STRUCTURAL AND MAGNETIC PROPERTIES STUDY OF Nd16Fe76—xNixB8 ALLOYS WITH LOW Ni CONTENTS
ESTUDIO DE LAS PROPIEDADES MAGNETICAS Y ESTRUCTURALES DE ALEACIONES Nd16Fe76—xNixB8 CON BAJOS CONTENIDOS DE Ni
Juana M. Jiménez, Germán Y. Vélez, Ligia E. Zamora and Germán A. Pérez
Departamento de Física, Universidad del Valle, A.A. 25360, Cali, Colombia
(Recibido: 04/2012. Aceptado: 06/2012)
Contacto: Germán Y. Vélez gpgeperez@gmail.com
Cómo citar: Jiménez, J.M., Vélez, G.Y., Zamora, L.E. & Pérez, G.A., Momento 44, 11 (2012)
Abstract
In this work we report a magnetic and structural study of Nd16Fe76—xNixB8 (x = 0, 2.5, 5, 7.5 and 10) alloys. This system was investigated by means of x–ray diffraction,¿ Mössbauer spectrometry, and magnetization. The samples were melted in an arc furnace and then annealed during three days at 1000 °C. The results show the majority formation of the hard Nd2Fe14B tetragonal phase with lattice parameters a = 8.810 Å and c = 12.210 Å; these parameters do not vary substantially with the addition of Ni. Mössbauer results show a ferromagnetic contribution with six sites of iron associated to the Nd2Fe14B phase and a paramagnetic contribution as a doublet attributed to the Nd1.1Fe4B4 phase. The hysteresis cycles show that all the samples present a hard magnetic behavior, and the additio of Ni decreases this property.
Keywords: Magnetism, Mössbauer spectrometry, Nd2Fe14B permanent magnets, magnetic materials.
Resumen
En este trabajo reportamos las propiedades magnéticas y estructurales de las aleaciones Nd16Fe76—xNixB8 (x=0, 2.5, 5, 7.5 y 10). Este sistema fue investigado por medio de difracción de rayos–x, espectrometría Mössbauer y magnetización. Las muestras fueron fundidas en un horno a arco y luego recocidas a 1000 °C durante tres días. Los resultados muestran la formación mayoritaria de la fase tetragonal dura Nd2Fe14B con parámetros de red a=8.810Å y c=12.210 Å; estos parámetros no varían sustancialmente al agregar Ni. Los resultados Mössbauer muestran una contribución ferromagnética constituida por seis sitios de hierro asociados a la fase Nd2Fe14B y una contribución paramagnética en forma de doblete atribuida a la fase Nd1.1Fe4B4. Los ciclos de histéresis muestran que todas las muestras presentan un comportamiento magnético duro, y al agregar Ni esta propiedad disminuye.
Palabras clave: Magnetismo, espectrometría Mössbauer, imanes permanentes de Nd2Fe14B, materiales magnéticos.
Introduction
Many researchers have been very attracted for permanent magnets development since the discovery of the Nd2Fe14B compound in 1984 [1]. Nanocomposite hard magnetic materials consist of a mixture of magnetically hard phase, like Nd2Fe14B, and soft phases, like α–Fe or Fe2B, with nanometric size, and they show a big increase of the remanence due the exchange interaction between these two phases [2]. The nanocomposite hard magnetic compound name was proposed by Kneller and Hawig [3]. The magnets based onvthe Nd2Fe14B are obtained by different preparation methods like magnetron sputtering [4, 5], melt–spinning [6, 7], mean beam epitaxy (MBE) [8], inductive melting followed by melt–spinning [9] and annealing [10], sintering [11, 12], and mechanical alloying [13–15]. The development of the current permanent magnets has involved a great effort to obtain an improvement in its magnetic properties, for example, substituting the Fe by magnetic or no–magnetic elements like Co, Ni, Mn, Ru and Al, or also by the substitution of Nd by rare earths such as Dy and Tb [16–18].
The nanocomposite permanent magnets are interesting because they exhibit unusual properties, experimentally detected [19, 20] and theoretically modeled [21–23], such as: remanence relation )Mr/Ms) larger than 0.5, coercive field values (Hc) between 2 and 4 kOe, and a maximum energy product (BH)max given by 14 MGOe [24]. The previous properties are sensible to the type of elements which form the hard material [25–30] as well as the soft material [25, 31], to the grain size of the hard material [18, 26, 27, 32–34], and the different techniques which had been used in the materials preparation [4–15]. Previously, some studies reported that powders of NdFeB present a majority phase of Nd2Fe14B, which shows a ferromagnetic behavior joined with another minority paramagnetic phase of Nd1.1Fe4B4 [35].
The purpose of present study is to replace Fe by Ni atoms in alloys with stoichiometry given by Nd16Fe76—xNixB8 (x=0, 2.5, 5.0, 7.5 and 10), and to investigate the Ni effect in the structural and magnetic properties of the system. In this work we report the experimental results obtained by x–rays diffraction (XRD), Mössbauer spectrometry (MS), and magnetization techniques.
Experimental
Samples with nominal compositions Nd16Fe76—xNixB8 (x=0, 2.5, 5.0, 7.5 and 10) were prepared by arc–melting under argon atmosphere. The purity of the Nd, Fe, Ni, and B elements were bigger than 99.9%. After melting the samples, they were encapsulated in evacuated quartz tubes and annealed for three days at 1000 °C. The samples were characterized by X–ray diffraction (XRD) using the Cu–Kα radiation and the patterns were refined by using the MAUD program [36]. The Mössbauer spectra were taken on a conventional spectrometer with a 57Co/Rh source of 25 mCi, and the spectra were fitted by using the MOSFIT program [37]. All the studies were realized at room temperature.
Results and discussion

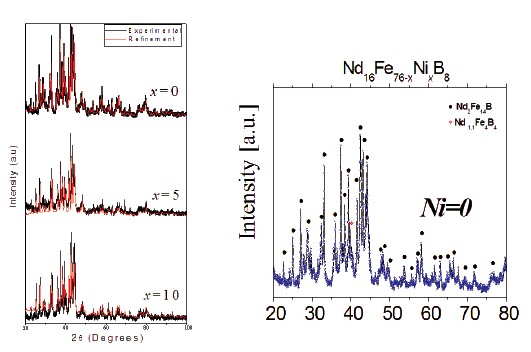
Figure 1. X–ray diffraction patterns of the Nd16Fe76—xNixB8 (x = 0, 5.0 and 10) samples.
The XRD analysis was carried out for all prepared Nd16Fe76—xNixB8 (x=0, 2.5, 5.0, 7.5, and 10) alloys, and some typical patterns with their corresponding refinements are shown in Fig. 1a. All the samples present principally the peaks of the Nd2Fe14B tetragonal phase (space group P42/mmm), and also the peaks of another minority phase (less than 2%) associated to the Nd1:1Fe4B4 phase (Fig. 1b).
Rietveld analysis permit to calculated structural parameters such as lattice parameters and the crystallite size (ø). These structural parameters are listed in Table 1. The lattice parameters a and c of the Nd2Fe14B phase remain approximately constant with the variation of the nickel concentration, meaning that Ni atoms do not induce a significant change on the structure of this phase. This can be attributed to the similar atomic size of the Ni and Fe atoms. The mean crystallite size varies between 50 and 60 nm without any tendency. These obtained values show the nanostructured character of the samples.
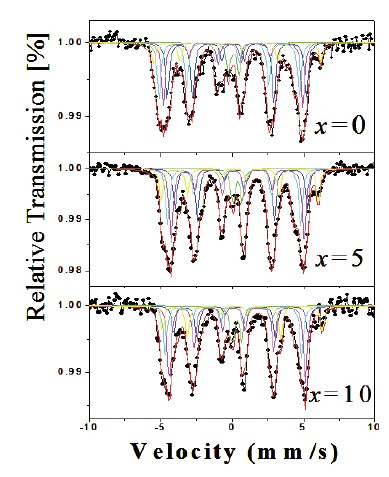
Figure 2. Mössbauer spectra of the Nd16Fe76—xNixB8 (x = 0, 5, and 10) samples.
Fig.2 shows the 57Fe Mössbauer spectra collected at room temperature of Nd16Fe76—xNixB8 (x=0, 5.0 and 10) samples. All spectra were best fitted with seven sub–spectra, one paramagnetic component (doublet) associated to the Nd1.1Fe4B4 phase [35] and
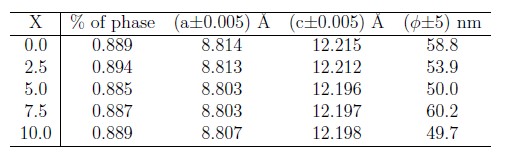
Table 1. Structural parameters of Nd16Fe76—xNixB8 (x = 0, 2.5, 5.0, 7.5 and 10) samples.
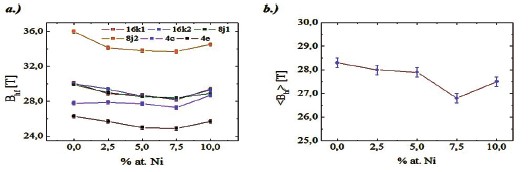
Figure 3. Behavior with the Ni concentration of a) the hyperfine magnetic field of the different sites of iron, and b) of the mean hyperfine field of the Nd16Fe76—xNixB8 alloys.
six sextets with hyperfine parameters, which correspond to the six non–equivalent Fe sites (16k1, 16k2, 8j1, 8j2, 4c, and 4e) associated to the Nd2Fe14B hard magnetic phase [18, 34, 38]. In all samples the hard Nd2Fe14B phase has a spectral area bigger than 90 %. Fig. 3a shows the behavior of the hyperfine magnetic field as a function of the Ni content for each one of the iron sites of the hard phase. All they slowly decrease with the Ni concentration increase up to 7.5 %, and then they present a small increase between 7.5 and 10 at. % Ni. The behavior of the mean hyperfine magnetic field, as a function of Ni content, is shown in Fig. 3b. This behavior is similar to that shown by the ferromagnetic sites, however the decrease up to 7.5 at. % Ni and the increase after this concentration are more accentuated.
In Fig. 4 is shown the obtained isomer shift values versus Ni content, for the different ferromagnetic sites of the hard phase. It
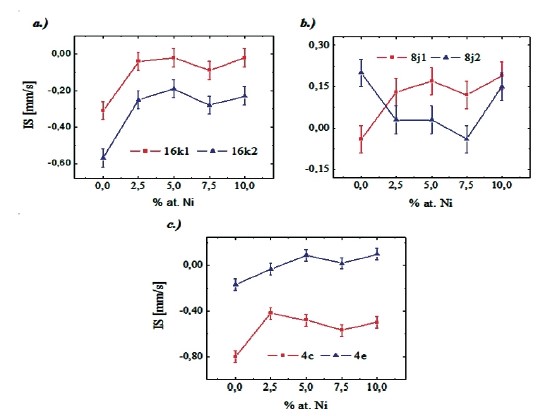
Figure 4. Isomer shift as a function of the Ni for the sites a) 16k1 and 16k2, b) 8j1 and 8j2, c) 4c and 4e.
can be noted that the isomer shift values of the different Fe sites of the Nd2Fe14B structure increase with the nickel concentrations up to 2.5 at. % Ni and then tends to remains constant thereafter. This is true for all the sites except the 8j2 one. These results indicate that nickel atoms replace mainly iron atoms in the majority of the sites, for the low nickel concentrations, and the effect of this substitution is an increase of the 3d electron density of the Fe atoms, which repeal the s electrons at the Fe nucleus decreasing in this way its density and then increasing isomer shift. For the 8j2 site it is possible that it must be enriched by Fe atoms explained in this way why its isomer shift decreases. Figure 5 shows the obtained hysteresis cycles of all the samples. It can be noted that the samples present cycles which saturated until an applied field of 15000 kOe (1.5 T) and this indicate that the Nd1.1Fe4B4 phase do not contribute appreciably with its paramagnetic character.
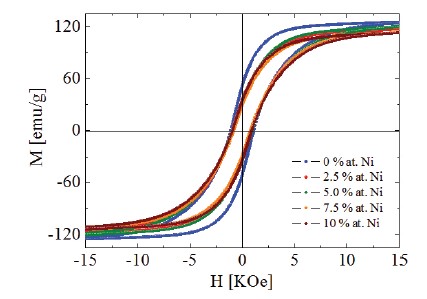
Figure 5. Hysteresis cycles of the Nd16Fe76—xNixB8 (x = 0, 2.5, 5.0, 7.5, and 10) samples.
The obtained values of the saturation magnetization vary from 130 to 105 emu/g and that of the coercive fields vary from 1.1 to 0.7 kOe when Ni content changes from 0 to 10 at. %. These results and that of the mean hyperfine field vs Ni concentration permit us to conclude that the samples behave as a magnetically hard magnet but the Ni presence inside them decreases this hard magnetic behavior.
Conclusions
It has been shown that the Nd16Fe76—xNixB8 alloys (with x(0, 2.5, 5.0, 7.5 and 10) consist of hard magnetic Nd2Fe14B phase and a paramagnetic Nd1.1Fe4B4 phase. The substitution of Fe by Ni in the Nd16Fe76B8 phase does not change its crystallographic structure but decreases the magnetic and hyperfine properties.
Acknowledgements
The authors would like to thank Universidad del Valle for financial support for the realization of this work.
References
[1] J. J. Croat, J. F. Herbst, R. W. Lee, and F. E. Pinkerton, J. Appl. Phys. 55, 2078 (1984).
[2] Z. Chen, Y. Zhang, Y. Ding, G. C. Hadjipanayis, Q. Chen, and B. Ma, J. Magn. Magn. Matter. 195, 420 (1999).
[3] E. Kneller and R. Hawig, IEEE Trans. Magn. 27, 3588 (1991).
[4] T. Fukagawa, T. Ohkubo, S. Hirosawa, and K. Hono, J. Magn. Magn. Matter. 322, 3346 (2010).
[5] W. Cui, Y. Takahashi, and K. Hono, Acta Materialia 59, 7768 (2011).
[6] T. Saito, J. Alloy Compd. 505, 23 (2010).
[7] Z. Chen, D. Miller, and J. Herchenroeder, J. Appl. Phys. 107, 09A730 (2010).
[8] D. J. Keavney, E. E. Fullerton, J. E. Pearson, and S. D. Bader, J. Appl. Phys. 81, 4441 (1997).
[9] T. Nishio, S. Koyama, Y. Kasai, and V. Panchanathan, J. Appl. Phys. 81, 4447 (1997).
[10] M. Corfield, A. Williams, and I. Harris, J. Alloy Compd. 296, 138 (2000).
[11] M. Yue, M. Tian, J. Zhang, D. Zhang, P. Niu, and F. Yang, Mater. Sci. Eng.: B 131, 18 (2006).
[12] S. Pandian, V. Chandrasekaran, G. Markandeyulu, K. J. L. Iyer, and K. V. S. R. Rao, J. Appl. Phys. 92, 6082 (2002).
[13] A. Przybyt, I. Wnuk, P. G?bara, and J. Wystocki, J. Achievements in Materials and Manufacturing Engineering 49, 210 (2011).
[14] V. Pop, S. Gutoiu, E. Dorolti, O. Isnard, and I. Chicinas, J. Alloy Compd. 509, 9964 (2011).
[15] V. Neu and L. Schultz, J. Appl. Phys. 90, 1540 (2001).
[16] J. L. Breton and J. Teillet, J. Magn. Magn. Matter. 101, 347 (1991).
[17] S. Hirosawa and M. Sagawa, Proc. MRS Int. Meet. Adv. Mater. 11, 17 (1989).
[18] J. Valcanover, C. Paduani, J. Ardisson, C. S. Pérez, and M. Yoshida, Acta Materialia 53, 2815 (2005).
[19] D. Brown, Z. Chen, P. Guschl, and P. Campbell, J. Magn. Magn. Matter. 303, e371 (2006).
[20] N. G. Akdogan, G. C. Hadjipanayis, and D. J. Sellmyer, Nanotechnology 21, 295705 (2010).
[21] R. Fischer, T. Schre, H. Kronmüller, and J. Fidler, J. Magn. Magn. Matter. 150, 329 (1995).
[22] E. H. Feutrill, P. G. McCormick, and R. Street, J. Appl. Phys. 75, 5701 (1994).
[23] H. Fukunaga and H. Inoue, Jpn. J. Appl. Phys. 31, 1347 (1992).
[24] D. Edgley, J. L. Breton, S. Steyaert, F. Ahmed, I. Harris, and J. Teillet, J. Magn. Magn. Matter. 173, 29 (1997).
[25] W. C. Chang, S. H. Wu, B. M. Ma, and C. O. Bounds, J. Appl. Phys. 81, 4453 (1997).
[26] X. C. Kou, M. Dahlgren, R. Grössinger, and G. Wiesinger, J. Appl. Phys. 81, 4428 (1997).
[27] V. Villas–Boas, S. A. Romero, and F. P. Missell, J. Appl. Phys. 81, 4434 (1997).
[28] Z. Wang, S. Zhou, M. Zhang, and Y. Qiao, J. Appl. Phys. 88, 591 (2000).
[29] H. Yan, F. Kong, W. Xiong, B. Li, J. Li, and L. Wang, Int. J. Hydrogen Energ. 35, 5687 (2010).
[30] Y. Hou, Z. Xu, S. Peng, C. Rong, J. Liu, and S. Sun, Adv. Mater. 19, 3349 (2007).
[31] I. Betancourt and H. A. Davies, Mater. Sci. Eng. 26, 5 (2010).
[32] C. Y. You, Y. K. Takahashi, and K. Hono, J. Appl. Phys. 108, 043901 (2010).
[33] B. Cui, X. Sun, L. Xiong, S. Cao, X. Zhang, W. Liu, D. Geng, and Z. Zhang, J. Alloy Compd. 340, 242 (2002).
[34] D. Oyola Lozano, L. Zamora, G. Pérez Alcázar, Y. Rojas, H. Bustos, and J. Greneche, Hyperfine Interact. 169, 1253 (2006).
[35] M. Raja, A. Narayanasamy, and V. Ravichandran, J. Magn. Magn. Matter. 159, 345 (1996).
[36] L. Lutterotti and P. Scardi, J. Appl. Crystallogr. 23, 246 (1990).
[37] F. Varret and J. Teillet, "MOSFIT program", (2012), Unpublished.
[38] G. J. Long, F. Grandjean, and O. Pringle, J. Magn. Magn. Matter. 125, L29 (1993).