ABSTRACT
Introduction: Spontaneous pneumomediastinum (SPM) is defined as the presence of air in the mediastinum. It is a rare entity considered benign and self-limiting, which mostly affects young adults. Its diagnosis is confirmed through clinical and radiological studies.
Case description: 21-year-old male patient with cough and greenish expectoration for four days, associated with dyspnea, chest pain, fever and bilateral supraclavicular subcutaneous emphysema. Chest X-ray suggested pneumomediastinum, which was confirmed by tomography. The patient was hospitalized for observation and treatment. After a positive evolution, he was discharged on the sixth day.
Discussion: SPM is a differential diagnosis in patients with chest pain and dyspnea. Its prevalence is lower than 0.01% and its mortality rate is low. It should be suspected in patients with chest pain and subcutaneous emphysema on physical examination. Between 70 and 90% of the cases can be identified by chest X-ray, while confirmation can be obtained through chest tomography. In most cases it does not require additional studies.
Conclusion: SPM is a little known cause of acute chest pain, and rarely considered as a differential diagnosis; it is self-limited and has a good prognosis.
Introduction
SPM is defined as the presence of air in the mediastinum without an apparent secondary cause (1). It is rare, benign and self-limiting, and affects mostly young adults with an average age of 25 years (2), ranging between 13 to 35 (3); a study by Cáceres et al. (4) reported a similar incidence between men and women. In 1944, Macklin et al. suggested that SPM originates after an alveolar rupture caused by increased intrathoracic pressure, with subsequent passage of air into the interstitium and bronchovascular tissues of the tracheobronchial tree (5).
The most frequent symptoms are chest pain, dysphagia, persistent cough and dyspnea, while risk factors include chronic obstructive pulmonary disease, asthma, and tobacco and illicit drugs use. In addition, precipitating factors such as nausea, vomiting, cough, upper respiratory tract infection and strenuous physical exercise have been observed (3). SPM cases have also been reported as complications of pneumonia by influenza A (H1N1) in children, mainly during the pandemic period of this infection in 2009 (6).
The goal of treatment is to control symptoms and may require observation. The length of hospital stay varies from a few hours to several days (2,4). This article presents a SPM case in a young adult.
Case description
21-year-old male patient from Garagoa (Boyacá), resident of Bogotá D.C. Colombia, an industrial automation student, mestizo, socioeconomic stratum 3, who presented a clinical picture of four days of evolution consisting of cough with greenish expectoration, dyspnea, chest pain, and unquantified fever. On physical examination he did not have respiratory distress and his vital signs were normal. Bilateral supraclavicular subcutaneous emphysema, decreased vesicular murmur and bilateral intermittent wheezing were identified; no other abnormal findings were observed. The patient had no relevant medical history.
Based on the clinical and epidemiological characteristics, an acute respiratory infection of viral origin was considered; in addition, due to the presence of subcutaneous emphysema and alterations in pulmonary auscultation, spontaneous pneumothorax was suspected. Leukocytosis with neutrophilia and mild oxygenation disorder was found in the requested paraclinical exams (Table 1), while left chest and left supraclavicular soft tissues were observed on the chest radiograph (Figure 1).
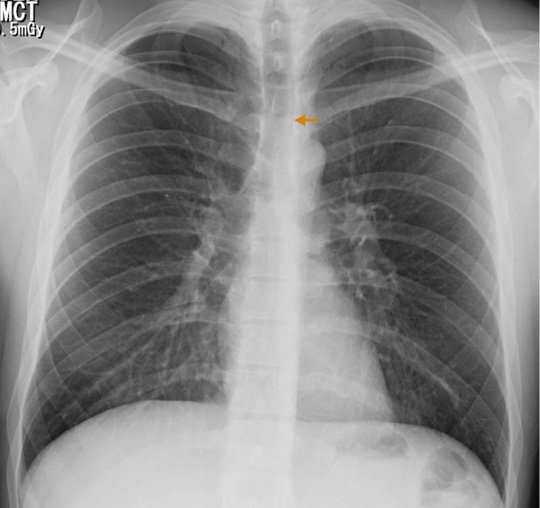
Figure 1. PA chest x-ray: pneumomediastinum, delimitation of anatomical structures allowing a neat visualization of its contours (arrow).
Source: Own elaboration based on the data obtained in the study.
Table 1. Paraclínical exams.
|
On admission |
Control at 72 hours |
|
|
Hematogram |
Leukocytes 14670 cell/mm3 Neutrophils 13670 cell/mm3 Hemoglobin 17 g/dL Hematocrit 48% Platelets 257000 cells/mm3 |
Leukocytes 12150 cell/mm3 Neutrophils 8240 cell/mm3 Hemoglobin 16.7 g/dL Hematocrit 47.7% Platelets 264000 cell/mm3 |
|
Arterial blood gas |
pH 7.43 PO2 55.8 mmHg FiO2 0.24 PCO2 35.6 mmHg PAFI 232.7 HCO3 23.3 mmol/L BE -0.4 mmol/L |
pH 7.43 PO2 62.6 mmHg FiO2 0.21 PCO2 33.7 mmHg PAFI 297 HCO3 22.3 mmol/L BE - 0.6 mmol/L |
Source: Own elaboration based on the data obtained in the study.
Later, a chest tomography was performed, which showed air in the anterior, middle, posterior and superior mediastinum, reaching the lower neck (Figure 2). Due to the absence of risk factors related to secondary causes, SPM secondary to an acute respiratory infection of viral origin was diagnosed; the patient was maintained under observation, and treatment including oxygen through nasal cannula, respiratory therapy, analgesia and rest was indicated.

Figure 2. Chest tomography, coronal plane: pneumomediastinum, presence of infracarinal and paratracheal air (sepia arrow). Left supraclavicular subcutaneous emphysema is also observed.
Source: Own elaboration based on the data obtained in the study.
The patient improved during follow-up, period in which leukocytosis and oxygenation disorder were corrected (Table 1), and was discharged after six days of hospitalization. Outpatient radiographic monitoring was requested and he was given recommendations and warning signs. The patient did not present adverse drug reaction or other events during hospitalization.
Discussion
Pneumomediastinum was first reported in 1819 by René Laennec while spontaneous pneumomediastinum was described in 1939 by Louis Hamman (7). Its incidence is less than 0.01% and has a recurrence rate of 1.6% per year (8,9). SPM is a differential diagnosis in patients with chest pain and dyspnea, and is believed to be caused by alveolar rupture due to increased intraalveolar pressure (1,10); therefore, its association with pneumothorax is frequent, being found in 32% of patients (11). In 44% of cases, patients have a history of congestive lung disease, such as asthma, chronic obstructive pulmonary disease, interstitial disease, pulmonary fibrosis, pneumonitis, among others (11).
The mean age at diagnosis is 25 years (11), similar to that of patients with spontaneous pneumothorax (9). In 34% to 49% of the cases, precipitating factors, such as inhaled drug abuse, acute respiratory infection, vomiting, asthmatic crisis and intense exercise are observed (9,11).
The most common clinical manifestations include chest pain (68-78.1%), dyspnea (28.1-44%), sore throat (14.1-28%) and cervical pain (54.7%) (9,12). Furthermore, subcutaneous emphysema is the most frequent symptom in about 40 to 100% of patients (9,12,13); in contrast, Hamman’s sign (systolic crackle heard with a stethoscope at the left sternal border) is found in only 20% of cases (14,15).
Its presentation is usually masked because of the low specificity of the symptoms and the lack of knowledge of this entity (16). The diagnosis is made based on clinical manifestations and radiological confirmation, in addition to searching for triggers (4,17). 79% of the patients are diagnosed in the emergency room, 11% in the critical care unit, 2% during hospitalization, and 8% in outpatient consultations (11).
Radiological studies of the thorax are important in the evaluation and exclusion of secondary causes (9), and are sufficient to confirm the diagnosis (18). Not all patients with pneumomediastinum require contrast radiographic imaging, which is reserved for patients who are suspected of having a tracheobronchial or esophageal injury, especially when vomiting, dysphagia, known gastrointestinal disease, trauma, fever, hemodynamic instability, pleural effusion or pneumoperitoneum are involved (19).
Around 70% to 90% of SPM cases can be identified by chest X-ray (20). The presence of mediastinal air creates an interface with the anatomical structures that allows to visualize its contours neatly. Radiological signs depend on the quantity and location of the air (21): when it surrounds the vascular structures, the ring sign and the tubular artery sign appear. The delimitation of the inner and outer wall of the bronchus is possible due to the presence of intra and extraluminal gas, generating a double wall sign. The continuous diaphragm sign is caused by air posterior to the pericardium.
Other radiological signs include subcutaneous emphysema, radiolucent lines in the upper mediastinum, pneumoprepericardium, “Naclerio V”, extrapleural air sign and, thymic wing sign caused by the delimitation of the thymus in children (16,21). Chest tomography delimits the extension of the pneumomediastinum, and provides information about its etiology and differential diagnoses (21,22).
In most cases studies that look for secondary causes are unnecessary, since, in general, there are no alterations of the respiratory or digestive tracts. Advanced diagnostic procedures, restricting diet, administering antibiotics and prolonging hospitalization stay are not appropriate measures (19). SPM has a good prognosis and can be treated conservatively (18), which has shown good results in different studies (2,9,19,23). Such treatment consists of analgesia, rest, oxygen and bronchodilators (24).
In theory, oxygen supplementation is of great importance for treatment, regardless of the presence of an oxygenation disorder, since it increases the pressure of nitrogen diffusion in the interstitium and promotes the absorption of free air (16) accelerating the resolution time.
The mean time of hospitalization is 4.6 days (9) and its management in a critical care unit is unnecessary unless required or in cases in which esophageal rupture is highly suspected (19). Once the patient is discharged, radiological follow-up can be performed until full resolution (16).
The case described here corresponds to a patient, whose epidemiological, clinical and radiological characteristics are the most frequently reported in the literature. This is a typical case that contributes to the diagnostic approach in young patients who present chest pain on arrival to the emergency room. It is important to mention that this case had several limitations, including the lack of microbiological isolation of the germ responsible for the acute respiratory infection, radiological control, and information on outpatient follow-up to objectify the resolution of pneumomediastinum. However, this report is important because it illustrates a radiologically confirmed clinical case of a rare disease causing chest pain.
Conclusion
SPM is a rare entity that requires high clinical suspicion for both diagnosis and radiological confirmation. Its treatment is symptomatic and has a good prognosis. SPM should be considered as a differential diagnosis in patients with chest pain.
Conflict of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgement
Hospital Universitario Nacional de Colombia. Bogotá, Colombia.
Referencias
1.Macia I, Moya J, Ramos R, Morera R, Escobar I, Saumench J, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 2007;31(6):1110-4. http://doi.org/fntj86.
2.Jougon JB, Ballester M, Delcambre F, Mac Bride T, Dromer CE, Velly JF. Assessment of spontaneous pneumomediastinum: experience with 12 patients. Ann Thorac Surg. 2003;75(6):1711-4. http://doi.org/b5h8qp.
3.Perna V, Vilà E, Guelbenzu JJ, Amat I. Pneumomediastinum: is this really a benign entity? When it can be considered as spontaneous? Our experience in 47 adult patients. Eur J Cardiothorac Surg. 2010;37(3):573-5. http://doi.org/dhj7vg.
4.Caceres M, Ali SZ, Braud R, Weiman D, Garrett HE Jr. Spontaneous pneumomediastinum: a comparative study and review of the literature. Ann Thorac Surg. 2008;86(3):962-6. http://doi.org/bmrrgw.
5.Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiment. Medicine. 1944;;23(4):281-358.
6.Hasegawa M, Hashimoto K, Morozumi M, Ubukata K, Takahashi T, Inamo Y. Spontaneous pneumomediastinum complicating pneumonia in children infected with the 2009 pandemic influenza A (H1N1) virus. Clin Microbiol Infect. 2010;16(2):195-9. http://doi.org/bjtq8j.
7.Hamman L. Spontaneous mediastinal emphysema. Bull Johns Hopkins Hosp. 1939;64:1-21.
8.Lee SS. An unusual cause of chest pain in army trainee - spontaneous pneumomediastinum. Med J Malaysia. 2016 [cited 2016 Jul 19];71(1):30-1. Available from: https://goo.gl/jxLuH4.
9.Kim KS, Jeon HW, Moon Y, Kim YD, Ahn MI, Park JK, et al. Clinical experience of spontaneous pneumomediastinum: diagnosis and treatment. J Thorac Dis. 2015;7(10):1817-24. DOI: 10.3978/j.issn.2072-1439.2015.10.58.
10.Ba-Ssalamah A, Schima W, Umek W, Herold CJ. Spontaneous pneumomediastinum. Eur Radiol. 1999;9(4):724-7.
11.Iyer VN, Joshi AY, Ryu JH. Spontaneous pneumomediastinum: analysis of 62 consecutive adult patients. Mayo Clin Proc. 2009;84(5):417-21. http://doi.org/fx5q9h.
12.Takada K, Matsumoto S, Hiramatsu T, Kojima E, Watanabe H, Sizu M, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102(9):1329-34. http://doi.org/cxbdps.
13.Miura H, Taira O, Hiraguri S, Ohtani K, Kato H. Clinical Features of Medical Pneumomediastinum. Ann Thorac Cardiovasc Surg. 2003 [cited 2016 Jul 19];9(3):188-91. Available from: https://goo.gl/sqPfQy.
14.Kelly S, Hughes S, Nixon S, Paterson-Brown S. Spontaneous pneumomediastinum (Hamman’s syndrome). Surgeon. 2010;8(2):63-6. http://doi.org/bm75vb.
15.Koullias GJ, Korkolis DP, Wang XJ, Hammond GL. Current assessment and management of spontaneous pneumomediastinum: experience in 24 adult patients. Eur J Cardiothorac Surg. 2004;25(5):852-5. http://doi.org/c76m89.
16.Sahni S, Verma S, Grullon J, Esquire A, Patel P, Talwar A. Spontaneous pneumomediastinum: time for consensus. N Am J Med Sci. 2013;5(8):460-4. http://doi.org/bxxj.
17.Maunder RJ, Pierson DJ, Hudson LD. Subcutaneous and mediastinal emphysema. Pathophysiology, diagnosis, and management. Arch Intern Med. 1984;144(7):1447-53. http://doi.org/dq9k93.
18.Pekcan S, Gokturk B, Uygun Kucukapan H, Arslan U, Findik D. Spontaneous pneumomediastinum as a complication in human bocavirus infection. Pediatr Int. 2014;56(5):793-5. http://doi.org/bxxk.
19.Al-Mufarrej F, Badar J, Gharagozloo F, Tempesta B, Strother E, Margolis M. Spontaneous pneumomediastinum: diagnostic and therapeutic interventions. J Cardiothorac Surg. 2008;3:59. http://doi.org/dd3krd.
20.Kaneki T, Kubo K, Kawashima A, Koizumi T, Sekiguchi M, Sone S. Spontaneous pneumomediastinum in 33 patients: yield of chest computed tomography for the diagnosis of the mild type. Respiration. 2000;67(4):408-11. http://doi.org/ds54t3.
21.Zylak CM, Standen JR, Barnes GR, Zylak CJ. Pneumomediastinum Revisited. RadioGraphics. 2000;20(4):1043-57. http://doi.org/bxxn.
22.Bakhos CT, Pupovac SS, Ata A, Fantauzzi JP, Fabian T. Spontaneous pneumomediastinum: an extensive workup is not required. J Am Coll Surg. 2014;219(4):713-7. http://doi.org/bxxp.
23.Banki F, Estrera AL, Harrison RG, Miller CC, Leake SS, Mitchell KG, et al. Pneumomediastinum: etiology and a guide to diagnosis and treatment. Am J Surg. 2013;206(6):1001-6. http://doi.org/bxxq.
24.Abolnik I, Lossos IS, Breuer R. Spontaneous pneumomediastinum. A report of 25 cases. Chest. 1991;100(1):93-5. http://doi.org/b9mxrh.