Resumen
Introducción. La trombosis venosa profunda (TVP) es una afección frecuente, especialmente en pacientes con factores de riesgo como cáncer. Su tratamiento es la anticoagulación, aunque en ocasiones está contraindicada y es necesario implantar un filtro de vena cava inferior para evitar el desarrollo de embolia pulmonar. La aparición de embolia pulmonar en un paciente que utiliza un filtro de vena cava inferior es poco frecuente (<2%); sin embargo, como este método terapéutico no está exento de complicaciones, existe una discusión acerca del riesgo-beneficio de su aplicación.
Presentación del caso. Hombre de 47 años con antecedente de glioblastoma grado IV y TVP en miembro inferior izquierdo quien asistió al servicio de urgencias de un hospital de tercer nivel de atención de Logroño (España) por aumento del diámetro de su pierna izquierda. Por diagnóstico de TVP, se inició tratamiento anticoagulante, el cual tuvo como complicación un hematoma agudo extra-axial en la convexidad del lóbulo frontal izquierdo. Se implantó un filtro de vena cava inferior; sin embargo, el paciente desarrolló embolia pulmonar con presencia de trombo en la vena cava inferior yuxtarrenal por encima del filtro. El paciente recibió manejo anticoagulante, se recuperó satisfactoriamente y fue dado de alta después de 12 días de estancia hospitalaria con tratamiento ambulatorio.
Conclusiones. La indicación de implantación de un filtro de vena cava inferior en pacientes con TVP es limitada, por lo que se sugiere usarlo únicamente en pacientes con enfermedad tromboembólica venosa aguda y contraindicaciones absolutas a los anticoagulantes o en pacientes con resultados inadecuados tras el tratamiento anticoagulante.
Abstract
Introduction: Deep vein thrombosis (DVT) is a highly prevalent disease, especially in patients with risk factors such as cancer. The treatment for this condition is anticoagulation, although it is sometimes contraindicated, making it necessary to implant an inferior vena cava filter to prevent the development of pulmonary embolism. The occurrence of pulmonary embolism in a patient using an inferior vena cava filter is rare (<2%), but since this therapeutic method is not exempt from complications, there is a discussion about the risk-benefit of its implementation.
Case presentation: A 47-year-old man with a history of grade IV glioblastoma and DVT in the left lower limb visited the emergency department of a tertiary care hospital in Logroño (Spain) due to an increase in the diameter of his left leg. Considering his DVT diagnosis, anticoagulant treatment was started, leading to an acute extra-axial hematoma in the convexity of the left frontal lobe as a complication. An inferior vena cava filter was implanted, but the patient developed pulmonary embolism with thrombus in the inferior vena cava and juxtarenal vein above the filter. The patient received anticoagulant therapy, recovered satisfactorily, and was discharged after 12 days of hospital stay with outpatient treatment.
Conclusion: The indication for inferior vena cava filter implantation in patients with DVT is limited, and it is therefore suggested to use it only in patients with acute venous thromboembolic disease and absolute contraindications to anticoagulants or in patients with inadequate results after anticoagulant therapy.
Introduction
Venous thromboembolism (VTE) is the third most common vascular condition after myocardial infarction and stroke. This condition includes deep vein thrombosis (DVT) and pulmonary embolism (PE), the latter being the most critical manifestation and the third leading cause of death in hospitalized patients (1). One of the main risk factors for developing PE is to suffer a DVT in the lower limbs.
Oncology patients are more susceptible to developing VTE because this disease induces a state of hypercoagulability; however, the appropriate treatment of cancer-associated VTE is controversial (2,3). For both the general population and oncology patients, the mainstays of treatments for VTE are anticoagulants and mechanical cava prophylaxis via inferior vena cava filter implantation (IVCF) (3). The use of these devices has increased in recent years, especially in patients in whom anticoagulant therapy is contraindicated. However, it is important to keep in mind that this technique could lead to complications, which are usually minor and include bleeding at the access site, hematoma, accidental arterial puncture, and infection. The rate of major complications is usually <1% (4) and the frequency of occurrence of PE is <2% (5) in patients with IVCF.
The objective of the present work was to describe a clinical case of PE in a patient with IVCF, who recovered satisfactorily.
Case presentation
A 47-year-old male with a history of craniotomy due to grade IV glioblastoma located in the frontal area of the brain, visited the emergency department of a tertiary care hospital in Logroño (Spain) 10 days after this procedure due to an increase in the diameter of his left lower limb. The patient did not have dyspnea or other associated symptomatology and on admission physical examination reported pain in the left calf with positive Homans sign. No circulatory alterations or change in temperature or coloration of the affected limb were observed.
To complete the study, a blood test was performed, revealing leukocytosis (13 700/mm3), neutrophilia (12 200/mm3), and elevated D-dimer levels (5975 ng/mL, normal value: 0-240 ng/mL). In view of these results, on the same day of admission, a Doppler ultrasound was performed, which revealed thrombosis in the proximal tibiofibular joint and complete occupation of the left popliteal vein. No alterations were seen in the superficial and common femoral veins, nor in the saphenous vein.
On the same day of admission, the patient was hospitalized and referred to the angiology and vascular surgery service to start anticoagulant treatment with enoxaparin at a dose of 1.5mg/kg (100mg/24 hours subcutaneous). A coagulation factor test was also performed, which ruled out an increase in factor VIII, and an antithrombin III blood test ruled out a decrease in the levels of this protein.
One day after admission, the patient presented with headache, so a computed axial tomography (CAT) scan of the brain was performed, revealing an acute extra-axial hematoma in the convexity of the left frontal lobe. This hematoma had a focus of acute intraparenquimatous hemorrhage of 10mm in the white matter in the area where the craniotomy was performed. The CAT scan also showed blood content in the descending portion of the occipital horn of the right lateral ventricle (Figure 1).
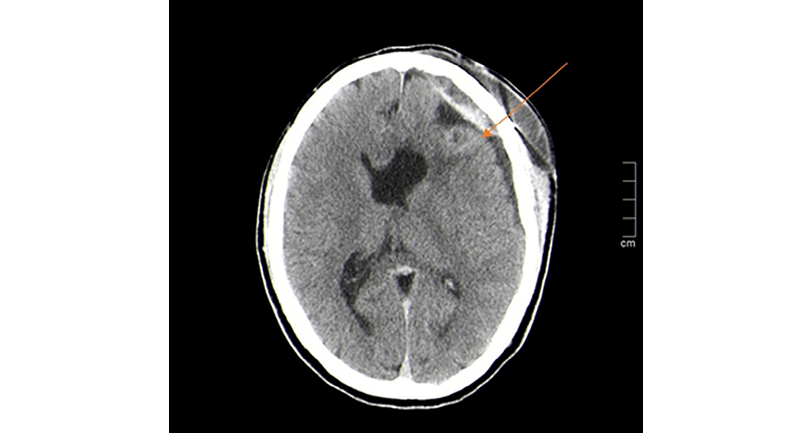
Figure 1. Computed axial tomography scan of the brain showing acute extra-axial hematoma in the convexity of the left frontal lobe with focus of acute intraparenquimatous hemorrhage (orange arrow).
Source: Image obtained while conducting the study.
On the fourth day of hospital stay, due to acute bleeding, anticoagulants were suspended and a filter was implanted via the femoral artery in the left inferior vena cava. Six days after this procedure, due to satisfactory progress, the patient was discharged without anticoagulant treatment.
Two days later, the patient was again admitted to the emergency department of the same institution with chest pain that increased with physical exertion. Physical examination revealed blood pressure of 123/86mmHg, heart rate of 84bpm, and oxygen saturation of 95%. A blood test was requested, which again revealed leukocytosis (16 600/mm3), neutrophilia (14 500/mm3), and elevated D-dimer levels (14 236 ng/mL).
Given the findings, a CAT scan of the abdomen and thorax was performed that same day, confirming bilateral PE with the presence of an extensive thrombus circulating between both main pulmonary arteries. In the right lung, thrombotic defects were observed in the right main artery, the lobar and segmental arteries of the right upper lobe, the middle lobe artery, the interlobar artery, and the segmental and subsegmental arteries of the right lower lobe. In the left lung, thrombotic defects were observed in the left pulmonary artery, the lobar and segmental arteries of the left upper lobe, the lingual artery, and the lobar and segmental arteries of the left lower lobe. The CAT scan also showed hemodynamic involvement in the right side of the heart with a right ventricular/left ventricular ratio >1 (Figure 2). In the abdomen, a thrombus was observed in the inferior vena cava and juxtarenal vein above the filter.
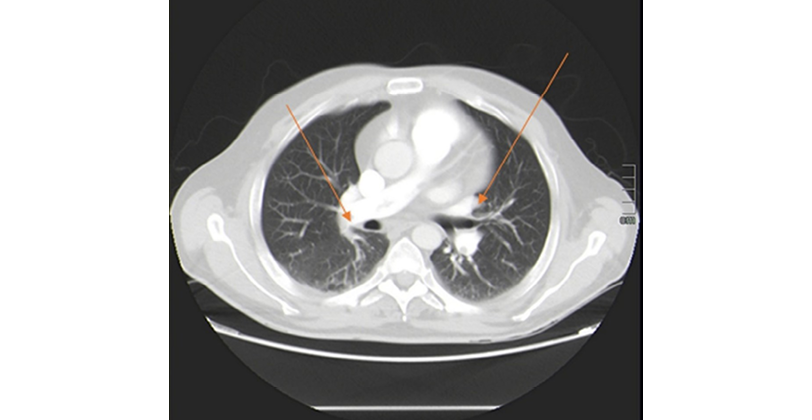
Figure 2. Computed axial tomography of the chest showing bilateral pulmonary embolism. Arrows indicate areas of thrombosis.
Source: Image obtained while conducting the study.
Due to the history of intraparenquimatous hemorrhage, the patient was hospitalized again on the same day of the second admission, was informed of the risk of starting anticoagulant treatment, and was referred to the pulmonology department, which started treatment with acenocoumarol 4mg based on the patient’s progress. This drug was chosen because of the possibilities of faster reversal of its effect and stricter control of anticoagulation levels. Five days after starting treatment, a new CAT scan of the abdomen and thorax was performed, which revealed that the intraparenquimatous hemorrhage had not increased, so anticoagulant management was modified to subcutaneous enoxaparin at a dose of 1.5mg/kg (100mg/24 hours subcutaneous) indefinitely. Due to the high risk associated with the surgery, the IVCF was not removed and, after a 12-day hospital stay, the patient was discharged with follow-up by the outpatient pulmonology, neurology and vascular surgery clinics, where he showed adequate progress.
Discussion
PE is one of the most important complications of DVT. To prevent this condition, physicians may order anticoagulant treatment or implantation of IVCFs. The latter is indicated in patients with VTE who cannot receive anticoagulants, as was the case of the patient in the present case due to his history of cerebral hemorrhage, or in those in whom this treatment did not achieve the expected objective (6). However, it should be kept in mind that the use of these devices can cause complications such as the development of thrombosis in the filter, which has an incidence ranging between 5% and 30% (7).
DVT is most frequently located in the lower limbs, as in the patient of the present case, and is only observed in about 5-10% of cases in the upper limbs (8).
The risk of developing VTE is 2 to 20 times higher in oncology patients (9), in whom low-molecular-weight heparin (LMWH) has been found to be the best treatment as it reduces the risk of recurrence without increasing the risk of bleeding, although patients treated with LMWH or vitamin K antagonists often experience recurrent VTE (3). These findings are consistent with the present case, in which the patient had a history of grade IV glioblastoma and developed PE despite anticoagulant treatment with LMWH.
According to Pandhi et al. (3), it is possible that novel anticoagulants may be effective in the treatment and prevention of VTE in oncology patients, as factor Xa inhibitors, such as rivaroxaban, apixaban and edoxaban, and the factor IIa inhibitor dabigatran offer potential medium- to long-term anticoagulation with lower risk of bleeding than standard therapy. Similarly, in a systematic review and meta-analysis comparing the efficacy and safety of direct oral anticoagulants and LMWH, Mulder et al. (10) found that direct oral anticoagulants are an effective treatment option for patients with cancer and acute VTE, although they should be used with caution in patients at high risk of bleeding.
The use of IVCFs has proven to be an effective therapy in certain cases for the prevention of VTE, and its indications are divided into absolute or relative. The former are mainly related to patients with contraindications to anticoagulant therapy, as in the present case given the patient’s history of intraparenquimatous hemorrhage, while the latter are related to patients with massive PE, limited cardiopulmonary reserve, floating inferior vena cava thrombus, or poor compliance with anticoagulant therapy (11). In the literature, the use of IVCFs is controversial due to the lack of prospective studies.
In a systematic review and meta-analysis including 11 studies, Bikdeli et al. (12) found that patients undergoing IVCF intervention have a lower risk of subsequent PE and an increased risk of DVT, but the use of these devices has no significant effect on overall mortality. However, it should be noted that the authors disclosed that their conclusions could be significantly biased due to differences between populations and the quality of the included studies.
In a case-control study aimed at determining the 1-year cumulative incidence of rehospitalization for DVT or PE among patients treated with IVCFs compared with a control population with VTE, White et al. (13) found that insertion of an IVCF was not associated with a significant reduction in the 1-year incidence of rehospitalization for PE but was associated with a higher incidence of rehospitalization for DVT in patients who initially manifested PE.
Liu et al. (14) conducted a systematic review and meta-analysis in which they included 7 studies for a total of 1274 patients, finding insufficient evidence to conclude that the use of IVCFs reduces mortality. However, they did observe that the incidence of PE decreased with the use of this device without increasing the rate of VTE or major bleeding. These findings are in contrast to what was observed in the patient in the present case, given that he developed PE despite IVCF implantation.
There is a possibility that the use of retrievable inferior vena cava filters (rIVCF) could reduce the incidence of PE and DVT; however, in prospective studies comparing both techniques, such as that of Kim et al. (15), no differences have been observed. It is worth noting that rIVCF are often not recovered in daily practice, especially in cancer patients, which increases the risk of complications associated with these devices (16).
Conclusions
The indication for IVCF implantation in patients with DVT is limited, being suggested only in patients with acute venous thromboembolic disease and absolute contraindications to anticoagulants, or in patients with inadequate results after anticoagulant therapy. If this treatment is implemented, possible complications such as filter thrombosis and development of PE should be considered. Future prospective studies are required to better understand the prevalence of this entity, as well as its associated mortality.
Ethical considerations
The patient’s informed consent was obtained for the preparation of this case report.
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgments
None stated by the authors.
References
1.Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388(10063):3060-73. https://doi.org/gjjw6f.
2.Becattini C, Agnelli G. Risk stratification and management of acute pulmonary embolism. Hematology Am Soc Hematol Educ Program. 2016;2016(1):404-12. https://doi.org/f964jj.
3.Pandhi MB, Desai KR, Ryu RK, Lewandowski RJ. The Role of Inferior Vena Cava Filters in Cancer Patients. Semin Intervent Radiol. 2016;33(2):71-4. https://doi.org/mrpx.
4.Van Ha TG. Complications of inferior vena caval filters. Semin Intervent Radiol. 2006;23(2):150-5. https://doi.org/dn94wm.
5.Andreoli JM, Lewandowski RJ, Vogelzang RL, Ryu RK. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol. 2014;25(8):1181-5. https://doi.org/f6cbfd.
6.Weinberg I, Kaufman J, Jaff MR. Inferior vena cava filters. JACC Cardiovasc Interv. 2013;6(6):539-47. https://doi.org/f46kvv.
7.Telich-Tarriba JE, Bolaños-Jiménez R, Arizmendi-Vargas J, Martínez-Schulte A. Trombosis masiva posterior a la colocación de filtro en vena cava inferior. Reporte de un caso. Rev. Fac. Med. (Méx.). 2019;62(1):19-22. https://doi.org/mrww.
8.Kastora SL, Oduyoye O, Mahmood S. Upper extremity deep venous thrombosis prevalence in the NHS Grampian Medical Ambulatory clinic: diagnostic, therapeutic, and prognostic considerations in oncology patients. Ir J Med Sci. 2022;191(4):1569-75. https://doi.org/mrw4.
9.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-64. https://doi.org/gqj5hg.
10.Mulder FI, Bosch FTM, Young AM, Marshall A, McBane RD, Zemla TJ, et al. Direct oral anticoagulants for cancer-associated venous thromboembolism: a systematic review and meta-analysis. Blood. 2020;136(12):1433-41. https://doi.org/mrxb.
11.Stern JR, Cafasso DE, Meltzer AJ, Schneider DB, Ellozy SH, Connolly PH. Prophylactic Inferior Vena Cava Filter Utilization and Risk Factors for Nonretrieval. Vasc Endovascular Surg. 2018;52(1):34-8. https://doi.org/gchtpp.
12.Bikdeli B, Chatterjee S, Desai NR, Kirtane AJ, Desai MM, Bracken MB, et al. Inferior Vena Cava Filters to Prevent Pulmonary Embolism: Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2017;70(13):1587-97. https://doi.org/gbzb35.
13.White RH, Zhou H, Kim J, Romano PS. A population-based study of the effectiveness of inferior vena cava filter use among patients with venous thromboembolism. Arch Intern Med. 2000;160(13):2033-41. https://doi.org/bn9d2k.
14.Liu Y, Lu H, Bai H, Liu Q, Chen R. Effect of inferior vena cava filters on pulmonary embolism-related mortality and major complications: a systematic review and meta-analysis of randomized controlled trials. J Vasc Surg Venous Lymphat Disord. 2021;9(3):792-800.e2. https://doi.org/gpvx4v.
15.Kim HS, Young MJ, Narayan AK, Hong K, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008;19(3):393-9. https://doi.org/d7fdsh.
16.Peterson EA, Yenson PR, Liu D, Lee AY. Predictors of attempted inferior vena cava filters retrieval in a tertiary care centre. Thromb Res. 2014;134(2):300-4. https://doi.org/f6f743.