Resumen
Introducción. El síndrome de la arteria mesentérica superior, también llamado síndrome de Wilkie, ocurre por la disminución del ángulo aortomesentérico con compresión de la tercera porción duodenal. La sintomatología en muchos casos es inespecífica y su etiología se presenta por causas relacionadas con disminución de la grasa mesentérica. El diagnóstico se basa en hallazgos imagenológicos y endoscópicos. Su tratamiento se enfoca en corregir la causa relacionada con la pérdida de peso y, en casos refractarios, está indicado el manejo quirúrgico.
Presentación del caso. Mujer de 19 años que asistió a una institución de tercer nivel de complejidad en la ciudad de Bucaramanga (Colombia), por sintomatología de 2 meses consistente en pérdida de peso y pérdida parcial del apetito. Las pruebas de sangre mostraron anemia e hiperleucocitosis con 37% de blastos, por lo que se hizo dictamen de leucemia linfoide aguda diagnosticada por aspiración de médula ósea. Durante su estancia hospitalaria presentó dolor abdominal, vómito e intolerancia a la vía oral. En la tomografía de abdomen se observó distensión de la tercera y cuarta porción duodenal y una reducción del ángulo aortomesentérico (18.8º), lo que generó una obstrucción mecánica proximal del intestino. Se inició manejo médico, nutrición parenteral total y terapia postural postprandial. En imágenes de vías digestivas de control se evidenció un adecuado paso de medio de contraste hasta el yeyuno. La paciente toleró el inicio de la vía oral y presentó una buena evolución clínica.
Conclusiones. El síndrome de la arteria mesentérica superior es una causa poco frecuente de obstrucción intestinal con características clínicas variables, por lo que se requiere una alta sospecha diagnóstica. Se deben realizar estudios imagenológicos para confirmar el diagnóstico e iniciar manejo médico de forma temprana para evitar posibles intervenciones quirúrgicas.
Abstract
Introduction: Superior mesenteric artery syndrome, also known as Wilkie’s syndrome, occurs due to a decrease in the aortomesenteric angle, resulting in compression of the third portion of the duodenum. In many cases, the symptomatology is nonspecific and its etiology is associated with a decrease in mesenteric fat. Diagnosis is based on imaging and endoscopic findings. Treatment is focused on correcting the cause related to weight loss and, in refractory cases, surgery is indicated.
Case presentation: A 19-year-old female attended a tertiary care institution in the city of Bucaramanga (Colombia) after experiencing weight loss and a decrease in appetite for two months. Blood tests showed anemia and hyperleukocytosis with 37% blasts, resulting in a diagnosis of acute lymphoid leukemia diagnosed via bone marrow aspiration. During her hospital stay, she presented abdominal pain, vomiting, and intolerance to oral intake. A CAT scan of the abdomen showed distension of the third and fourth portions of the duodenum and a reduction of the aortomesenteric angle (18.8º), which generated a proximal mechanical obstruction of the bowel. Medical treatment, total parenteral nutrition and postprandial postural therapy were initiated. Follow-up digestive tract imaging showed adequate passage of contrast medium to the jejunum. The patient tolerated the initiation of the oral intake and had a good clinical progress.
Conclusions: Superior mesenteric artery syndrome is a rare cause of intestinal obstruction with variable clinical features, so high diagnostic suspicion is required. Imaging studies should be performed to confirm the diagnosis and early medical treatment should be initiated to avoid possible surgical interventions.
Introduction
Superior mesenteric artery (SMA) syndrome is a relatively rare clinical condition in which a proximal intestinal obstruction of vascular origin occurs (1). It is characterized by an extrinsic compression of the third portion of the duodenum caused by a narrowing of the aortomesenteric angle, which occurs mainly due to a decrease in retroperitoneal fat tissue (2).
The SMA syndrome, also known as Wilkie’s syndrome, was first described by von Rokitansky in 1842 and, years later, characterized by David Wilkie, who made an exhaustive description of this disease in 75 patients in 1927 (3). It may be found incidentally on imaging examination or be associated with symptoms such as abdominal pain and distention (acute or chronic), vomiting, and weight loss. Its etiology is more frequently associated with acquired causes, mainly related to a decrease in mesenteric fat, as in constitutional syndromes, hypercatabolic states, eating disorders, and other documented congenital conditions (4).
Its diagnosis is based on imaging findings of vascular compression of the duodenum by the superior mesenteric artery (SMA), duodenal dilatation, and decreased aortomesenteric angle (4,5). Conservative management with nutritional supplementation for weight gain is the first line of treatment; however, in certain chronic and refractory cases, surgery is required (6).
The following is a case of SMA syndrome in a patient recently diagnosed with acute lymphoblastic leukemia (ALL), who was successfully treated with parenteral hyperalimentation, gastric decompression with a feeding tube, and postural management for improved gastric emptying.
Case presentation
A 19-year-old female attended the emergency room of a tertiary care institution in the city of Bucaramanga (Colombia) complaining of weight loss and poor tolerance to oral intake for two months. Physical examination on admission showed the following vital signs: blood pressure: 110/70 mmHg, heart rate: 96 bpm, respiratory rate: 19 rpm. Conjunctival pallor was also observed. Admission laboratory tests showed moderate anemia (7 mg/dL) along with hyperleukocytosis of 62 360 mm³ with 37% blasts, prompting an advanced hematological study whose results allowed making the diagnosis of precursor B-cell ALL (common phenotype found via bone marrow aspiration).
Since her symptoms were initially attributed to her newly diagnosed ALL, the patient was treated by the hematology and oncology service. However, due to the persistence of abdominal pain in addition to multiple episodes of vomiting and intolerance to oral intake, on the sixth day the inpatient general surgery service requested a contrasted computed axial tomography (CAT) scan of the abdomen. The results showed gastric distension of the third and fourth portions of the duodenum, so a proximal intestinal pseudo-obstruction was suspected and confirmed that same day by means of a computerized tomography scan of the abdomen, which documented a significant 18.8º reduction of the aortomesenteric angle and a distance between the aorta and the SMA of 50.7mm (Figure 1). Although it was greater than 8mm, it generated a compressive effect on the third portion of the duodenum and caused a proximal mechanical obstruction of the intestine.
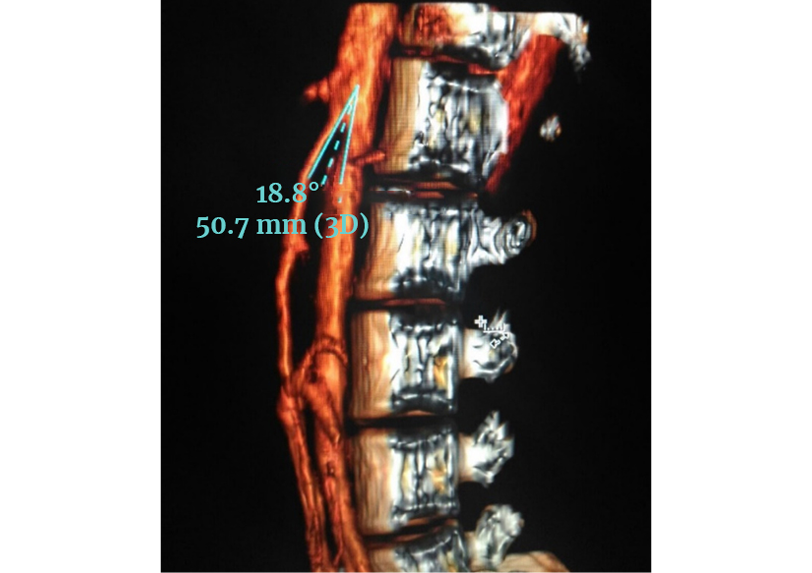
Figure 1. Computerized tomography scan of the abdomen. Aortomesenteric angle of 18.8º. Distance between the aorta and the SMA of 50.7mm.
Source: Image obtained while conducting the study.
In view of these findings, on the seventh day of hospital stay, medical management with a nasogastric tube was started in order to achieve duodenal decompression. Additionally, postprandial postural therapy in left lateral decubitus, genupectoral position, and total parenteral nutrition (TPN) were instituted for 11 days. The protocol for establishing treatment with TPN in our institution involves a continuous infusion of a nutritional bag (approximately 18 hours per bag), and the nutrient components of each bag are described in Table 1.
Table 1. Nutrient components of the NPT nutritional bag.
|
Component |
Amount per bag |
|
Proteins (g/Kg) |
1.5 |
|
Carbohydrates (Glucose) |
2.4 |
|
Lipids (g) |
0.7 |
|
Sodium and Potassium (mEq) |
1 |
|
Calcium (mg) |
210 |
|
Fat soluble vitamins (mL) |
10.00 |
|
Water-soluble vitamins (mL) |
10.00 |
|
Trace elements (mL) |
5.00 |
|
Calories/kg of weight |
25 |
Patient’s weight: 42kg.
Source: Own elaboration.
On the eighteenth day of hospitalization, a follow-up X-ray of the digestive tract was requested, which showed adequate passage of the contrast medium into the jejunum (Figure 2). For that reason, TPN was suspended that same day and the patient was started on oral intake with good tolerance. The patient made adequate clinical progress, without complications, and was discharged on the twenty-eighth day of hospitalization. The patient was followed up for 6 months with monthly outpatient check-ups, resulting in a weight gain of 9kg and complete resolution of the gastrointestinal symptoms. Consequently, it was decided to discharge the patient from the general surgery department.
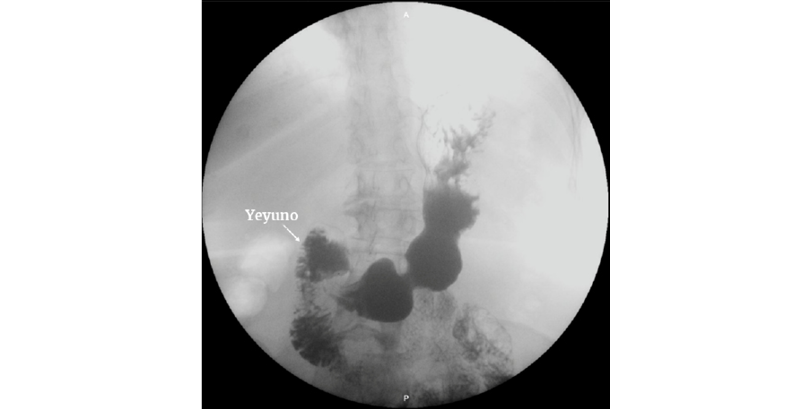
Figure 2. X-ray of the digestive tract. There is evidence of adequate passage of contrast medium into the jejunum.
Source: Image obtained while conducting the study.
Discussion
SMA syndrome or Wilkie’s syndrome is a rare cause of upper bowel obstruction, with variable clinical characteristics, resulting from compression of the third portion of the duodenum between the abdominal aorta and the SMA (7). An incidence of less than 0.4% has been reported, which can reach up to 4.7% after spinal surgery, being more frequent in women (with an incidence of 3:2). It is also more common in the second decade of life, as in the reported case (8).
Symptoms are nonspecific, so they are challenging for surgeons and require a high index of suspicion. However, it usually presents with postprandial epigastric pain (59%), bilious vomiting (50%), nausea (40%), early satiety and abdominal distension (32%), and anorexia (18%) with secondary weight loss. These symptoms may be alleviated when the patient is in the genupectoral and left lateral decubitus position or exacerbated in the supine decubitus position (9).
The etiology of this condition is more frequently associated with acquired causes, mainly related to a decrease in mesenteric fat, such as constitutional syndromes and hypercatabolic states (cancer, tumors, extensive third-degree burns, polytrauma, brain and/or spinal cord injury) (10); eating disorders such as anorexia nervosa and malabsorption syndrome (11,12); postoperative states (bariatric surgery, esophagectomy, aortic aneurysm repair, scoliosis with kyphosis sagittal, proctocolectomy); and other congenital conditions such as anatomical abnormalities, greater or lesser length of the suspensory muscle of the duodenum (ligament of Treitz), duodenal malrotation, low origin of the SMA, and compression due to peritoneal adhesions (bands of Ladd) (13-15).
The SMA originates from the anterior aorta, at the level of the lumbar vertebrae L1 and L2 (inferior to the origin of the celiac trunk) and descends forming an acute angle towards the mesentery (16). This angle varies from 38º to 65º, with a distance from the aorta to the SMA (at the duodenal junction) of 13mm to 34mm (17). If there is a relative decrease in the space between the aorta and the SMA, compression of the third portion of the duodenum may occur. Diagnostic criteria for SMA syndrome include a decrease in the distance between the aorta and the SMA of less than 8mm (sensitivity 100% and specificity 100%) and an aortomesenteric angle of less than 22° (sensitivity 42.8%, specificity 100%) (18). In the clinical case reported, an angle of 18.8º was observed in the computed tomography angiography of the abdomen.
Despite its low incidence, this condition can be overdiagnosed if appropriate clinical and radiological criteria are not considered. In incomplete obstruction, symptomatology and physical examination findings may not be specific, making diagnosis very difficult. The following radiographic criteria have been established for the diagnosis of SMA syndrome: dilatation of the first and second portions of the duodenum (with or without gastric dilatation), abrupt vertical and oblique compression of the duodenal mucosal folds, antiperistaltic flow of contrast medium (barium) proximal to the obstruction, delayed gastroduodenal transit of 4-6 hours, and relief of the obstruction in the prone, genupectoral, or left lateral decubitus position. However, these findings are not pathognomonic as they can be found in other diseases such as scleroderma, lupus, small bowel obstruction, pancreatitis, peptic ulcer disease, and diabetes (19,20).
Conventional barium studies play an important role in diagnosis, although results are little specific (16). The diagnostic method used in the case presented was CAT scan of the abdomen, which revealed duodenal obstruction secondary to a decrease in the aortomesenteric angle, confirming the diagnostic suspicion by means of computed tomography angiography (detailed study of the mesenteric vasculature and the angle between the aorta and the superior mesenteric artery). These imaging studies are considered in the literature as valuable non-invasive diagnostic tools (20).
Computed tomography (CT) is very useful since it allows detecting duodenogastric distension, defining anatomical relationships of the superior mesenteric vessels, determining the intra-abdominal and retroperitoneal fat component, measuring the distance and the aortomesenteric angle, and detecting complications that may require immediate surgery such as pneumatosis intestinalis and pneumatosis portalis (13). Other complications described are acute gastric dilatation with or without rupture, cardiovascular collapse, and compression of the renal vein with secondary thrombosis, also known as the nutcracker syndrome (19,21). Ultrasound has also been described as a diagnostic method; however, the advantage of the CT over the latter is that it can provide a global assessment of the abdominal cavity, allowing the detection of other associated conditions (22,23). Konen et al. (24) pointed out the advantage of using CT with three-dimensional angiography reconstructions, which can help to rule out misdiagnoses originating from angulations of the superior mesenteric artery. Furthermore, endoscopy is indicated to look for major damaging events due to gastroparesis and biliary reflux (gastritis, duodenal ulcer) and to rule out an intrinsic cause of duodenal compression (25).
Multiple therapeutic opinions have been proposed for the management of this condition, ranging from surgery to conservative management, the latter being considered as the first line of treatment especially when the cause is significant weight loss (26-28). Nasogastric tube placement for duodenal and gastric decompression and mobilization in the prone or left lateral decubitus position are often effective measures for the treatment of acute symptomatology (19). Medical treatment involves electrolyte correction, positive nitrogen balance, nasogastric tube decompression, and an increase in body weight to promote restoration of retroperitoneal adipose tissue with a consecutive increase in the aortomesenteric angle. Both nasojejunal enteral feeding and TPN have proven to be successful in the treatment of SMA syndrome (25,29).
Surgical treatment is indicated in the following cases: conservative management failure, chronic disease with progressive weight loss together with duodenal dilatation and stasis, complicated peptic ulcer disease, pancreatitis secondary to stasis and biliary reflux, and other associated disorders requiring management by laparotomy (22). There is no clear time frame for the duration of medical treatment; however, symptom relief has been observed between 2 to 12 days, although treatments of up to 169 days have also been documented (19).
The different surgical options include side-to-side duodenojejunostomy between the duodenum and the first loop of the jejunum, Strong’s procedure in the section of the ligament of Treitz, duodenoduoenostomy, and gastrojejunostomy. Most surgeons prefer duodenojejunostomy, which is considered superior to other options due to its 80-100% success rate (25,30).
In the case described, the initial gastric decompression associated with postural measures and TPN not only favored weight gain, but also improved the vascular space by increasing mesenteric fat and adipose tissue around the SMA, preventing surgical intervention.
Conclusions
SMA syndrome is a rare cause of intestinal obstruction that presents with variable clinical features, so a high diagnostic suspicion is required. Imaging studies should be performed to confirm the diagnosis and to start early medical treatment focused on recovering mesenteric fat and adipose tissue around the SMA, in order to avoid possible surgical interventions.
Ethical considerations
The authors state that they have followed the protocols on the publication of patient data. Informed consent was obtained from the patient.
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
References
1.Ibarra F, Arriagada D. Síndrome de la Arteria Mesentérica Superior: Caso Clínico y Revisión. Bol Esc Med U.C. Pon Univ Cat Chile. 2006;31(1):42-6.
2.Gopal M, Fisher RM. A case report of B-cell lymphoma masquerading as superior mesenteric artery syndrome. J Pediatr Surg. 2007;42(11):1926-7. https://doi.org/fnz694.
3.Biank V, Werlin S. Superior mesenteric artery syndrome in children: a 20-year experience. J Pediatr Gastroenterol Nutr. 2006;42(5):522-5. https://doi.org/ccr2dc.
4.Rodriguez A, Romero-Vidomlansky S, Ferrarotti C, Larrañaga N, Gallo JC, Kozima S. Síndrome de la arteria mesentérica superior. Presentación de un caso. Rev Argent Radiol. 2014;78(2):96-8. https://doi.org/f2s54z.
5.Barchi LC, Alves AM, Jacob CE, Caldas-Bresciani CJ, Yagi OK, Nogueira TG, et al. Favorable minimal invasive surgery in the treatment of superior mesenteric artery syndrome: Case report. Int J Surg Case Rep. 2016;29:223-6. https://doi.org/mrp9.
6.Veysi VT, Humphrey G, Stringer MD. Superior mesenteric artery syndrome presenting massive gastric dilatation. J Pediatr Surg. 1997;32(12):1801-3. https://doi.org/brnx82.
7.Fernandez-López MT, López-Otero MJ, Bardasco-Alonso ML, Alvarez-Vázquez P, Rivero-LuisMT, López-Barros G. Síndrome de Wilkie: A propósito de un caso. Nutr Hosp. 2011;26(3):646-9. https://doi.org/mrqk.
8.Grabala P, Grabala M, Kossakowski D, Malinowski P, Larysz D. A case report of a 13-year-old girl diagnosed with superior mesenteric artery syndrome after undergoing spine correction with posterior fusion for rapidly progressed juvenile idiopathic scoliosis. Polish Ann Med. 2016;23(2):165-71. https://doi.org/mrqm.
9.Izaskun-Vergara R, Ninette-Pezo R. Síndrome Pinzamiento Mesentérico: a propósito de un caso de hiperemesis. Asoc Nac Cient Estud Med Chile. 2013;7(2):92-5. Available from: https://bit.ly/3ylNFQJ.
10.Rosa-Jimenez F, Rodriguez-Gonzáles FJ, Puente-Gutiérrez JJ, Muñoz-Sánchez R, Adarraga-Cansino MD, Zambrana-García JL. Duodenal compression caused by superior mesenteric artery: study of 10 patients. Rev Esp Enferm Dig. 2003;95(7):480-4. Available from: https://bit.ly/4b8J8Q1.
11.Alhadi AN, Shuqdar RN. Anorexia Nervosa versus Superior Mesenteric Artery Syndrome in a Young Woman: Case Report and Literature Review. J Taibah Univ Med Sci. 2008;3(1):55-60. https://doi.org/mrsm.
12.García AJ, Pérez IA, Sánchez CRM. Síndrome de la arteria mesentérica superior. Informe de una paciente. Cir Gen. 2000;22(4):347-50. Available from: https://bit.ly/4boFhyA.
13.Castaño-Llano R, Chams-Anturi A, Arango-Vargas P, García-Valencia A. Síndrome de la arteria mesentérica superior o síndrome de Wilkie. Rev Colomb Gastroenterol. 2009;24(2):200-9.
14.Penco JMM, Murillo JC, Calle Pato U, Masjoan D. Un posible caso de origen congénito de síndrome de la arteria mesentérica superior (SAMS). Cir Pediatr. 2008;21(4):228-31.
15.Dash N. An Unusual Cause of Superior Mesenteric Artery Syndrome in a Young Boy. Med J Armed Forces India. 2011;67(1):92. https://doi.org/dgq8dx.
16.Ozkurt H, Cenker MM, Bas N, Erturk SM, Basak M. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat. 2007;29(7):595-9. https://doi.org/cjwtgm.
17.Pivawer G, Haller JO, Rabinowitz SS, Zimmerman DL. Superior mesenteric artery syndrome and its ramifications. Comput Med Imaging Graph. 2004;28(1):8-10. https://doi.org/dxc22f.
18.Sabbagh C, Santin E, Potier A, Regimbeau JM. The superior mesenteric artery syndrome: A rare etiology for proximal obstructive syndrome. J Visc Surg. 2012;149(6):428-9. https://doi.org/mrsr.
19.Welsch T, Büchler MW, Kienle P. Recalling superior mesenteric artery syndrome. Dig Surg. 2007;24(3):149-56. https://doi.org/cfxjdw.
20.Agrawal GA, Johnson PT, Fishman EK. Multidetector row CT of superior mesenteric artery syndrome. J Clin Gastroenterol. 2007;41(1):62-5. https://doi.org/bbmgrw.
21.Ko KH, Tsai SH, Yu CY, Huang GS, Liu CH, Chang WC. Unusual complication of superior mesenteric artery syndrome: spontaneous upper gastrointestinal bleeding with hypovolemic shock. J Chin Med Assoc. 2009;72(1):45-7. https://doi.org/cgsx5w.
22.Mandarry MT, Zhao L, Zhang C, Wei ZQ. A comprehensive review of superior mesenteric artery syndrome. Eur Surg. 2010;42(5):229-36. https://doi.org/fqr7vk.
23.Unal B, Aktaş A, Kemal G, Bilgili Y, Güliter S, Daphan C, et al. Superior mesenteric artery syndrome: CT and ultrasonography findings. Diagn Interv Radiol. 2005;11(2):90-5.
24.Konen E, Amitai M, Apter S, Garniek A, Gayer G, Nass S, et al. CT Angiography of superior mesenteric artery syndrome. ARJ Am J Roentgenol. 1998;171(5):1279-81. https://doi.org/mrsx.
25.Le Moigne F, Lamboley JL, Vitry T, Stoltz A, Galoo E, Salamand P, et al. Superior mesenteric artery syndrome: A rare etiology of upper intestinal obstruction in adults. Gastroenterol Clin Biol. 2010;34(6-7):403-6. https://doi.org/cq7scx.
26.Ricca RL, Kasten J, Javid PJ. Superior mesenteric artery syndrome after minimally invasive correction of pectus excavatum: Impact of post-operative weight loss. J Pediatr Surg. 2012;47(11):2137-9. https://doi.org/mrsz.
27.Villar-Ramos J, Altadill-Bermejo A, Rubio-Sánchez S, Fernández-Lobo V. Otras causas de dolor abdominal: síndrome de la arteria mesentérica superior. Rev Clin Med Fam. 2019;12(2):93-96. https://bit.ly/3ysl63Q.
28.Lozano-Vega JJ, Mejía-Sanguino S, Gaviria-Gallego DA, Polanco-Cabrera JP. Síndrome de la arteria mesentérica superior (Síndrome de Wilkie): a propósito de un caso en una adolescente. Rev Colomb Cir. 2024;39. https://doi.org/mvfm.
29.Naseem Z, Premaratne G, Hendahewa R. “Less is more”: Non operative management of short term superior mesenteric artery syndrome. Ann Med Surg (Lond). 2015;4(4):428-30. https://doi.org/mrs2.
30.Aranda-Escaño E, Perfecto-Valero A, Tallaeche-de la Iglesia M, Gómez-Cruzado LF, Santidrian-Martínez JI. Síndrome de la pinza aorto-mesentérica (Sind. De Wilkie). Análisis de una serie de 7 casos. Cir Esp. 2020;98(1):48-57. https://doi.org/mvfp.