
Magda Torres1, Yuri Chipatecua2, Diana Maritza Marulanda Cardona3 y Jhon Jairo Olaya Florez4
1 Engineering Physics. M.Sc., In Materials and Processes, Universidad Nacional de Colombia. mtorresluque@gmail.com
2 Chemical Engineering, Universidad América, Bogotá, Colombia. M.Sc., In Materials and Processes, Universidad Nacional de Colombia, Bogotá, Colombia. Universidad Nacional de Colombia. ylchipatecuag@unal.edu.co
3 Electronic Engineer. Ph.D., Student in Materials Science and Technology, Universidad Nacional de Colombia. Bogotá, Colombia. Universidad Nacional de Colombia. dmaritzamc@gmail.com
4 M.Sc., Materials and Processes, Universidad Nacional de Colombia. Ph.D., in Engineering, Universidad Nacional Autónoma de México. Universidad Nacional de Colombia.jjolayaf@unal.edu.co
ABSTRACT
This work was aimed at comparing the corrosion resistance of CrN and CrN/Cr coatings deposited through unbalanced magnetron sputtering (UBM), Cr industrial coatings and epoxy paints. UBM coatings were optimised and produced at room temperature, using 400 mA discharge current. Ar and N2 fl rates were set at 9 standard cubic centimetres per minute (SCCM) and 3 SCCM, respectively. Deposition times were set to produce CrN monolayers and nanometric multilayers having 1 μm total thickness and 100 nm period. Coating microstructure was determined through scanning electron microscopy as texture and crystalline phases were determined using x-ray diffraction and infrared spectroscopy. Corrosion resistance was studied with anodic polarisation tests using 0.5M H2SO4 and 0.05M KSCN solution. Nanometric multilayers improved stainless steel corrosion resistance and it was observed that coated CrN steel A36 could be an alternative for replacing stainless steel in acid environments. Corrosion mechanisms for the coatings so deposited are discussed.
Keywords: CrN, hard chromium, multilayer, corrosion, UBM.
Received: april 20th 2009
Accepted: november 15th 2010
Introduction
The electrochemical technique for producing chromium coatings is the most common one in Colombia (also called galvanoplasty) due to its low cost, high efficiency and mass production possibility. However, it is environmentally harmful and deposition conditions are difficult to control. This is why coatings and techniques for substituting hard chromium have been studied during the last few decades, trying to keep the technique´s advantages but improving properties such as corrosion resistance (Alfonso, Torres et al., 2009). On the other hand, industries using galvanoplasty are being pressed by environmental authorities to control contamination of the residual water used in the electrochemical manufacturing of coatings. For example, electrodeposited hard chromium produces highly toxic waste contaminating water and air. Cr+6 used in electrochemical deposition has been confirmed as being carcinogenic in humans and constitutes an environmental contamination source (Alfonso, Torres et al., 2009).
This is why several countries eliminated its use back in the 1980s and 1990s. There are alternatives for replacing electrochemical chromium but the most studied ones are related to physical vapour deposition (PVD) and chemical vapour deposition (CVD). Amongst PVD processes, unbalanced magnetron sputtering (UBM) can be used for producing chromium (Lee, 2006). This technique allows depositing nanometric Cr, CrN monolayers and CrN/Cr multilayers having higher wear and corrosion resistance than hard chromium.
CrNx coatings have replaced TiN coatings in several applications because their mechanical properties are similar, but CrNx has better oxidation resistance at high temperatures and deposition rates are up to 3 times higher than TiN (Olaya, Rodil et al., 2005). Preliminary studies have shown that CrN has high density leading to be-tter corrosion resistance compared to galvanic layers so that CrN could replace electrochemical hard chromium, mainly because of its higher hardness and corrosion resistance (Olaya, Rodil, et al., 2006).
Nanometric multilayers significantly increase corrosion, wear and fatigue resistance (Olaya, Rodil et al., 2005). This is mainly due t nano-multilayer interface enhancement and interaction which behave differently regarding their volume or higher thickness monolayer counterparts (Flores, 2004; Kot, Rakowsi et al., 2008). It is known that nano-multilayers prevent dislocation movement as this produces plastic deformation in materials and the propagation of micro-cracks responsible for fractures in ceramics (Zhang and Liu, 2009). Re-nucleation present in multilayer structures reduces the amount of pores, thereby resulting in better corrosion resistance.
The aim of this research was thus to study the corrosion behaviour of UBM deposited nanometric scale crystalline CrN films and CrN/Cr multilayers. The advantage of using UBM was the possibility of systematically varying ionic bombardment (ion energy and flow) for producing high quality nano-multilayers regarding hardness, adhesion, toughness and density. Industrial coatings widely used in Colombia were used to determine CrN and CrN/CrN coating performance. It was thus possible to establish UBM coating applicability as an efficient and environmental alternative for replacing electrochemical coatings.
Experimental setup
Coatings deposition
CrN and Cr coatings were produced through UBM using a 10 cm diameter chromium target (99.9 % purity). Working pressure was 5 x 10-1 Pa and all coatings were grown at room temperature using 400 mA discharge current and ∼150 W discharge power for Cr deposition as ∼160 W was the discharge power for CrN coatings. Ar and N2 flow rates were set at 9 standard cubic centimetres per minute (sccm) and 3sccm, respectively. Target-sample distance was set at 5 cm and deposition time was adjusted to obtain ~1 μm thickness for multilayers with 20 nm bilayer period.
The coatings obtained using such deposition conditions presented FCC crystalline phase and good substrate adherence. A Cr buffer layer was deposited before CrN layer to improve adhesion. CrN coatings were simultaneously deposited on steel (AISI 304, ASTM A36) and silicon (100) as nanometric multilayers were deposited on AISI 304 and silicon (100). Steel substrates were mirror polished and ultrasonically cleaned in acetone and alcohol in sequence for 1 min before being placed in the deposition chamber. Table 1 presents the chemical composition for the steel used as substrate.

Electrochemical coatings were provided by ALFACROM S.A. (Colombia) and the paint system was provided and applied by SIKA Colombia S.A.; it consisted of red epoxy resin (code 137008) and urethane enamel (A36 series). Both coatings were deposited on ASTM A36.
Corrosion resistance was evaluated in selected systems using these coatings and considering good substrate adherence, as shown in Table 2.

Electrochemical test
Corrosion resistance was evaluated by polarisation test using ASTM G5 standard reference (ASTM G5-87, 1999). A saturated calomel electrode (SCE) and high purity graphite were used as reference and counter electrodes, respectively. The exposed area was 0.8 cm2. After 45 minutes of immersion in a 0.5M H2SO4 + 0.05M KSCN electrolyte, scans were conducted in the -0.3 to 1.0 V range, having a 30 mV/min potential sweep (Chou, Yu et al., 2003; Kaciulis, Mezzi et al., 2006). Corrosion velocity and potential were obtained by Tafel´s extrapolation (Meas, 2008) using a Gamry reference 600 potentiostat- galvanostat
The corrosion products and cross-sectional morphology of coatings grown on silicon were studied using a FEI-KUANTA 200 scanning electron microscope (SEM) operating at 30 kV. Chemical studies before and after chemical attack were performed using the same equipment operating at 20 kV in EDS mode. Coating thickness was measured using a DEKTAK 150 profilometer.
Characterisation
Coatings were structurally studied by x-ray diffraction (XRD) using an X-PertPro panalytical system in the grazing incidence and Bragg-Brentano configuretions and using monochromatised CuK radiation (1.540998 Å) working at 45 kV and 40 mA. Infrared studies were performed on IR Perkin Elmer Paragon 500 equipment.
Results and discussion
Figure 1 presents XRD patterns for CrN/SS, CrN/Cr/SS and hard chromium coatings. The CrN pattern was characterised by FCC NaCl phase (Barshilia, Selvakumar et al., 2006; Inoue, Okada et al., 2002) preferential orientation (111). The presence of CrN (200) and (111) and Cr BCC phase with preferential orientation (110) was observed for CrN/Cr multilayers (Saravanan and Mohan, 2009).

Figure 2 shows IR spectrum for paints. Alcohol (3,364.2 cm-1) and epoxy (1,240.4 cm-1 and 1,111.9 cm-1) functional group peaks were observed; these are characteristic for epoxy resin. Aromatic (1,637.4 cm-1 and 1,519.5 cm-1) and ester (1724.4 cm-1) functional groups which are basic components of urethane enamel were also observed. Other peaks were present due to enlargement or flexion of more common organic functional groups such as alkenes (876.4 cm-1) or single carbon-hydrogen bonds (2,926.1 cm-1, 2,858.4 cm-1 by enlargement and 1,454.1 cm-1, 1,380.1cm-1 by flexion) which are present in both system components (Silverstein, 2005, pp. 71-111).

Cross-sectional microstructure is shown in Figure 3.(a) shows CrN monolayer presenting a dense film of compact columnar grains. Figure 3(b) corresponds to CrN/Cr deposited with 20 nanolayers, homogeneous layers and columnar growth being observed with no equiaxial grains, starting from the interface without interruption when changing layer. White films correspond to Cr and gray to CrN.

Figure 4 presents CrN, CrN/Cr and hard chromium coating surface microstructure. Each coating revealed different morphology; for example, hard chromium coatings presented nodular structure and high roughness, while UBM-deposited coatings were more homogenous and less rough. This was possibly due to deposition parameters. UBM-deposited coatings were produced with higher adatom mobility in high vacuum which decreases impurities (Ahn, Choi et al., 2002).

This promotes formation of more dense and less rough films Potentiodynamic polarisation test results (Tafel polatisation) are shown in Figure 5. Table 3 presents potential and current density for the systems studied. CrN coatings reduced current density for both steels (A36 and AISI 304); however, they did not improve corrosion protection. This could have been due to the porous columnar microstructure obtained for CrN films that is common for PVD coatings and makes them permeable, resulting in accelerated pitting corrosion by the formation of a galvanic pair: noble coating active substrate (Kaciulis, Mezzi et al., 2006; Liu, Bi et al., 2003; Bertrand, Mahdjoub et al., 2000)

On the other hand, CrN/Cr/SS and paints presented lower Icorr values. The good behaviour of nanometric multilayers was probably due to the re-nucleation generated layer by layer that reduced the size and amount of pores reducing paths for the acid solution to reach the substrate. Paints presented lower Icorr values which were a direct response to their organic chemical composition, which is inert to several corrosion environments. These measurements need to be complemented by loss weight tests and other electrochemical techniques for studying their behaviour for longer periods of time in different corrosive environments. Hard chromium coatings did not improve steel A36 corrosion resistance, probably due to the high porosity common for these coatings. Nevertheless, hard chro-mium coatings could improve steel durability in different corrosion environments, which needs further investigation.
Figure 6 presents coating morphology after corrosion tests. Figure 6 (a) and 6(b) show steel corrosion products on coating defects. Corrosive solution becomes diffused towards the substrate through these defects, producing film-substrate interface corrosion and loss of adhesion (Liu, Bi et al., 2003).
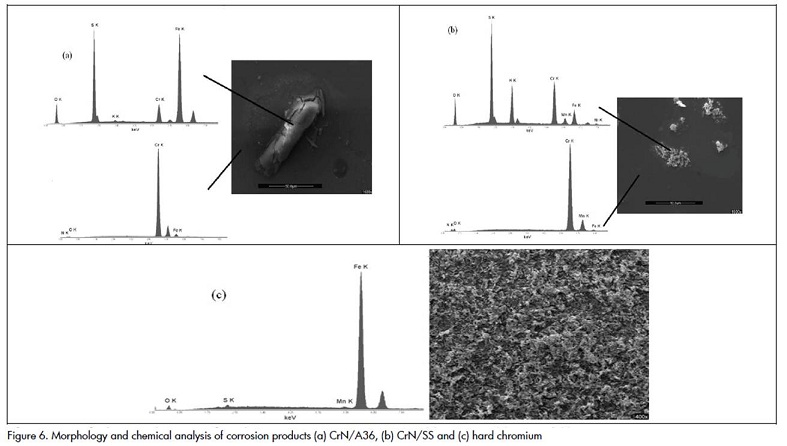
This phenomenon is known as galvanic pair and is formed as a consequence of Ecorr disparity between coating and steel. (c) clearly shows total hard chromium coating corrosion because of the acid solution, thereby recommending that this coating not be applied to acid environments.
Conclusions
CrN and CrN/Cr coatings were successfully produced using the unbalanced magnetron sputtering technique. Stainless steel corrosion resistance was improved using nanometric Cr and CrN coatings and they are thus proposed as an environmental alter-native for replacing electrochemical hard chromium in some applications. Another alternative for industrial applications is the use of CrN coated steel A36 as a replacement for stainless steel in acid environments.
Corrosion was produced in the coatings when the corrosive solution diffused through coating defects thereby generating localised corrosion because of the potential disparity between coating and substrate This led to an accelerated attack on the filmsubstrate interface and subsequent coating delamination
Paints presented the best performance in acid environments in the test conditions used in this research. However, further measurements are needed to evaluate their performance when temperature and corrosion test time are varied.
Acknowledgments
This research was carried out with financial support provided by the Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnología (COLCIENCIAS) through projects CT 206-2006 and Universidad Nacional de Colombia DIB grant 21101009338