region milky-brown covered with brown, irregular, diffused, small blotches; dorsum of front limbs, thighs, hands, feet, and all hidden surfaces orange brown covered by brown blotches; blotches on dorsum of front limbs well defined, dark, nearly forming reticulation pattern, forming dark continuous band along external edges of humerus and ulna; upper part of vent flap traversed by white blotches; narrow black band extends along supratympanic fold and canthus between eye and nostril; dark band on upper lip absent; dark pigment on posterolateral part of throat. Ventrally, belly, chest and throat yellowish cream, limbs yellowish orange.
One specimen (UMMZ 239648) has flank tan yellowish; paler blotches on flank; scattered peppered pigmentation on lateral parts of chest and belly; vent flap thick, grooved medially, opening near ventral level of thighs. Two specimens (UMMZ 2396501) have blunt rostri.
DISCUSSION
We considered all the collected specimens to be a single species because the tadpoles of the Carrizal de Bravo and Yextla populations are nearly identical. We assign all the collected specimens to Plectrohyla chryses by their shared phenotypic features, including that juvenile specimens (UMMZ 239648-51) have the following characters: thoracic fold; eyes prominent; vent flap short, wide, thin, not grooved medially; vent opening at upper level of thighs; skin of dorsum smooth; tympanum and tympanic ring evident; webbing of fingers vestigial; webbing of toes I2--21/2II13/4-31/3III2-3IV3-11/2V (UMMZ 239649); ulnar tubercles distinct, forming row; tarsal fold distinct; flanks and dorsum with same coloration and pigmentation pattern in preservative (Straughan & Wright 1969). Juvenile specimens (UMMZ 239648-51) differ from the diagnostic combination of P. chryses by having a smaller tympanum, dark small blotches on dorsum of body, head and limbs, and a well-defined mottling on flanks and dorsum, and by lacking an axillary membrane.
The phylogenetic analysis (Fig. 3) shows that P. chryses is nested within the P. bistincta group, i.e. it is inside the thick-skinned species. Although bootstrap values are low throughout the tree, likely due to the small number of base pairs examined, P. chryses falls within the P. bistincta group in both analyses, and Plectrohyla itself is monophyletic in both analyses. Thus, although the monophyly of the P. bistincta group, and the inclusion of P. chryses within this group, is supported by molecular synapomorphies, no concordant morphological synapomorphy is presently known. Duellman & Campbell (1992) proposed that the frogs of the P. guatemalensis group and the thick-skinned frogs of the P. bistincta group, i.e. all species except P. calvicollina, P. charadricola, P. chryses, P. labedactyla, P. sabrina, and P. thorectes, form a monophyletic group. This hypothesis was supported by four synapomorphies: medial ramus of pterygoid in contact with otic capsule; dorsal skin thick: absence of median papillary hiatus; presence of accessory labial papillae (=submarginal papillae) between marginal papillae and tooth rows. Our phylogenetic analysis suggests that Duellman & Campbell's (1992) clade is paraphyletic with respect to P. chryses and also with respect to P. thorectes because this thin-skinned species (Table 1) appear to be nested within the thick-skinned species of the P. bistincta group (Figs 3 and 4). Our morphological observations also suggest that P. chryses should not have been excluded from Duellman & Campbell's (1992) clade because it has three of the four synapomorphies that support it: medial ramus of pterygoid in contact with otic capsule, a tadpole lacking median papillary hiatus, and a tadpole having submarginal papillae between marginal papillae and tooth rows (Figs. 4 and 6). Finally, our results suggest that the validity of the fourth synapomorphy supporting Duellman & Campbell's clade, i.e. "thick skin" is questionable because some species in this clade, e.g. P. ixil and P. matudai, have thinner skins than those of some species excluded from this clade such as P. charadricola.
Adler (1965) used the character "dorsal skin thickness" in the diagnostic combination of P. chryses. Toal & Mendelson (1995:13) questioned the usefulness of this character for the taxonomy of any species of the P. bistincta group because previous conclusions as to whether a species has thin or thick skins were reached subjectively using qualitative external examinations, not quantitative measurements. Our results (Table 1, columns 4 and 6) show that previous assessments of the skin thickness using external examination are largely correct and are thus credible. Only in P. ameibothalame was the qualitative external assessment erroneous. Therefore, we consider the character "skin thickness," and its states "thin skin" and "thick skin," and the proposed taxonomic distribution of these states currently valid for use in the taxonomy and systematics of the P. bistincta group. In other words, the state "thin skin" is only present in P. calvicollina, P. charadricola, P. chryses, P. labedactyla, P. sabrina, and P. thorectes.
Toal & Mendelson (1995) also questioned the utility of the character "dorsal skin thickness" for taxonomy and systematics in the P. bistincta group by suggesting that the recognition of the states "thin skin" and "thick skin" is arbitrary because the skin thickness varies continuously in this group. Table 1 suggests that the variation of skin thickness in the P. bistincta group is discrete because the difference in thickness between the skins of some species is much larger than that between the skins of other species such as P. charadricola and P. sabrina whom are different by 68.6% versus a difference between P. sabrina and P. thorectes of 7.7%. However, it is still premature to conclude that the variation in skin thickness is discrete in the P. bistincta group or that only the states "thin skin" and "thick skin" exist because not enough species and individuals per species have yet been examined. The skin thickness of P. arborescandens, P. cyclada, P. hazelae, and P. thorectes was never assessed using external examinations or quantitative measurements. Our results (Table 1, column 6) suggest that P. thorectes has thin skin and P. arborescandens, P. cyclada, and P. hazelae have thick skins.
Mendelson & Toal (1996) concurred with the original diagnosis of P. chryses (Adler 1965), except that they found that adults have a thoracic fold and that an axillary membrane and rostral keel are absent. We found that the adult individuals of P. chryses have a thoracic fold (Fig. 2a) and an axillary membrane (Figs. 1e, f), but that a rostral keel is absent (both paratypes present few small, dispersed, not-coalesced tubercles on their rostrum). We also found that all juveniles have a thoracic fold and that the axillary membrane and rostral keel are absent (Figs. 5a, b). These findings confirm that a thoracic fold and an axillary membrane are present in P. chryses and that a rostral keel is absent and suggest that the character "presence of axillary membrane" in this species should be redefined as "presence of axillary membrane in adults".
It appears that the characters "presence of thoracic fold", "presence of axillary membrane in adult", and "absence of rostral keel" cannot currently be used for the diagnosis of P. chryses because, when narrowly defined (see Materials and Methods), as the correct conditions of these characters are still unknown in most species of the P. bistincta group. Also, it is still unclear whether a thoracic fold is present in juveniles of species of the P. bistincta group that are considered to lack this character (a distinct possibility, see Kaplan et al. 2015: 218). Only a comprehensive assessment of the correct conditions of the narrowly defined characters "thoracic fold," "axillary membrane," and "rostral keel" in most species of the P. bistincta group, and a better understanding of their intraspecific, sexual, and ontogenetic variation, will allow us to determine if these characters have any value for the diagnoses of P. chryses and the other species of the P. bistincta group.
With the removal of the characters of the thoracic fold, axillary membrane, rostral keel, and tarsal fold (see Kaplan et al. 2015) from the diagnosis of P. chryses (Mendelson & Toal 1996), it is unclear if this diagnosis is still useful. Part of the difficulty in assessing whether the diagnosis of P. chryses is useful is the questionable nature of the same characters for the diagnoses of all the other species of the P. bistincta group as they are part of all the current diagnoses. However, it appears that P. chryses can still be distinguished from the other species of the P. bistincta group by the following combination of characters: thin skin (0.14-0.25 mm); tadpole with LTRF 2(2)/4; nuptial excrescences finely granular, not extending beyond Finger II (i.e., contra Mendelson & Toal [1996] excrescences can be present on Finger I [MVZ 112331, 162273-74; CAS 142943] or/and Fingers I and II [MVZ 162275]); maximum SVL of males 41.1 mm; tympanum large in adults (tympanum diameter 57% of eye diameter; 35% in juveniles); tympanic annulus visible; anal opening at upper level of thighs; anal flap short, wide, thin, not grooved medially; dorsum golden yellow to dark greenish-brown in life; mottling on flank and dorsum (contra Mendelson & Toal [1996] adults and juveniles preset mottling [Fig. 2c); flanks and dorsum with same, or very similar, coloration and pigmentation pattern; pale blotches traverse upper part of anal flap; foot webbing formula I2-21/2II2-31/3III2-3IV3-11/2V, eyes prominent. In light of the challenge to the characters thoracic fold, axillary membrane, rostral keel, and tarsal fold (see Kaplan et al., 2015 for discussion), a re-evaluation of the diagnoses of most species of the P. bistincta group is in order.
The tadpole of P. chryses differs from that of other species of the Plectrohyla bistincta group with known tadpoles (i.e., all except P. calvicollina (Toal, 1994), P. charadricola; P. labedactyla (Mendelson and Toal, 1996), P. miahuatlanensis Meik et al. 2006, P. pachyderma (Taylor, 1942), P. psarosema (Campbell and Duellman, 2000), and P. sabrina) by having marginal papillae not interrupted by a gap and LTRF 2(2)/4. Therefore, in the context of the phylogenetic hypothesis of Hylini by Faivovich et al. (2005) and Wiens (2005), the presence of complete marginal papillae and LTRF 2(2)/4 appears to have evolved independently in P. chryses and Charadrahyla nephila (Mendelson and Campbell, 1999) (KU 104124). The tadpole KU 87605 from Campamento Vista Hermosa Oaxaca also has LTRF 2(2)/4 and complete marginal papillae, but its taxonomic identity is still unknown (Kaplan & Heimes 2011 showed it is neither P. arborescandens nor P. cyclada). We think KU 87605 is Charadrahyla nephila because it is nearly identical to the tadpole of this species (compare Duellman 2001; Figs. 184B and 185B to Mendelson & Campbell 1999; Fig. 3) and it inhabits the Sierra de Juarez in Oaxaca.
Adler & Dennis (1972) suggested that the identities of several individuals from the Sierra Madre del Sur west of Chilpancingo, Guerrero (i.e., IPN CB 149-156; UIMNH 38023-25; UMMZ 125376), all of which had been previously referred to P. bistincta (Duellman 1964, Adler 1965), were still unknown. These authors were unable to assess if these specimens constitute one species, nor were they able to assign them to any of the known species of the area such as P. chryses or P. mykter. Nor could they determine if the specimens represent a new species because, with one exception (i.e., UMMZ 125376), the specimens are juveniles or poorly preserved adults (Adler & Dennis 1972), making them not comparable to species with undescribed juvenile forms.
We did not examine the specimens IPN CB 149-156, UIMNH 38023-25, or UMMZ 125376 in this study. However, it would appear that the adult specimens IPN CB 149156 are P. mykter because males have nuptial excrescences on the prepollex and on all the fingers (Adler & Dennis 1972, Kaplan et al. 2015), a very rare condition in Plectrohyla known only in P. ameibothalame (Canseco et al. 2002). Adler & Dennis (1972:15) doubted that these specimens are P. mykter because they lack a rostral keel, webbing on fingers, and mottling on venter and flank. However, this conclusion is questionable because a rostral keel can be either present or absent in P. mykter, the webbing on the fingers is vestigial and hard to compare (Kaplan et al. 2015), and specimens can often lose their pigmentation over time (Simmons 1993). The juvenile specimens UIMNH 38023-25 appear to be P. chryses because they have a mottled pattern on the dorsum (Adler & Dennis 1972, Kaplan et al. 2015, this study), a character that is absent in P. bistincta and less clearly defined in P. mykter. Examination of UIMNH 38023-25 is needed to fully identify these specimens. Finally, the adult female UMMZ 125376 appears to be P. mykter because it has a dark venter in preservative and small body size (likely SVL< 40 mm) (Adler & Dennis 1972). Adler & Dennis (1972) did not identify UMMZ 125376 as P. mykter because it lacks a prepollical spine, as determined via X-Ray. However, the prepollical spine could still be present in this specimen but not visible through X-Ray because of poor ossification, which we found in several individuals of P. bistincta (e.g., UMMZ 85453, USNM 114520, CNHM 111076).
In summary, we propose a new diagnostic combination for P. chryses that includes newly observed morphological characters (e.g., tadpole oral disc completely surrounded by marginal papillae and LTRF 2(2)/4) and excludes some previously proposed characters (e.g., thoracic fold). We show that P. chryses is part of the P. bistincta group (contra Duellman and Campbell 1992) and that it is nested within the thick-skinned species of this group (e.g., P. bistincta). Finally, we found evidence that the character states "thin skin" and "thick skin" can be used in the taxonomy and systematics of the P. bistincta group.
EXAMINED SPECIMENS
Plectrohyla ameibothalame MEXICO: Oaxaca: San Juan Teposcolula (UMMZ 239718 [1 male]).
P. arborescandens: MEXICO: Veracruz: 0.5 km from Tepiolulco on road to Zaragoza, 6940 ft. (UMMZ 239810 [1 male]).
P. bistincta: MEXICO: Michoacan: Uruapan Parque Nacional (UMMZ 112839 [7105] [1 male]); Uruapan (UMMZ 85453 [1 female], USNM 114520 [1 male]). Puebla: near Laguna San Bernardino (CNHM 111076 [1 male]).
P. calthula: MEXICO: Oaxaca: Sierra Mixe, Totontepec (KU 289212 [1 male]).
P. charadricola: MEXICO: Puebla: 7.3 mi SW Huauchinango (UMMZ 121567 [9738] [1 male]).
P. crassus: MEXICO: Oaxaca: 1.9 km S El Estudiante, 1850 m (KU 148698 [1 male]).
Plectrohyla chryses: MEXICO: Guerrero: Carrizal de Bravo to Jaleaca road (UMMZ 239648-51 [4 juveniles], 239880 [2 tadpoles], 239881 [2 tadpoles]; "el Puente" on Carrizal de Bravo to Chichihualco road (UMMZ 239959 [1 tadpole]); Between Puerto Chico and Asoleadero (UMMZ 125374, 125373, 125375 [3 males]); 4 km WNW Carrizal de Bravos (MVZ 162273-75 [3 males]); Sierra Madre del Sur, 1 km from Carrizal de Bravos (MVZ 112331 [1 male]); Sierra Madre del Sur, 5.5 km SW Carrizal de Bravos (CAS 142943 [1 male]).
P. cyclada: MEXICO: Oaxaca: 42.9 km S Valle Nacional, 1850 m (UMMZ 124866 [6958] [1 male]).
P. hazelae: MEXICO: Oaxaca: El Punto, Sierra de Juarez (UMMZ 239802 [1 male]).
P. ixil: MEXICO: Chiapas: N Pueblo Nuevo Soliasthuacan (UMMZ 121518 [8817] [1 male]).
P. matudai: GUATEMALA: San Marcos: Finca La Paz, near La Reforma, 1325 m (UMMZ 107738 [1 male]).
P. mykter: MEXICO: Guerrero: near the Carrizal de Bravo-Chichihualco road, 4 km W from deviation to Asoleadero (UMMZ 238295 [1 female]).
P. pentheter: MEXICO: Oaxaca: road 175 slightly N. Jalatengo, 1470 m (UMMZ 239773 [1 male]).
P. robertsorum: MEXICO: Hidalgo: El Chico Parque Nacional (UMMZ 106401 [5585] [1 male]).
P. sabrina: MEXICO: Oaxaca: 11.9 km S. Vista Hermosa, 1920 m (KU 137068 [1 male]).
P. thorectes: MEXICO: Oaxaca: ca. 37 km N. San Gabriel Mixtepec (UMMZ 125388 [1 male]).
Tlalocohyla loquax: MEXICO: Oaxaca: 2.3 mi N Donaji, 91.4 m (UMMZ 115282 [10256] [1 male]).
AUTHORS PARTICIPATION
MK collection of specimens, morphological studies, design and writing of manuscript, PH collection of specimens and review of manuscript, EZ sequencing of DNA, phylogenetic analysis and review of manuscript, JM sequencing of DNA, phylogenetic analysis and final correction of manuscript.
ACKNOWLEDGEMENTS
Oscar Flores-Villela for collecting permits in Mexico. Rafael Aguilar for help in the field. Ron Nussbaum, Jens Vindum, Linda Trueb, Ron Heyer, Alan Resetar, and Greg Schneider for loaning museum specimens.
LITERATURE CITED
Adler, K. 1965. Three new frogs of the genus Hyla from the Sierra Madre del Sur of Mexico. Occasional Papers of the Museum of Zoology University of Michigan 642:1-18.
Adler, K., & D. M. Dennis. 1972. New tree frogs of the genus Hyla from the cloud forests of western Guerrero, Mexico. Occasional Papers of the Museum of Natural History University of Kansas 7:1-19.
Altig, R. & R. W. McDiarmid. 1999. Body plan: development and morphology. In R. W. McDiarmid & R. Altig. (eds). Tadpoles: the biology of anuran larvae: 25-51, University of Chicago Press, Chicago.
Caldwell, J. 1974. A re-evaulation of the Hyla bistincta species group, with descriptions of three new species (Anura: Hylidae). Occasional Papers of the Museum of Natural History, The University of Kansas 28:1-37.
Canseco-Marquez, L., J. R. Mendelson II & G. Gutierrez-Mayen. 2002. A new species of Hyla (Anura: Hylidae) from the Mixteca Alta, Oaxaca, Mexico. Herpetologica 58: 260-269.
Duellman, W. E. & J. A. Campbell. 1992. Hylid frogs of the genus Plectrohyla: systematics and phylogenetic relationships. Miscellaneous Publications of the Museum of Zoology University of Michigan 181:1-32.
Duellman, W. E. 2001. Hylid frogs of Middle America. Society for the Study of Amphibians and Reptiles, Ithaca, NY. 1170 pp.
Duellman, W. E., A. B. Marion & S. B. Hedges. 2016. Phylogenetics, classification, and bio-geography of the treefrogs (Amphibia: Anura: Arboranae). Zootaxa 4104: 1-109.
Faivovich, J., C. F. B. Haddad, P. C. A. Garcia, D. R. Frost, J. A. Campbell, & W. C. Wheeler. 2005. Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bulletin of the American Museum of Natural History 294: 1-240.
Frost, D. R. 2015. Amphibian species of the world: an online reference. Version 6.0 (August 6, 2015). Electronic Data Base accessible at http://research.amnh.org/herpetology/amphibia/index.html. American Museum of Natural History, New York, USA.
Gosner, K. L. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183-190.
Kaplan, M., P. Heimes, & J. McCormack. 2015. Contributions to the morphology of Plectrohyla mykter (Anura: Hylidae) with comments on some controversial characters and the diagnosis of this species. Caldasia 37: 211-220.
Katoh, K. & D. M. Standley. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772-780.
Kocher, T. D., W. K. Thomas, A. Meyer, S. V Edwards, S. Pabo, F. X. Villablanca & A. C. Wilson. 1989. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proceedings of the National Academy of Sciences USA 86: 6196-6200.
Mendelson III, J. R. & J. A. Campbell. 1999. The taxonomic status of populations referred to Hyla chaneque in southern Mexico, with the description of a new treefrog from Oaxaca. Journal of Herpetology 33: 80-86.
Mendelson III, J. R. & K. R. Toal. 1996. A new species of Hyla (Anura: Hylidae) from the Sierra Madre del Sur of Oaxaca, Mexico, with Comments on Hyla chryses and Hyla mykter. Journal of Herpetology 30: 326-333.
Moritz, C., C. J. Schneider & D. B. Wake. 1992. Evolutionary relationships within the Ensatina eschscholtzii complex confirm the ring species interpretation. Systematic Biology 41: 273-291.
Simmons, J. E. 1993. Blithe spirits: problems and potentials of fluid preservation. In Snyder, A.M. (ed). The 1992 American Society of Ichthyologists andHerpetologists Workshop on Collections Care and Management Issues. 1227 ASIH.
Stamatakis, A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312-1313.
Straughan, I. R. & J. W. Wright. 1969. A new stream frog from Oaxaca, Mexico (Anura, Hylidae). Contributions in Sciences, Los Angeles County Museum 169: 1-12.
Toal, K. R & J. R. Mendelson III. 1995. A new species of Hyla (Anura: Hylidae) from cloud forest in Oaxaca, Mexico, with comments on the status of the Hyla bistincta group. Occasional Papers of the Natural History Museum, The University of Kansas 174: 1-20.
Wessner, F. M. 1960. General Zoological Microtechniques. The Williams and Wilkins Company, Baltimore. 230 pp.
Recibido: 11/12/2015 Aceptado: 02/10/2016