DETERMINATION OF VERAPAMIL THROUGH LC-ESI-MS/MS IN A CASE OF FATAL INTOXICATION
National Institute of Legal Medicine and Forensic Sciences, Special Edition
Sindy Varon1,
Diana Mariño2
1. Pharmacological chemist.
National Institute of Legal Medicine and Forensic Science
- Bogotá Office -
Toxicology Group.
2. Chemist. M.Sc.
National Institute of Legal Medicine and Forensic Science
- Office Bogotá -
Toxicology Group
Correspondence to:
Sindy Varón.
Toxicology Group,
National Institute of Legal Medicine and Forensic Science.
Email: svaron@medicinalegal.gov.co
SUMMARY
Verapamil is a synthetic derivative of papaverine, which is used therapeutically as a hypertensive, antiarrhythmic and antianginal. This study describes an analytical method for the determination of verapamil in biological matrices of blood and urine, which consists of a liquid-liquid extraction of samples for analysis using liquid chromatography-mass spectrometry (LC-ESI-MS/ MS), with flurazepam as an internal standard. The method was applied to the acute fatal intoxication of a 17-year-old young woman who consumed 170 tablets of verapamil; the concentration of this medication found in the blood was 18.261mg/L and 0.369mg/L in the urine. This study also puts forth the use of LC-ESI-MS/MS in the analysis of verapamil in biological samples for applications in forensic toxicology.
Keywords: Verapamil; Liquid chromatography with electrospray ionization and tandem mass spectrometry (LC-ESI-MS/MS); Forensic toxicology.
INTRODUCTION
A large number of cases of unspecified deaths which arrive at the National Institute of Legal Medicine and Forensic Science (INMLCF for it’s acronym in Spanish) in Bogotá lack adequate information to establish an apparent cause of death. Toxicological analysis is used to identify the substances of habitual consumption to assist in determining the cause of death. In the experience of the Toxicology Laboratory at the INMLCF, some suicide deaths are associated with the consumption of pest-control substances and various pharmaceutical drugs.
Verapamil is a drug that acts by inhibiting slow calcium channels, which are dependent on the voltage of cardiac muscle cells, reducing the intracellular ion concentration. This medication is classified as a calcium blocker which acts on the vascular system, heart, and conduction tissue; it has clinically useful effects in relaxing blood vessels, reducing the need for the heart to pump with such force; it also increases blood flow and oxygenation of the heart while diminishing the electrical activity to control heart rate (1,2,3,4).
Verapamil is administered orally and the therapeutic dosage is determined by pharmacokinetics as well as the clinical actions and characteristics of the patient. Overdosing can be fatal, which is why the analysis to determine biological matrices is very important in forensic toxicology to establish the cause of death.
A quick and simple method was developed to determine the quantity of verapamil in blood and urine using LC-ESI-MS/MS. The analytical methodology was obtained by reviewing articles that analyze these substances (5,6,7,8), which were then applied to a case of fatal intoxication presumably caused by this drug.
Presentation of the case
A young woman of 17 years of age with a 53kg weight was brought to the emergency room due to intoxication symptoms after consuming a mixture of drugs including metformin, gemfibrozil and verapamil, which produced vomiting, tonic-clonic seizures, abundant secretions from the airways and finally, a cardio- respiratory arrest.
In the medical-legal examination, the patient presented signs of minor blunt trauma in the inferior extremities with different stages of evolution. There were also nonspecific signs, both internal and external, of marked hypoxia, pulmonary oedema and cerebral and pulmonary lesions suggesting pulmonary hemorrhaging. From the medical forensic opinion, the findings were nonspecific and were not enough to determine the cause of death; therefore the toxicological analysis was necessary, considering that, along with the cadaver, 17 packs of 120mg of verapamil were found, which correspond to 170 tablets (20.4g), 3 packs of 850mg of metformin, which correspond to 30 tablets (25.5g), and 4 packs of 600mg of gemfibrozil, which correspond to 40 tablets (24g). The research was then narrowed down to verapamil since literature reports a lethal dose for this drug, in contrast with the other substances that were ingested (Table 1).
Based on the number of tablets consumed, the volume of distribution of each substance, the concentration in plasma and the weight of the deceased, theoretical values of the concentrations of each substance in the blood were calculated supposing that they were totally absorbed.
It is not known if any other attempts at disintoxication, aside from vomiting, were made in the emergency room to eliminate the absorption of the active principles in the gastric content (Table 2). These calculations allow for the establishment of an approximate value of the concentration in the blood of each drug supposing a total absorption of these substances. As the ingested dose of verapamil was so high and the lethal dose low, it is likely that a total absorption did not occur since the organs failed quickly.
|
Substance in blood |
Therapeutic concentration (μg/mL) |
Toxic concentration (μg/mL) |
Lethal concentration (μg/mL) |
|
Verapamil |
0.08-0.3 |
0.36 |
1 |
|
Gemfibrozilo |
Not reported |
Not reported |
Not reported |
|
Metformina |
1-4 |
45-70 |
Not reported |
Table 1. Therapeutic, toxic and lethal concentrations of the drugs in this study.
Source: (9,10).
|
Substance in blood |
Volume of distribution (L/kg)3,9 |
Dose taken (g) |
Maximum concentration in blood (mg/L) |
|
Verapamil |
2-6 |
20.4 |
64.15 |
|
Gemfibrozilo |
Not reported |
24.0 |
--- |
|
Metformin |
1-4 |
25.5 |
120.28 |
Table 2. Volume of distribution, dose taken of each drug and maximum concentration in blood of the deceased.
Source: (3).
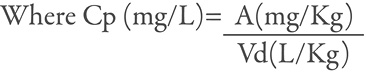
Cp (mg/L): Concentration in plasma.
A (mg/L): Dose taken over weight.
Vd (L/Kg): Volume of distribution for each active component.
METHODOLOGY
Procedure
The levels of the calibration curve were prepared in triplicate by taking 2mL of blank blood in test tubes and adding verapamil to obtain concentrations of 5, 10, 15, 20 and 25μg/mL and 0.1μg/mL of flurazepam (enriched blood). For the urine, the same preparation was done with concentrations of 0.1, 0.3, 0.5, 0.7, 1.0 and 1.5 μg/mL of verapamil and 0.1μg/mL of flurazepam (enriched). Flurazepam was added to the blood and urine samples of the cadaver as internal standard with a concentration of 0.1μg/mL.
Levels of enriched blood and urine, along with the respective samples from the cadaver, were submitted to liquid-liquid extraction to recover the verapamil by adding a pH 6 buffer of 4.0 mL of phosphates and 6.6 mL of extraction solvent (dichloromethane/isopropanol/ammonium hydroxide 80/20/2). They were then submitted to sonication for 30 minutes and centrifuged at approximately 2000 rpm. The upper organic layer was then transferred to clean and dry test tubes of 6 mL, evaporated at 60°C, and agitated to achieve an approximate volume of 0.5 mL. Maximum vacuum was applied until dry. Finally, the evaporated extracts were reconstituted with 50μL of ACN/H20 solution (50:50 v/v) with 0.1% formic acid.
Each solution of verapamil and flurazepam analytes were prepared at 1mg/mL in methanol, both reactions are standard. The reagents were at analytic grade with the exception of methanol and acetonitrile (ACN), which were at HPLC grade. Blank urine was obtained through volunteers and blank blood was obtained from a 50:50 dilution of concentrated red blood cells with deionized water.
Conditions of the liquid chromatography-mass spectrometry
PA liquid chromatography-mass spectrometry (LC-MS) of the Thermo Electron Corporation brand and Thermo Surveyor-LCQ Advantage Max model was used. The conditions were: Column HPLC Hypersil Gold PFP of (50mm X 2.1mm, 5μm); the temperature of the column was 40°C. An acetonitrile gradient was used with 0.1% formic acid and a 10 mm solution of ammonium formiate with 0.1% of formic acid at a constant flowrate of 200 μL/min. The programming was the following: 0-0.5 min 5% of ACN, 0.5-5.5 increase of 5-95%, 5.5-8.5 minutes remaining at 95% of ACN, 8.6-13 minute decrease of 95-5%. The solvents of the mobile phase had been vacuum filtered earlier using a hydrophilic polyvinylidene fluoride (PVDF) membrane with a pore size of 0.22μm. The injection volume was 10μL.
Mass spectometry was conducted in tandem with the ion trap analyzer using a product ion scan, equipped with an electrospray ionization source (ESI) in positive mode. The conditions were optimized for the verapamil through infusion to the mass spectrometer. The conditions of the main parameters were: capillary voltage 9.00V, source voltage of 5.00kV, capillary temperature of 160ºC, lense voltage of 5V, flow of ionization gas of 55 units and flow of auxiliary gas of 15 units. Table 3 shows the values of ions (m/z), retention time (RT) and collision energy (CE) and the isolation width optimized for the identification of each composite.
|
Composite |
Transition (m/z) |
CE (%) |
isolation width |
Polarity |
RT (min) |
|
Verapamil |
455.1→303.2 455.1→165.1 |
35 |
1 |
positive |
6.23 |
|
Fluazepam (SI) |
388.1→315.1 388.1→317.1 |
35 |
1 |
positive |
5.52 |
Table 3. Data of the LC-ESI-MS/MS
Source: Author.
RESULTS
The correlation coefficients for the calibration curve were 0.0047 and 0.9960 in blood and urine respectively, with a variation coefficient lower than 5% in each level. The concentration of verapamil found in the blood of the deceased was 18,26mg/L and 0.37mg/L in the urine. The chromatogram and calibration curve of the verapamil in blood are presented in Figure 1.
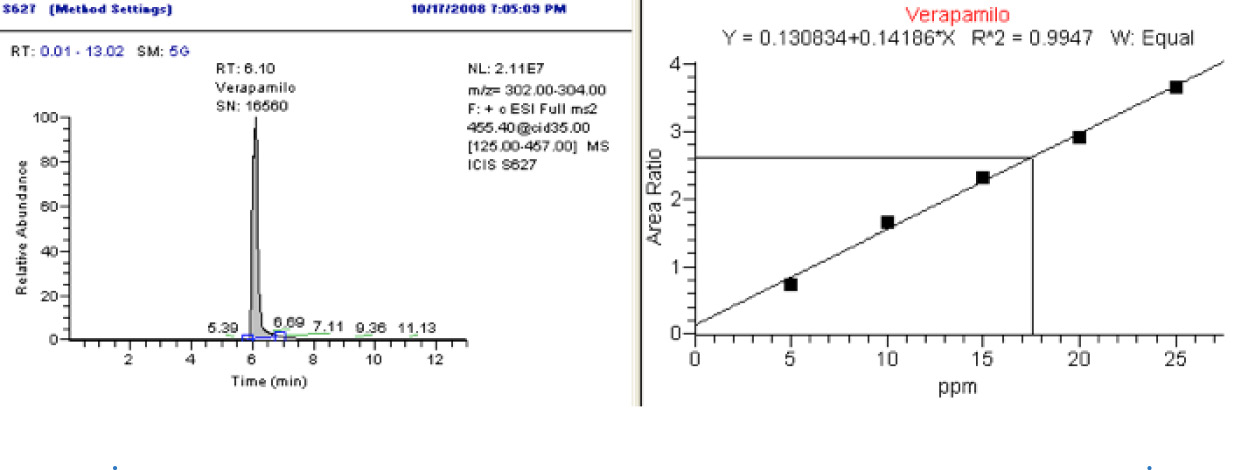
Fig 1. Chromatogram and calibration curve of the verapamil in blood (LC-MS)
Source: Author.
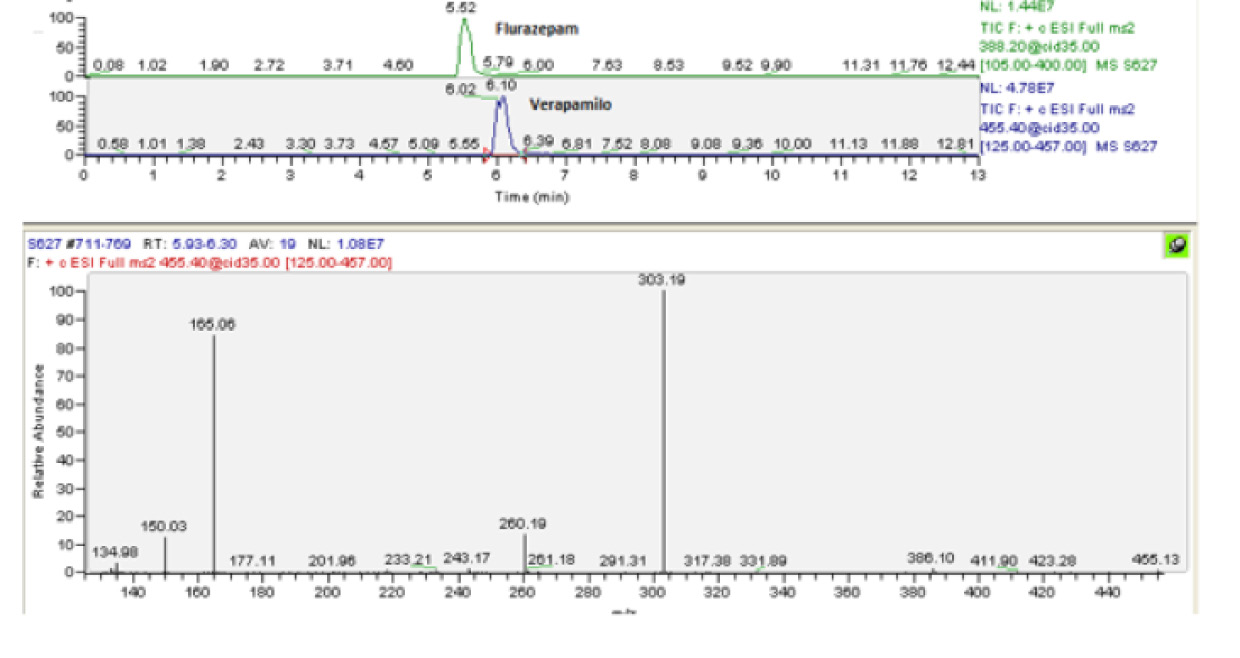
Fig 2. Chromatogram of the verapamil and flurazepam, and mass spectrum of the verapamil
Source: Author.
DISCUSSION AND CONCLUSIONS
A new method was established to determine verapamil in blood and urine through LC-ESI-MS/MS. The method presents linear results that comply with the acceptance criteria of bioanalytical guidelines (11).
LC-ESI-MS/MS is a sensitive technique to detect verapamil in biological samples; the method is highly selective and allows for the unequivocal determination of the drug in question, which is a fundamental requirement of forensic toxicology.
According to information found in bibliographical references, the lethal concentration of verapamil is 1μg/mL and in the results obtained, the concentration of verapamil was 18.26mg/L (18.26μg/mL) after the deceased had consumed 170 tablets of 120 mg of verapamil for a total dosage of 20.4g, which was potentially modified by vomiting. Plasma concentration levels could have theoretically reached 64.15mg/L if the drug had been completely absorbed.
The lethal dose of verapamil can be considered the cause of rapid organ failure, indicating that she suffered from an acute intoxication that led to her death.
Finally, in the forensic context, it is good practice to determine the presence of a substance with different matrices or, if there is only one sample, to undertake analyses with different techniques or assays repeated on different days (12). While components are not necessarily present in all matrices (according to the variables involved in toxicokinetics), in the current case, the concentration of verapamil in blood and urine definitely proved the cause of death by intoxication with this drug.
REFERENCES
1. Isaza C, Isaza G, Fuentes J. Fundamentos de Farmacología en Terapéutica. 4th ed. Pereira: Postergraph; 2002.
2. Allendes C, Maureira F. Inusual Caso de Intoxicación por Hipoglicemiantes Orales. In: III Encuentro Regional de Toxicología Forense (TIAFT); 2007 Oct 23-26; Bogotá, D.C.; 2007.
3. Moffat A, Osselton D, Widdop B. Clarke’s Analysis of Drugs and Poisons: In Pharmaceuticals, Body Fluids, and Postmortem Material. 3rd ed. London: Pharmaceutical Press; 2004.
4. Baselt RC. Disposition of Toxic Drugs and Chemicals in Man. 7th Ed. Foster City: Biomedical Publications; 2004.
5. Shin HS, Oh-Shin YS, Kim HJ, Kang YK. Sensitive assay for verapamil in plasma using gas-liquid chromatography with nitrogen-phosphorus detection. J. Chromatogr. B. Biomed. Appl. 1996;677(2):369-73. http://doi.org/czsfwb.
6. Chytil L, Strauch B, Cvačka J, Marešová V, Widimský J Jr, Holaj R, et al. Determination of doxazosin and verapamil in human serum by fast LC-MS/MS: application to document non-compliance of patients. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2010;878(30):3167-73. http://doi.org/chw3c7.
7. Von-Richter O, Eichelbaum M, Schönberger F, Hofmann U. Rapid and highly sensitive method for the determination of verapamil, [2H7] verapamil and metabolites in biological fluids by liquid chromatography-mass spectrometry. J. Chromatogr. B. Biomed. Sci. Appl. 2000;738:137-147. http:// doi.org/d9skwt.
8. Mullett WM, Walles M, Levsen K, Borlak J, Pawliszyn J. Multidimensional on-line sample preparation of verapamil and its metabolites by a molecularly imprinted polymer coupled to liquid chromatography– mass spectrometry. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2004;801(2):297- 306. http://doi.org/bpv568.
9. Repetto MR, Repetto M. Tabla de concentraciones de xenobióticos en fluidos biológicos humanos como referencia para el diagnóstico toxicológico. In: M Repetto, ed. Ampliación de Toxicología de Postgrado. Actualización 2007. Sevilla: Instituto Nacional de Toxicología Ciencias Forense, Area de Toxicología de la Universidad deSevilla; 2007.
10. Winek CL, Wahba WW, Jr. Winek CL, Winek- Balzer T. Drug and chemical blood-level data 2001. Forensic Science International. 2001;122(2- 3):107-23. http://doi.org/b8qk84.
11. U.S. Food and Drug Administration. Guidance for Industry Bioanalytical Method Validation. Washington, D.C.: U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM); 2001.
12. Society of Forensic Toxicologists Inc, American Academy of Forensic Sciences, Toxicology Section. SOFT/AAFS Forensic Laboratory Guidelines. Forensic Toxicology Laboratory Guidelines. 2006:1-24