Resumen
Introducción. La colangiopatía tras COVID-19 grave es una patología hepática descrita recientemente; esta se atribuye a factores adicionales a los ya descritos en la colangitis esclerosante secundaria en pacientes críticamente enfermos (CES-PCE), en la que el alto requerimiento de fármacos vasoactivos y de ventilación con presión positiva al final de la espiración conlleva a una mayor prevalencia y severidad.
Presentación del caso. Mujer de 55 años quien acudió al servicio de urgencias de una clínica de III nivel de complejidad de Cali, Colombia, por cuadro clínico consistente en anosmia, diarrea, fiebre (39°C), tos seca y disnea progresiva. La paciente, que fue diagnosticada con COVID-19 y requirió soporte ventilatorio, vasopresor y hemodinámico en la unidad de cuidados intensivos, tuvo sobreinfección bacteriana y estancia hospitalaria prolongada. Al día 24 de hospitalización presentó ictericia en mucosas y escleras, así como elevación de los niveles de bilirrubinas, fosfatasa alcalina y γ-glutamiltranferasa. Se le realizó una colangiorresonancia y una biopsia hepática que evidenciaron hallazgos compatibles con colangitis esclerosante, por lo que se le dio un manejo conservador con vigilancia y observación. Luego del alta tuvo mejoría de su compromiso hepático e infeccioso y continuó con hemodiálisis ambulatoria.
Conclusiones. La colangiopatía esclerosante secundaria tras COVID-19 grave es multifactorial e inusual, por tanto es necesario incluir al SARS-Cov-2 como etiología a descartar en pacientes con síntomas relacionados para así instaurar un manejo temprano y disminuir la morbilidad hepática.
Abstract
Introduction: Cholangitis following severe COVID-19 is a newly described liver disease. It is attributed to factors other than those already described in secondary sclerosing cholangitis in critically ill patients (SSC-CIP), in which the high requirement of vasoactive drugs and positive end-expiratory pressure ventilation leads to a higher prevalence and severity.
Case presentation: A 55-year-old female attended the emergency service of a tertiary care center in Cali, Colombia, due to anosmia, diarrhea, fever (39°C), dry cough, and progressive dyspnea for 6 days. The patient, who was previously diagnosed with COVID-19 and required ventilatory, vasopressor and hemodynamic support in the intensive care unit, had a bacterial superinfection that required a prolonged hospital stay. On the 24th day of hospitalization, she presented with jaundice in mucous membranes and sclera, as well as elevated bilirubin, alkaline phosphatase, and γ-glutamyl transferase. Cholangiopancreatography and liver biopsy revealed findings compatible with sclerosing cholangitis, so she was given a conservative treatment, with surveillance and observation. After being discharged, her liver condition and the infectious disease improved, and she continued with outpatient hemodialysis.
Conclusions: Secondary sclerosing cholangitis after severe COVID-19 is multifactorial and rare. Therefore, it is necessary to include SARS-CoV-2 as an etiology that must be ruled out in patients with related symptoms in order to establish early treatment and reduce liver morbidity.
Introduction
COVID-19 is a disease caused by the SARS-CoV-2 virus and is known for causing pneumonia in moderate-severe cases (1,2). However, its clinical spectrum is broad, as some patients are asymptomatic or have mild symptoms, while others experience severe disease with multisystemic involvement (2).
Post-COVID-19 liver disease includes manifestations such as elevated transaminase levels, hypoalbuminemia and prolonged prothrombin time, which are the result of the cytopathic action of the virus (3-6). Secondary sclerosing cholangitis in critically ill patients (SSC-CIP) is a rare entity, with a prevalence of 0.05% in patients requiring hospitalization in the intensive care unit (ICU) (7) and as high as 12% in patients with severe COVID-19 (7-10). This may be explained by the fact that, apart from the already known effects associated with SSC-CIP (requirement of invasive mechanical ventilation and vasopressors, shock, and sepsis), these patients experience direct damage to the cholangiocyte endothelium, which is facilitated by the high expression of angiotensin-converting enzyme receptors (ACE-2) in the cholangiocytes (8,11). Furthermore, it should be kept in mind that the doses of vasoactive agents and positive end-expiratory pressure used in mechanical ventilation in patients with COVID-19 are considerably higher than usual (11,12).
The following is the case of a female patient who, after suffering from severe COVID-19, developed SSC-CIP, a complication recently reported in these patients. Recent literature demonstrates that frequency and clinical presentation of SSC-CIP differ from that reported prior to the emergence of SARS-CoV-2; therefore, further studies evaluating this entity are needed in the future to better understand the disease.
Case presentation
This is the case of a 55-year-old mestizo woman from Cali (Colombia), a former smoker with a history of arterial hypertension (under treatment with losartan 50 mg/day) and morbid obesity. Her family history of relevance to the case included that her son and husband had recently tested positive for SARS-CoV-2 by reverse transcription polymerase chain reaction test. The patient attended the emergency department of Clínica Imbanaco, a tertiary care center in Cali, on December 30, 2020, complaining of a 6-day history of anosmia, fever (39°C), dry cough, progressive dyspnea and diarrhea, for which she had self-medicated with ibuprofen and azithromycin.
On admission physical examination, the following findings were noted: respiratory rate of 45 rpm (tachypnea), heart rate of 160 rpm (tachycardia), blood pressure of 102/64 mmHg, low oxygen saturation (SO2: 58%), and bilateral basal hypoventilation; the patient weighed 112kg and was 158cm tall. Laboratory tests on admission to the emergency department showed elevated levels of C-reactive protein, ferritin, and lactate dehydrogenase (LDH); impaired renal function; liver function within normal parameters; hyperglycemia; and severe hypoxemia (Table 1).
Table 1. Laboratory tests on admission.
|
Test |
Result |
|
Complete blood count |
Leukocytes: 8.270/mm3, neutrophils: 85%, lymphocytes: 10.8%, eosinophils: 0.03%, basophils: 0.10%, platelets: 282 000/mm3, hemoglobin: 14.3 gr/dL, and hematocrit: 43.1% |
|
Kidney function tests |
Creatinine: 4.40 mg/dL, BUN: 65.20 mg/dL |
|
Liver function tests |
AST 37 U/L (reference value 0-40 U/L), ALT 30 U/L (reference value 0-33 U/L) |
|
Blood albumin test |
3.40 gr/dL (reference value 3.5-5.2 gr/dL) |
|
Blood glucose |
121 mg/dL (reference value 60-100 mg/dL) |
|
Electrolyte panel |
Chloride: 111 mmol/L, potassium: 4.35 mmol/L, sodium: 144 mmol/L |
|
Procalcitonin test |
0.24 ng/MI (reference value 0.5-2.0 ng/MI) |
|
C-reactive protein test |
25.53 mg/dL (reference value <5 mg/dL) |
|
SARS-CoV-2 antigen test* |
Positive |
|
Lactate dehydrogenase test (LDH) |
607 U/L (reference value 270-480 U/L) |
|
Total creatinine kinase test |
53 U/L (reference value 0-170 U/L) |
|
Ferritin test |
>2 000 mg/dL (reference value 15-150 mg/dL) |
|
Quantitative troponin T test |
5.6 ng/mL (reference value 0-14 ng/mL) |
|
D-dimer test |
0.52 µg/MI |
|
Arterial blood gas test |
pH: 7.45, lactate: 1.23, PCO2: 30 mmHg, PO2: 65 mmHg, HCO3-: 19, TCO2: 19.9 mmol/L, BEecf: -5.6 mmol/L, BE: -4.6 mmol (ref -2-3 mmol/L), PAO2: 586 mmHg, P/F ratio: 65 mmHg, FiO2: 100% |
|
Polymerase chain reaction panel test for respiratory pathogens |
Negative |
BUN: blood urea nitrogen; AST: aspartate aminotransferase; ALT: alanine aminotransferase.
* Test validated by the Colombian National Institute of Health.
Source: Own elaboration.
During the initial examination in the emergency department, a diagnostic impression of type I respiratory failure due to severe COVID-19 pneumonia and acute renal failure classified as AKIN III was established, so the patient was admitted to the ICU where she was treated with mechanical ventilation and administration of fentanyl (1mcg/kg) and ketamine (1mg/kg) in order to perform a rapid intubation sequence. Likewise, treatment with prophylactic enoxaparin (subcutaneous injection of 40 mg/day during the entire hospital stay), dexamethasone (intravenous injection of 6 mg/day for 10 days), omeprazole (intravenous injection of 40 mg/day), and intravenous fluids (initially 1 bolus of 500mL of Hartman and then infusion at 1 mL/kg/hour during the ICU stay) was indicated. On the fifth day of admission, a computed tomography angiography of the thorax was performed, and pulmonary thromboembolism was ruled out.
On the seventh day of hospitalization, the patient’s clinical condition deteriorated: she developed a fever reaching up to 39°C, required vasopressor support, and her kidney function worsened, requiring a set of blood cultures and empirical antibiotic treatment and renal replacement therapy with hemodialysis. On the ninth day of hospitalization, it was established that two of the three blood cultures were positive for multisensitive Enterobacter aerogenes and Enterobacter cloacae, so antibiotic therapy was replaced by intravenous piperacillin-tazobactam at a dose of 2.25g 3 times a day for 7 days, and the use of vasopressors was maintained for 72 hours. Fourteen days after being admitted to the ICU, she was successfully extubated and the following day she was transferred to the general ward.
On the 24th day of hospitalization, she presented with generalized pruritus and jaundice in mucous membranes and sclera; in addition, laboratory tests performed due to this episode showed hyperbilirubinemia due to an increase in direct bilirubin (Table 2).
Table 2. Laboratory tests performed on the 24th day of hospital stay during an episode of jaundice.
|
Test |
Result |
|
Liver function tests |
Total bilirubin: 8.56 mg/dL, direct bilirubin: 8.54 mg/dL, indirect bilirubin: 8.54 mg/dL: 0.002 mg/dL). ALT: 174 U/L, AST: 89 U/L, LDH: 425 U/L, FA: 1 908 U/L (reference value 35-105 U/L), PT: 13.0 seg (reference value 9.1-13 s), PTT: 23.9 seg (25.1-31.1 s). |
|
Amylase test |
196 U/L (reference value 28-100 U/L) |
|
Hepatitis B test |
Surface antigen [Ag-HbS (-)] |
|
Hepatitis C test |
Hepatitis C antibodies (IgG and IgM) (-) |
|
Hepatitis A test |
Hepatitis A antibodies (IgG and IgM) (-) |
|
Gamma-glutamyl transferase |
796 U/L (reference value 5-95 U/L) |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; LDH: lactate dehydrogenase; FA: alkaline phosphatase; PT: prothrombin time, PTT: partial thromboplastin time.
Source: Own elaboration.
On the 26th day of hospitalization, a cholangiopancreatography was performed, revealing extrahepatic bile duct dilatation and saccular dilatation of the distal intrahepatic bile ducts with an obstructive pattern, which suggested sclerosing cholangitis. Considering the findings, on the 28th day of hospitalization, a liver biopsy was performed to determine the cause of the biliary involvement. The histopathological report described liver parenchyma with preserved architecture and widening of portal spaces; some bile ducts showed dilatation and atrophy, and their epithelium showed reactive changes with minimal periductal fibrosis and chronic inflammatory infiltrate, with no evidence of microthrombotic events (Figure 1).
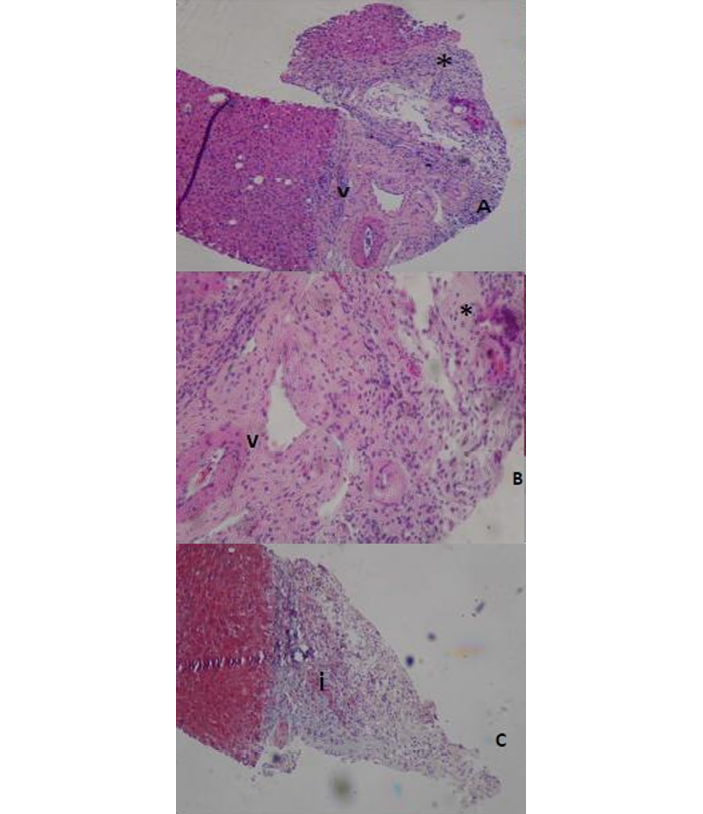
Figure 1. A) distortion and damage to cholangiocytes; B) portal space widening with fibrosis; C) chronic inflammatory infiltrate.
V: capillary; I: interstitium.
* Cholangiocytes.
Source: Image obtained during the study.
Considering these histopathological findings compatible with mild, possibly sclerosing, cholangitis, the number of differential diagnoses increased. Therefore, after ruling out HIV infection, bacterial or parasitic infections associated with cholangitis, or a history of biliary disease, SSC-CIP was considered to be caused by both SARS-CoV-2 damage to the biliary epithelium and multiple factors related to the patient’s critical condition during her ICU stay due to COVID-19, including mechanical ventilation, septic shock, and high vasopressor requirement. Given the impossibility of establishing a specific treatment, it was decided to opt for permanent observation, clinical surveillance, and symptom management.
The patient was discharged 30 days after admission with indication to undergo daily hemodialysis. One month later, in a follow-up appointment with the hepatology service, based on follow-up laboratory tests and physical examination, it was established that the patient’s condition had stabilized as normal levels of liver and biliary function parameters were observed.
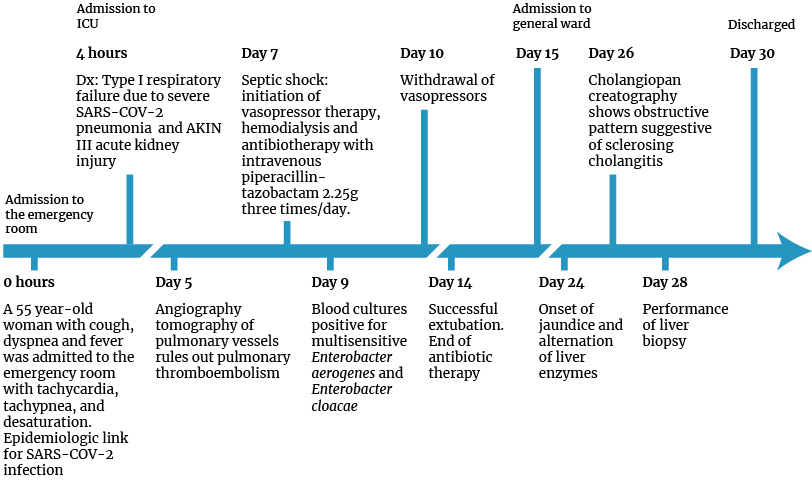
Figure 2. Timeline of patient care.
ICU: intensive care unit; Dx: diagnosis; Dx: diagnosis.
Source: Own elaboration.
Discussion
Liver damage associated with SARS-CoV-2 infection is usually mild, transient and asymptomatic, and although it usually does not progress to acute liver injury, some cases of liver injury have been reported, mainly in elderly patients with severe COVID-19 (4,6,12,13). The direct cytotoxicity of SARS-CoV-2 viral replication in the liver and bile ducts causes immune-mediated damage by severe/systemic inflammatory response in patients with COVID-19 (5,6). In this sense, systemic sepsis and respiratory failure induce ischemic hepatitis and promote immunological, inflammatory and toxic aggravating mechanisms, as well as vascular changes resulting from coagulopathy, endotheliitis, and right-sided heart failure (5,13).
COVID-19 may contribute to primary liver damage; however, ACE-2 expression is increased in patients with pre-existing liver disease, which further increases the hepatic tropism of SARS-CoV-2 (12). In this sense, secondary liver disease after severe COVID-19 manifests with elevation of bilirubin in 35% of cases, as well as of cholestatic liver enzymes: alkaline phosphatase in 6.1% of cases and γ-glutamyl transferase in 21.1% (3,5).
The literature on post-COVID-19 cholangiopathy is very scarce. However, Roth et al. (10) published a study describing the clinical course and histologic features of three adults who developed prolonged and severe cholestasis during their recovery from severe COVID-19, in which one of the cases developed SSC-CIP.
Liver damage associated with COVID-19 expresses a biphasic pattern in which, at the beginning of the infectious process, respiratory failure elevates transaminase levels. However, it is the increase in cholestatic liver enzymes during hospitalization, induced by the inflammatory response after SARS-CoV-2 binds to the endothelium of hepatocytes and cholangiocytes, that is associated with decreased survival and, therefore, it requires greater surveillance (14). The cholangiopancreatography of the patient reported here showed extrahepatic bile duct dilatation and saccular dilatations in the intrahepatic bile ducts, findings suggestive of cholangitis that could be associated with the aforementioned condition. Factors favoring SSC-CIP include respiratory failure and hypoxic state (5).
In some patients with COVID-19 in whom liver tests were abnormal, microthrombi were reported; however, in the case described here, histology did not reveal liver failure or bile duct injury, suggesting that these may not be primary mechanisms of hepatobiliary involvement (12).
The patient’s comorbidities could condition the severity of COVID-19, since obesity, diabetes and liver disease have been associated with retinoid accumulation, which favors irreversible damage to hepatocytes and bile ducts mediated by acute neutrophilic infiltration and activation of apoptosis and necrosis cascades. Additionally, massive apoptosis of neutrophils and lymphocytes decreases the functionality of the immune system and facilitates superinfection, as in the case reported here (15).
On the other hand, it has been proposed that liver and bile duct damage is induced by drugs such as antivirals, antibiotics (quinolones, macrolides), antipyretics, and hydroxychloroquine (13). The patient reported here took multiple medications for the treatment of COVID-19 symptoms prior to admission, several of which were hepatotoxic, such as acetaminophen, diclofenac, ibuprofen and azithromycin, which, together with those administered in the ICU for hemodynamic and ventilatory support, could have facilitated liver damage. In turn, an association between cholangiopathy and ketamine intake, a drug that was used in this patient during mechanical ventilation, has been suggested; however, the reported cases are related to the chronic use of this drug (16) or corticosteroids.
As observed in the biopsy of the reported patient, marked mitotic activity, apoptosis and periductal lymphocytic infiltration of cholangiocytes are histopathological findings suggestive of inflammation-mediated damage in cholestasis, which consequently leads to ductal dilatation and fibrosis associated with a chronic inflammatory infiltrate (17,18). Edema in portal spaces and vacuolization have also been described (10).
Infected cholangiocytes induce the activation of the inflammatory cascade by stimulating the secretion of proinflammatory and profibrotic cytokines, ultimately causing ongoing immunological reactions with excessive deposition of scar tissue and, subsequently, biliary cirrhosis (18). Systemic inflammatory response syndrome (SIRS) induces cholestasis and causes bile duct alterations, which depend on the cytokine storm, with tumor necrosis factor alpha and interleukins 1 and 6 being the major contributors to intrahepatic cholestasis and vascular endothelial damage (5).
In short, bile ducts in patients with severe COVID-19 are affected by hypoxia from respiratory failure (potentially aggravated by obliteration of the peribiliary plexus through blood vessel changes), by systemic SIRS resulting in inflammation-reactive cholangiocytes, by fibrosis, and by possible viral infection of the cholangiocytes themselves (5). On the other hand, sclerosing cholangitis may occur in critically ill patients with severe trauma or burns requiring hemodynamic support (19,20). Impaired perfusion and hypoxia are well-known inflammatory stimuli that condition the destruction of the biliary epithelium in sclerosing cholangitis and are found in patients with severe COVID-19 (21).
Conclusions
SSC-CIP is a widely studied disease whose prevalence and severity has increased in patients with severe COVID-19. Consequently, given that this association has a worse prognosis, a higher rate of suspicion, surveillance, and comprehensive management are required to reduce morbidity and mortality.
Possibly, cholangiopathy in the reported patient may have been the result of the accumulation of all the factors described as pathological, pharmacological, and associated with the direct action of the disease, becoming manifest in the recovery stage of COVID-19.
Secondary sclerosing cholangitis after severe COVID-19 is multifactorial and rare, so it is necessary to include SARS-Cov-2 as an etiology to be ruled out in patients with related symptoms in order to establish early management and reduce liver morbidity.
Ethical considerations
For the preparation of this case report, informed consent was obtained from the patient, who agreed to the disclosure of the information presented in this article.
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgments
None stated by the authors.
References
1.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. https://doi.org/ggqgd3.
2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-9. https://doi.org/ggkh48.
3.Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52(4):584-99. https://doi.org/gg4fp4.
4.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428-30. https://doi.org/ggpx6s.
5.Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41(1):20-32. https://doi.org/grrx45.
6.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998-1004. https://doi.org/ggpx23.
7.Pizarro-Vega NM, Valer Lopez-Fando P, de la Poza-Gómez G, Piqueras-Alcol B, Gil-Santana M, Ruiz-
Fuentes P, et al. Colangitis esclerosante secundaria: una complicación tras la infección severa por
COVID-19. Gastroenterol Hepatol. 2022;13:S0210-5705(22)00144-3. https://doi.org/j8d2.
8.Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARSCoV-2 infection. BMJ Case Rep. 2020;13(11):e237984. https://doi.org/gmzw5k.
9.Bütikofer S, Lenggenhager D, Wendel-Garcia PD, Maggio EM, Haberecker M, Reiner CS, et al.
Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41(10):2404-17. https://doi.org/gj5dfq
10.Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, et al. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116(5):1077-82. https://doi.org/gj9p3b.
11.Chai X, Hu LF, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. BioRxiv. 2020. https://doi.org/ggq626.
12.Li P, Liu Y, Cheng Z, Yu X, Li Y. COVID-19-associated liver injury: Clinical characteristics, pathophysiological mechanisms and treatment management. Biomed Pharmacother. 2022;154:113568. https://doi.org/gq9hg8.
13.Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18-24. https://doi.org/ghcv24.
14.Bernal-Monterde V, Casas-Deza D, Letona-Giménez L, de la Llama-Celis N, Calmarza P, Sierra-
Gabarda O, et al. SARSCoV- 2 Infection Induces a Dual Response in Liver Function Tests: Association with Mortality during Hospitalization. Biomedicines. 2020;8(9):328. https://doi.org/gmzzqr.
15.Mahase E. Covid-19: what treatments are being investigated? BMJ. 2020;368:m1252.
https://doi.org/ggq8j4.
16.Nyirenda TJ, Shirazi-Nejad A, Soliman AS. Persistent Ketamine-Induced Cholangiopathy: An Approach to Management. Cureus. 2020;12(11):e11611. https://doi.org/j8fr.
17.Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39(2):302-10. https://doi.org/bjwkvv.
18.Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16(5):269-81. https://doi.org/gk35bn.
19.Gelbmann CM, Rümmele P, Wimmer M, Hofstädter F, Göhlmann B, Endlicher E, et al. Ischemic-
like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007;102(6):1221-9. https://doi.org/fdm6hz.
20.Zilkens C, Friese J, Köller M, Muhr G, Schinkel C. Hepatic Failure After Injury - A Common Pathogenesis With Sclerosing Cholangitis? Eur J Med Res. 2008;13(7):309-13.
21.Horvatits T, Drolz A, Trauner M, Fuhrmann V. Liver Injury and Failure in Critical Illness. Hepatology. 2019;70(6):2204-15. https://doi.org/j8ft.