Introduction
According to the Global Cancer Observatory, gastric cancer (GC) is the fifth most common type of cancer and the fourth leading cause of death for this reason worldwide.1 GC in Colombia is considered a public health problem since it ranked fourth in incidence among all cancers in 2020 with 8 214 new cases and first in terms of mortality with 6 451 deaths in both sexes and all ages.2
Treatment of advanced GC consists of radical gastrectomy with removal of the perigastric nodes and the branches of the celiac trunk, which is combined with the administration of chemotherapeutic drugs; however, this treatment has a low success rate due to multidrug resistance.3,4 Some patients are diagnosed in advanced stages with unresectable cancers and their therapeutic options are limited to combination chemotherapy, palliative radiotherapy and perhaps the use of molecular targeted therapies in conjunction with immunotherapy, but the clinical benefit is variable and limited.5,6 In this regard, it has been reported that the median overall survival in GC patients treated with chemotherapy is 13-16 months and that they also have a poor quality of life due to toxicity, the occurrence of adverse effects, and the limited efficacy of these drugs as demonstrated through the occurrence of metastases and relapses.4,7-9 In view of the above, there is a need to develop new treatment options for patients with advanced GC.
Research on GC treatment has focused on the search for adjuvant or co-adjuvant biological therapeutic options. In this context, the field of virotherapy has emerged, focusing on the use of oncolytic viruses (OV) to treat solid tumors, with satisfactory results demonstrated in clinical trials due to its multimodal approach.10-12 OVs are attenuated viruses derived from prevalent pathogens or viruses that have been genetically modified to reduce their pathogenicity, enhance specificity towards cells with malignant phenotype, increase their oncolytic activity, and/or sensitize malignant cells to different chemotherapeutic agents.13-18
Rotavirus (RV) is a double-stranded non-enveloped RNA virus of the family Reoviridae that causes gastroenteritis.19,20 In particular, Guerrero et al.21 developed the RV Wt1-5 through a directed evolution experiment and using multiple serial passages of 5 wild-type isolates in cultures of different human tumor cell lines. In this study, the authors demonstrated that RV Wt1-5 selectively and more efficiently infects the tumor cell lines tested compared to the parental strains.21 Furthermore, other research has reported that the mechanisms of cell death induced by RV Wt1-5 are associated with increased cell membrane permeability, chromatin condensation and DNA fragmentation, suggesting that this rotavirus may induce cytotoxicity and apoptosis.21,22
However, some limitations arise when assessing the oncolytic potential of RV in primary cultures of cells isolated from gastric tumors:
i) Difficulty in disaggregating and isolating tumor cells due to tissue desmoplasia.
ii) Mechanical and/or chemical disaggregation methods that may stress cells and generate biochemical modifications that are likely to alter the assessment of susceptibility to
RV infection.
iii) Loss of much of the tumor heterogeneity, including extracellular matrix, immune system, and mucinous components present in the tumor microenvironment (TME).
iv) The limitation in the selection of a tumor marker with high sensitivity and specificity to characterize disaggregated tumor cells.
v) Increased risk of cell culture contamination due to mechanical manipulation and the use of enzymes.23
vi) The loss of histological architecture, biochemical signaling and interconnection between the wide diversity of cells with different degree of genomic instability and variability, both phenotypic and genotypic, present in any solid tumor.24
Given these limitations, the use of the explant culture method has been proposed as an ex vivo model that can provide more realistic results and offer more advantages in the preclinical evaluation of OVs.25,26 However, it remains necessary to consider the current challenges of virotherapy for solid tumors, such as insufficient selectivity toward tumor cells; inability to penetrate and disseminate in the tumor mass; premature viral clearance; low oncolytic potency; and poor stimulation of tumor-specific immunity.27 In addition, it should be noted that the efficacy of RV Wt1-5 has not been evaluated in an ex vivo explant model of solid tumors. Therefore, the objective of the present study was to evaluate the penetration ability, selectivity and oncolytic efficiency of RV Wt1-5 using an ex vivo infection model in tumor samples obtained from patients diagnosed with diffuse and intestinal gastric adenocarcinoma.
Materials and methods
Study type and sample
Experimental laboratory study performed on surgical specimens from the pyloric antrum obtained from 2 patients, a 54-year-old man with a diagnosis of diffuse gastric adenocarcinoma and a 64-year-old man with a diagnosis of intestinal gastric adenocarcinoma. Samples were collected after radical gastrectomy was performed at the Hospital Universitario de La Samaritana (HUS) in Bogotá, Colombia, in 2018. The tumors were >5cm in size and were poorly differentiated, which is consistent with a high tumor grade. In addition, the cancers were stage IIIB T3N1M0 and the patients had not received previous treatment for this disease.
Procedures
Histopathological analysis
The sample of gastric tumor tissue (>5cm) was collected, as well as unaffected gastric tissue located at the most distal end of the tumor. The sample was divided into two sections, one was used for histological diagnosis and the other for cellular disaggregation and obtaining explants for experimental evaluation by immunohistochemistry. Histopathological diagnosis was performed by embedding tissue sections in kerosene and staining with hematoxylin and eosin (H&E). Tumor histotype was categorized according to the criteria stipulated in the Lauren classification, which makes a distinction between two subtypes: intestinal and diffuse.28 Finally, the tumor-nodule-metastasis (TNM) system was used to establish cancer staging.29
Tissue preparation and culture
Both tumor and non-tumor surgical specimens were washed with RPMI 1640 medium. Then, under sterile conditions, in a Petri dish, necrotic tissue was removed and ∼2mm3-thick fragments were cut using scissors and a number 22 scalpel blade. For the primary cell culture study model (model 1), these fragments underwent enzymatic disaggregation with trypsin (5 mg/mL; Invitrogen, Madison, WI, USA) for 10 minutes at 37°C and 5% CO2, which were subsequently filtered through a Falcon™ 100µm Cell Strainer (Corning, NY, USA). The obtained cells were centrifuged at 700 x g for 7 minutes.
Cell count and viability were assessed by trypan blue exclusion staining (Sigma Chemical Co., St Louis, MO, USA) at 0.4% (%v/v) in a hemocytometer on inverted microscope with a 40x objective (Euromex, Arnhem, The Netherlands). Then, 5x104 cells per well were seeded in Costar® multiwell microplates in RPMI 1640 medium (Invitrogen, Carlsbad, USA) supplemented with 1% penicillin and streptomycin without fetal bovine serum (FBS) at 37°C with 5% CO2.
For the explant model (model 2), slices ∼2mm thick, equivalent to 8mm3 explants (∼0.1g), were distributed into different wells of a 12-well Corning™ flat-bottom cell culture plate with 3mL of RMPI 1640 medium supplemented with 1% penicillin and streptomycin without SFB.
Infection of tumor cells and gastric explants
RV Wt1-5 was obtained as described in the article by Guerrero et al.21 Viral titer was determined by analyzing the focus-forming units by serial dilution assay of the stock solution in the gastric cell line NCI-N87[N87] (ATCC®, CRL-5822).30 In addition, to evaluate whether gastric tumor cells were permissive to RV Wt1-5 infection, the following process was performed: 5x104 cells disaggregated from tumors or non-tumor tissue were seeded and, once adhered to the plate, were inoculated with RV Wt1-5 (MOI 0.8) activated with trypsin 1 μg/mL in RPMI medium without SFB; subsequently, they were incubated for 12 hours at 37°C and 5% CO2. Cells not inoculated with RV Wt1-5 were used as controls.
In the ex vivo infection model of gastric explants (model 2), RV Wt1-5 (MOI 0.8), diluted in 25µl of medium, was inoculated directly onto each explant, or phosphate buffered saline (PBS) was added and incubated for 1 hour at 37°C with 5% CO2. Subsequently, each explant was washed with RPMI 1640 medium to remove unbound virus.
Tissue sections were collected at 0, 12, 24, 24, 48 and 60 hours post infection (h.p.i). After each of these times, cultured cells and explants were fixed for 1 hour with 10% neutral buffered formalin with sodium acetate and analyzed by immunocytochemical assay using hyperimmune serum from rabbits (produced at the facilities of the Universidad Nacional de Colombia) to determine the presence of rotaviral antigens by evaluating structural proteins. Assays (in vitro viral replication) were performed on 3 independent samples, and each experiment (i.e., assay) was repeated twice (i.e., 3 experiments in total per sample); infected cells were used as control in all experiments.26,31
Immunohistochemical test
Cell cultures and fixed explants were embedded in kerosene and cut into 5μm thick sections, which were subjected to immunohistochemical analysis using the Ventana BenchMark XT automated slide staining device (Ventana Medical Systems, Inc. - Roche Diagnostics Division Tucson, USA) containing hydrogen peroxide substrate based on non-biotin horseradish peroxidase multimer and 3.3’-diaminobenzidine tetrahydrochloride chromogen (UltraViewTM Universal DAB Detection Kit, Ventana Medical Systems, Tucson, USA).
The following mouse monoclonal antibodies directed against human proteins were used: CEA (cat # GA622, dilution 1:100), Ck7 (cat # M7018, dilution 1:100), CA 19-9
(cat # M3517, dilution 1:100), CD4 (cat # M7310, dilution 1:100), as well as the rabbit polyclonal anti-CD3 antibody (cat # IS503, dilution 1:100) from Dako (Agilent Technologies, Inc. Santa Clara, CA, USA). In addition, hyperimmune serum from rabbits was used as primary antibody against RV structural proteins (1:1 000 v/v) for the detection of rotaviral antigens. The slides were counterstained with H&E.
The corresponding positive controls were used in all experiments, in addition to the internal positive controls. Slides were examined with a Bx51 TF optical microscope (Olympus Corporation) at 2x, 10x, 20x, 40x or 60x magnification, and image acquisition was performed with the aid of CellSens software (Olympus Corporation). Immunohistochemical reactivity was interpreted blindly by 2 independent investigators. Immunohistochemical analysis was performed using the ImageJ2 software version 2.3.0/1.53f. Finally, the immunohistochemical optical density score was determined using a scale of 1 to 4.32
Infection with RV Wt1-5 in primary cultures (model 1) and in explant culture of gastric adenocarcinoma (model 2) was assessed by immunohistochemical assay. Also, where necessary, immunohistochemical testing was performed to detect fungi and/or bacteria by Gomori-Grocott staining.
Flow cytometry
Both gastric tumor cells and non-tumor gastric tissue cells were analyzed by flow cytometry. Cells were disaggregated using serial passages performed with sterile 5mL syringes and 15, 19 and 21 GA needles, and a 0.5mL insulin syringe with 31 GA needle (BD Ultra-fine™). Cells obtained in suspension were analyzed with the FACScanto II™ flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), and data were analyzed with FACSDiva™ version 8.0 software (Becton-Dickinson, Franklin Lakes, NJ, USA).
Statistical analysis
Data were analyzed in Stata 12® (StataCorp, Texas, USA) and are described using means and standard deviations (SD). In addition, a bivariate analysis was performed using the Mann-Whitney U test to determine the differences between the data from the tests performed for the 2 models and the control used in each one. A statistical significance level of p<0.05 was considered.
Ethical considerations
The study followed the ethical principles for biomedical research involving human subjects established in the Declaration of Helsinki33 and the scientific, technical and administrative standards for health research contained in Resolution 8430 of 1993 of the Colombian Ministry of Health.34 Furthermore, the experimental protocols were approved by the Ethics Committee of the HUS through Minutes No. 2 of February 18, 2016. Since no interventions were performed on the patients, informed consent was not required.
Results
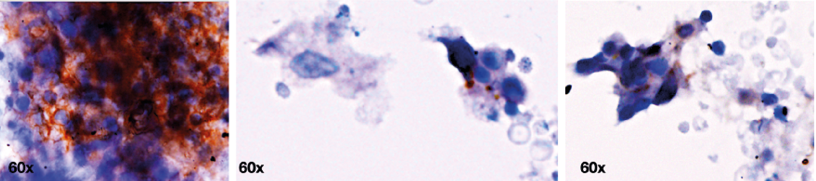
RV Wt1-5 was derived from a human parental rotavirus as previously described.21 In model 1, when evaluating the immunocytochemistry of disaggregated gastric adenocarcinoma cells inoculated with RV Wt1-5, intense immunoreactivity to RV structural proteins and cytopathic effects were observed. However, it was not possible to determine the type and characteristics of the infected cells, nor their interaction with the TME in this model (Figure 1).
Figure 1. Infection of gastric cancer tumor cells by rotavirus Wt1-5 in primary cell culture (model 1).
Note: disaggregated diffuse gastric adenocarcinoma cells were infected with rotavirus Wt1-5 (MOI 0.8) and the images represent horseradish peroxidase immunochemical staining of rotavirus structural antigens at 12 hours post infection.
Source: Image obtained while conducting the study.
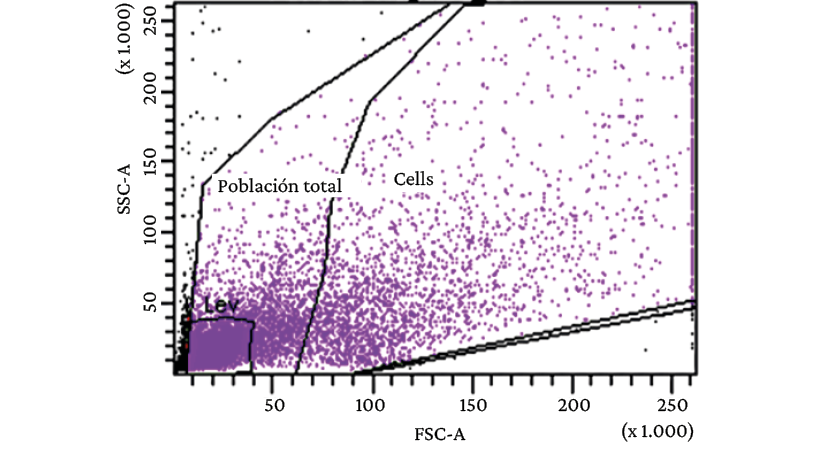
When analyzed using flow cytometry, there was evidence of great heterogeneity in the cell population obtained from the primary cell culture of diffuse gastric adenocarcinoma (model 1) (Figure 2).
Figure 2. Flow cytometry analysis of cells obtained from primary culture of diffuse gastric adenocarcinoma (model 1).
Source: Image obtained while conducting the study.
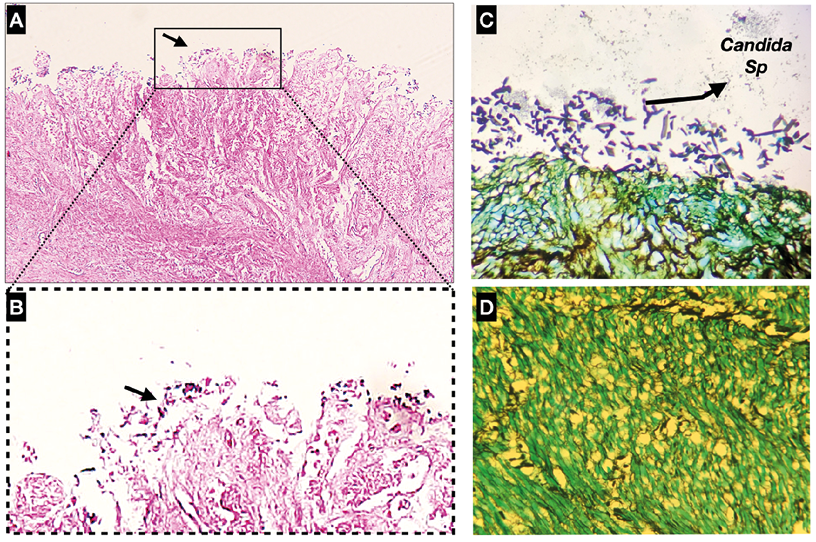
Since there was a noticeable increase in the very small cell population, an immunohistochemical test was performed to evaluate the possibility of fungal and/or bacterial contamination. Thus, the presence of yeasts and pseudohyphae of Candida species was found on the mucosal surface of the gastric tumor, although they did not invade the muscle layer (Figure 3).
Figure 3. A) Immunohistochemical test of the primary culture of diffuse gastric adenocarcinoma with hematoxylin and eosin staining x10; B) Immunohistochemical test of the primary culture of diffuse gastric adenocarcinoma with hematoxylin and eosin staining x60; C) and D) Presence of yeasts and pseudohyphae on the mucosal surface of the tumor according to immunohistochemical test with Gomori-Grocott staining.
Source: Image obtained while conducting the study.
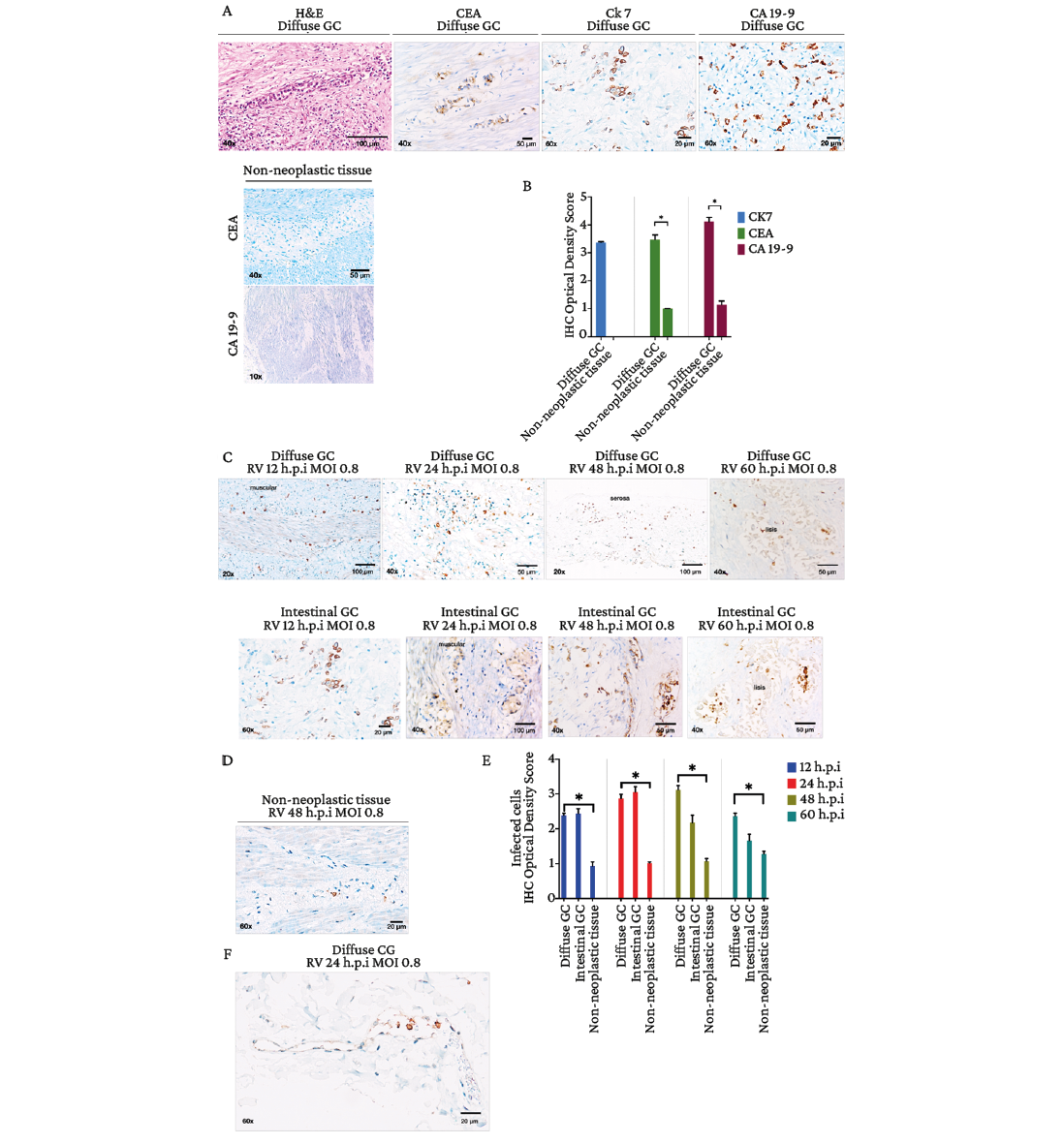
Considering that the presence of a wide heterogeneity in the cell population obtained in the primary culture is a limitation because it makes it difficult to establish the true susceptibility to RV Wt1-5 infection, it was decided to use the tissue explant model (model 2) to carry out the infection. Tissue tumor cells were identified by histomorphological analysis with H&E, and the expression of GC tumor markers CEA, Ck-7 and CA-19-9 was determined by immunohistochemistry tests (Figure 4A). Figure 4B shows that the immunohistochemistry optical density scores in malignant lesions of the diffuse subtype in the gastric wall were: 3.35 (Ck7), 3.40 (CEA) and 4.06 (CA 19-9), while the values in adjacent non-tumor tissue were 1 (CEA) and 1.19 (CA 19-9).
Subsequently, explants were infected with RV Wt1-5 (MOI 0.8) at different times (12, 24, 48 and 60 h.p.i), as detailed in the materials and methods section. Infected non-tumor tissue explants were also used as controls. Increased immunoreactivity to rotaviral antigens was observed in different areas of diffuse and intestinal tumors compared to non-tumor tissue (Figure 4C and 4D). The immunohistochemical optical density score was significantly higher in diffuse (2.36, 2.87, 3.12 and 2.34, at 12, 24, 48, and 60 h.p.i, respectively) and intestinal (2.44, 3.05, 2.18, and 1.66, respectively) GC compared to non-tumor tissue (1, 1, 1, 1.06, and 1.23, respectively) (p<0.05) (Figure 4E).
Images obtained from immunohistochemical testing of infected tumor specimens evidenced the presence of large areas of viral spread and infection in all tumor layers. Moreover, the presence of rotaviral antigens was detected in immune cells located in the blood vessels of the TME (Figure 4F), and necrosis was observed in the neoplastic tissues from 48 h.p.i. onwards. In contrast, non-tumor tissues inoculated with PBS did not show signs of tissue lysis (Figure 4C and 4E).
Figure 4. Ex vivo infection of tissue explants of diffuse and intestinal gastric adenocarcinomas with rotavirus Wt1-5 (MOI 0.8). A) Hematoxylin and eosin staining and immunohistochemical staining (CEA, CK-7, and CA 19-9); B) Immunohistochemical optical density score in tumor tissue and non-tumor tissue (data are shown as mean percentages ± standard deviation from three experiments performed on independent samples and repeated twice; Mann-Whitney U test with a p-value<0.05); C) Horseradish peroxidase immunochemical staining of rotavirus structural antigens at 12, 24, 48 and 60 h.p.i. in diffuse and intestinal gastric adenocarcinoma explants; D) Horseradish peroxidase immunochemical staining of rotavirus structural antigens at 48 h.p.i. in non-tumor tissue; E) Quantification of infection determined based on immunohistochemical optical density score as the number of cells positive for rotavirus structural antigens (data are shown as mean percentages ± standard deviation of three experiments performed on independent samples and repeated twice; Mann-Whitney U-test with a p-value<0.05); F) Immunohistochemical staining for rotaviral antigens in gastric adenocarcinoma (24 h.p.i, MOI 0.8), showing a strong cytoplasmic signal in immune cells in the lumen of a blood vessel.
Source: Images obtained while conducting the study.
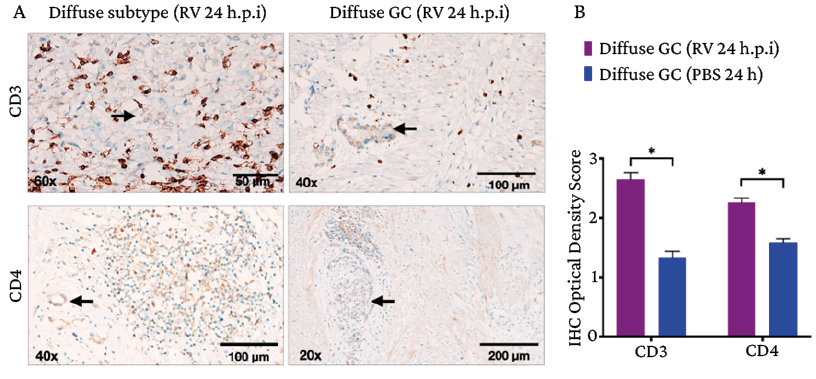
Furthermore, immunohistochemical analysis of CD3+ and CD4+ surface marker expression on RV-infected gastric adenocarcinoma explants revealed immunohistochemical optical density scores of 2.64 and 2.25, respectively. In turn, in the control group (tumor tissues inoculated with PBS), the values were 1.32 and 1.58, respectively (Figure 5).
Figure 5. Immune microenvironment in gastric adenocarcinoma after infection with rotavirus Wt1-5. A) CD3+ and CD4+ lymphocyte infiltration in the peritumoral area of diffuse gastric adenocarcinoma after infection with rotavirus Wt1-5 (24 h.p.i, MOI 0.8); tissues inoculated with saline phosphate buffer are shown as a control; the black arrows show tumor cell aggregates; B) Quantification of CD3 and CD4 immune cells with positive immunostaining in tumor; calculation made according to the immunohistochemical optical density score (data are presented as mean percentages ± standard deviation of three experiments performed on independent samples and repeated twice; Mann-Whitney U test with a significance level set at p*<0.05).
Source: Image obtained while conducting the study.
Discussion
As of 2020 in Colombia, GC was the fourth most frequent type of cancer and the leading cause of death related to cancer; it was also the second most frequent cancer in men and the fifth most frequent in women.2 According to Washington et al. (cited by Sun et al.35), the 5-year survival rate in patients with stage IV GC is 4%. However, in a study performed in Japan, Katai et al. (also cited by Sun et al.35) report that the 5-year survival rate in patients with stage IV GC can increase to 16.4% after gastrectomy. Despite this, the possibility of surgical resection and its efficacy remains limited in advanced GC, a stage in which the median survival rate is between 13 and 16 months.9 Therefore, the development of effective and safe therapeutic strategies to treat GC at advanced stages remains the main challenge for current biomedical science.
The success of oncolytic viral therapy lies in different coordinated mechanisms of action involving tumor penetration and dissemination, as well as reactivation of the immune system.36,37 The TME is a complex and dynamic ecosystem that is constantly evolving, in which tumor cells, cancer stem cells, immune cells (neutrophils, macrophages, eosinophils, mast cells, dendritic cells, T lymphocytes and, occasionally, NK cells and B lymphocytes) and stromal cells (gastric epithelial cells, endothelial cells, and cancer-associated fibroblasts) coexist. These cells establish interactions directly from cell to cell or through the secretion of cytokines, chemokines, reactive oxygen species or exosomes, all of which determine the natural history of the tumor.
In the field of virotherapy, these interactions could trigger premature viral clearance;38,39 in addition, replication and spread of OVs within the tumor may also be compromised by the restrictive properties of the solid tumor architecture and microenvironment, such as extracellular matrix, hypoxia, high interstitial tumor pressure, and acidic pH. Therefore, the first days of OV infection and its amplification within the tumor are of vital importance, as both the innate and adaptive immune systems eventually reduce the spread of the virus.40
In the present study, the ex vivo infection model of tumor tissue explants overcame the difficulties associated with the primary culture of disaggregated cells, facilitating the analysis of RV activity in the TME. This model is crucial to obtain a more accurate assessment of the RV’s ability to spread throughout the tumor, its infectivity, its cytopathic effects and the immune response, which, in turn, are influenced by the tissue heterogeneity of the TME.25
Similarly, this study demonstrates that RV Wt1-5 can spread through all tumor layers within just 12 hours, which promotes the infection of tumor cells. In addition, viral concentrations in the tumor were sufficient to reach the threshold necessary to initiate a cytopathic effect. It is noteworthy that rotaviral antigens were observed within circulating immune cells in the blood vessels of gastric tumor tissue, as well as a change in the tissue lymphocyte infiltrate. This finding is relevant since previous studies41-44 have shown that RV can activate macrophages, which are involved in the secretion of proinflammatory cytokines, and dendritic cells, favoring the presentation of antigens and the induction of effector T lymphocytes and B lymphocytes. These mechanisms could be fundamental to conducting future studies using the ex vivo infection model developed in the present work to demonstrate the ability of the RV to reactivate immunosurveillance in the tumor.
The present study also showed that the dissemination of the RV and its cytopathic effects were not affected by the TME, since the virus spread through all layers of the gastric tumor, including the mucosa, submucosa, muscle and serosa, causing necrosis of the neoplastic tissue at 48 and 60 h.p.i.
The tropism and safety of RV use were also demonstrated by observing that adjacent non-tumor gastric tissues were negative for infection with RV Wt1-5. These results are consistent with the validity of ex vivo culture of patient tissues as a model to evaluate therapeutic responses in tumors, in which cell viability and metabolic activity of the tissue remain intact for up to 72 hours, as demonstrated in previous studies.45-47 These findings support the evident cytopathic ability of RV Wt1-5 in the ex vivo tumor explant model used in this study, suggesting that oncolytic viral therapy could be considered as an alternative neoadjuvant therapy in the treatment of advanced GC.
The selectivity of RV could decrease unwanted side effects associated with conventional therapies, while possibly reducing tumor size and allowing resection in initially inoperable cases. Moreover, oncolytic viral therapy could also induce an antitumor immune response, which would increase the efficacy of treatment and prevent tumor recurrence, thus improving the survival rate and quality of life of patients.
One of the main limitations of the present study is that, as it is an ex vivo study, it focuses on local responses in the tissue analyzed and, therefore, does not evaluate how RV Wt1-5 affects the immune system in other parts of the body. Overall, this study indicates that RV Wt1-5 infects, replicates and spreads throughout the tumor, enabling its efficacy in vivo.
Conclusions
The results of the ex vivo model of infection in gastric adenocarcinoma explants demonstrate that RV Wt1-5 selectively and efficiently infects both diffuse and intestinal tumor cells, reaching its highest infective capacity at 24 h.p.i. Furthermore, it has been observed that RV can boost and/or reactivate the patient’s immune response, suggesting its potential usefulness as an oncolytic therapy in the treatment of advanced GC. Therefore, it is necessary to delve deeper into the study of RV Wt1-5 as an oncolytic therapy in advanced GC with the aim of improving the survival rate and quality of life of patients with this disease.
Note: the present article is derived from the PhD thesis entitled Estudio del potencial oncolítico del aislamiento rotaviral humano Wt1-5 en adenocarcinoma gástrico (Study of the oncolytic potential of the human rotaviral isolate Wt1-5 in gastric adenocarcinoma).48
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgments
To Jaydi Acosta-Álvarez from the HUS Pathology Laboratory for her assistance in the processing of the immunocytochemistry samples, and to Luis Gamboa-Martínez for his help with the essential statistics for the preparation of the manuscript.
References
1.Global Cancer Observatory (Gobocan). World Fact Sheet. Lyon: Globocan; 2020 [cited 2023 Oct 18]. Available from: https://bit.ly/3McHDpF.
2.Global Cancer Observatory (Gobocan). Colombia Fact Sheets. Lyon: Globocan; 2020 [cited 2023 Oct 18]. Available from: https://bit.ly/3scDH17.
3.Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019;39(1):10. https://doi.org/gh6dxv.
4.Zangouei AS, Moghbeli M. MicroRNAs as the critical regulators of cisplatin resistance in gastric tumor cells. Genes Environ. 2021;43(1):21. https://doi.org/gkfwmg.
5.Yu J, Jung J, Park SR, Ryu MH, Park JH, Kim JH, et al. Role of palliative radiotherapy in bleeding control in patients with unresectable advanced gastric cancer. BMC Cancer. 2021;21(1):413. https://doi.org/kx28.
6.Zhang Z, Liu Z, Chen Z. Comparison of Treatment Efficacy and Survival Outcomes Between Asian and Western Patients With Unresectable Gastric or Gastro-Esophageal Adenocarcinoma: A Systematic Review and Meta-Analysis. Front Oncol. 2022;12:831207. https://doi.org/grg4xq.
7.Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumor Biol. 2017;39(7):1010428317714626. https://doi.org/gbkg43.
8.Rowsell A, Sodergren SC, Vassiliou V, Darlington AS, Guren MG, Alkhaffaf B, et al. Systematic review of health-related quality of life (HRQoL) issues associated with gastric cancer: capturing cross-cultural differences. Gastric Cancer. 2022;25(4):665-77. https://doi.org/gsgtwj.
9.Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, et al. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018;21(2):315-23. https://doi.org/gc634k.
10.Hong B, Sahu U, Mullarkey MP, Kaur B. Replication and Spread of Oncolytic Herpes Simplex Virus in Solid Tumors. Viruses. 2022;14(1):118. https://doi.org/kx29.
11.Oh CM, Chon HJ, Kim C. Combination Immunotherapy Using Oncolytic Virus for the Treatment of Advanced Solid Tumors. Int J Mol Sci. 2020;21(20):7743. https://doi.org/gnc853.
12.Liu X, Zhang J, Feng K, Wang S, Chen L, Niu S, et al. Efficacy and safety of oncolytic virus combined with chemotherapy or immune checkpoint inhibitors in solid tumor patients: A meta-analysis. Front Pharmacol. 2022;13:1023533. https://doi.org/kx3b.
13.Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28(7):326-33. https://doi.org/c63f3j.
14.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2016;15(9):660. https://doi.org/gqsn6r.
15.Santos-Apolonio J, Lima-de Souza Gonçalves V, Cordeiro-Santos ML, Silva-Luz M, Silva-Souza JV, Rocha-Pinheiro SL, et al. Oncolytic virus therapy in cancer: A current review. World J Virol. 2021;10(5):229-55. https://doi.org/kx3c.
16.Cao GD, He XB, Sun Q, Chen S, Wan K, Xu X, et al. The Oncolytic Virus in Cancer Diagnosis and Treatment. Front Oncol. 2020;10:1786. https://doi.org/gjtq77.
17.Bourgeois-Daigneault MC, St-Germain LE, Roy DG, Pelin A, Aitken AS, Arulanandam R, et al. Combination of Paclitaxel and MG1 oncolytic virus as a successful strategy for breast cancer treatment. Breast Cancer Res. 2016;18(1):83. https://doi.org/f8wxd5.
18.Binz E, Berchtold S, Beil J, Schell M, Geisler C, Smirnow I, et al. Chemovirotherapy of Pancreatic Adenocarcinoma by Combining Oncolytic Vaccinia Virus GLV-1h68 with nab-Paclitaxel Plus Gemcitabine. Mol Ther Oncolytics. 2017;6:10-21. https://doi.org/gbrhz4.
19.Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, et al. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083. https://doi.org/gcn82d.
20.Suzuki H. Rotavirus Replication: Gaps of Knowledge on Virus Entry and Morphogenesis. Tohoku J Exp Med. 2019;248(4):285-96. https://doi.org/kx3d.
21.Guerrero CA, Guerrero RA, Silva E, Acosta O, Barreto E. Experimental Adaptation of Rotaviruses to Tumor Cell Lines. PLoS One. 2016;11(2):e0147666. https://doi.org/f8q2n3.
22.Guerrero R, Guerrero C, Acosta O. Induction of Cell Death in the Human Acute Lymphoblastic Leukemia Cell Line Reh by Infection with Rotavirus Isolate Wt1-5. Biomedicines. 2020;8(8):242. https://doi.org/kx3f.
23.Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. 2016;22(8):2403-14. https://doi.org/f8c232.
24.Smyth EC, Nilsson M, Grabsch HI, van Grieken NCT, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635-48. https://doi.org/ghjdmz.
25.Kloker LD, Yurttas C, Lauer UM. Three-dimensional tumor cell cultures employed in virotherapy research. Oncolytic Virother. 2018;7:79-93. https://doi.org/kx3h.
26.Diallo JS, Roy D, Abdelbary H, De Silva N, Bell JC. Ex vivo infection of live tissue with oncolytic viruses. J Vis Exp. 2011;(52):2854. https://doi.org/cvgsg3.
27.Sanjuan R, Grdzelishvili VZ. Evolution of oncolytic viruses. Curr Opin Virol. 2015;13:1-5. https://doi.org/kx3j.
28.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31-49. https://doi.org/gjpcbn.
29.Liu JY, Peng CW, Yang XJ, Huang CQ, Li Y. The prognosis role of AJCC/UICC 8th edition staging system in gastric cancer, a retrospective analysis. Am J Transl Res. 2018;10(1):292-303.
30.Arnold M, Patton JT, McDonald SM. Culturing, storage, and quantification of rotaviruses. Curr Protoc Microbiol. 2009;Chapter 15:Unit 15C 3. https://doi.org/d85h6h.
31.Introini A, Vanpouille C, Fitzgerald W, Broliden K, Margolis L. Ex Vivo Infection of Human Lymphoid Tissue and Female Genital Mucosa with Human Immunodeficiency Virus 1 and Histoculture. J Vis Exp. 2018;(140):57013. https://doi.org/gh8tgc.
32.Seyed-Jafari SM, Hunger RE. IHC Optical Density Score: A New Practical Method for Quantitative Immunohistochemistry Image Analysis. Appl Immunohistochem Mol Morphol. 2017;25(1):e12-e3. https://doi.org/gjqjs2.
33.World Medical Association (WMA). WMA Declaration of Helsinki – Ethical principles for medical research involving human subjects. Fortaleza: 64th WMA General Assembly; 2013.
34.Colombia. Ministerio de Salud. Resolución 8430 de 2013 (octubre 4): por la cual se establecen las normas científicas, técnicas y administrativas para la investigación en salud. Bogotá D.C.; october 4 1993.
35.Sun J, Nan Q. Survival benefit of surgical resection for stage IV gastric cancer: A SEER-based propensity score-matched analysis. Front Surg. 2022;9:927030. https://doi.org/kx3n.
36.Guo ZS, Liu Z, Kowalsky S, Feist M, Kalinski P, Lu B, et al. Oncolytic Immunotherapy: Conceptual Evolution, Current Strategies, and Future Perspectives. Front Immunol. 2017;8:555. https://doi.org/kx3p.
37.Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18(8):498-513. https://doi.org/gdzp62.
38.Rezalotfi A, Ahmadian E, Aazami H, Solgi G, Ebrahimi M. Gastric Cancer Stem Cells Effect on Th17/Treg Balance; A Bench to Beside Perspective. Front Oncol. 2019;9:226. https://doi.org/kx3q.
39.Giraldo NA, Sanchez-Salas R, Peske JD, Vano Y, Becht E, Petitprez F, et al. The clinical role of the TME in solid cancer. Br J Cancer. 2019;120(1):45-53. https://doi.org/cxqf.
40.Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15(9):1686-93. https://doi.org/ctmqbc.
41.Sen A, Ding S, Greenberg HB. The Role of Innate Immunity in Regulating Rotavirus Replication, Pathogenesis, and Host Range Restriction and the Implications for Live Rotaviral Vaccine Development. In: Kiyono H, Pascual DW, editors. Mucosal Vaccines. 2nd ed. Elsevier; 2020. p. 683-97. https://doi.org/kx3r.
42.Di Fiore IJ, Holloway G, Coulson BS. Innate immune responses to rotavirus infection in macrophages depend on MAVS but involve neither the NLRP3 inflammasome nor JNK and p38 signaling pathways. Virus Res. 2015;208:89-97. https://doi.org/f7q3j5.
43.Narvaez CF, Angel J, Franco MA. Interaction of rotavirus with human myeloid dendritic cells. J Virol. 2005;79(23):14526-35. https://doi.org/dcfz8m.
44.Hakim MS, Ding S, Chen S, Yin Y, Su J, van der Woude CJ, et al. TNF-alpha exerts potent anti-rotavirus effects via the activation of classical NF-kappaB pathway. Virus Res. 2018;253:28-37. https://doi.org/gdzdnf.
45.Powley IR, Patel M, Miles G, Pringle H, Howells L, Thomas A, et al. Patient-derived explants (PDEs) as a powerful preclinical platform for anti-cancer drug and biomarker discovery. Br J Cancer. 2020;122(6):735-44. https://doi.org/ghg9mt.
46.Stackhouse CT, Gillespie GY, Willey CD. Cancer Explant Models. Curr Top Microbiol Immunol. 2021;430:131-60. https://doi.org/kx3s.
47.Barber G, Anand A, Katarzyna O, Phelan JJ, Heeran AB, Flis E, et al. Characterizing caspase-1 involvement during esophageal disease progression. Cancer Immunol Immunother. 2020;69(12):2635-49. https://doi.org/gn6frv.
48.Sossa-Rojas H. Estudio del potencial oncolítico del aislamiento rotaviral humano Wt1-5 en adenocarcinoma gástrico [thesis]. Bogotá D.C.: Facultad de Ciencias, Universidad Nacional de Colombia; 2022.
 Pedro Gabriel Franco-Maz2,3
Pedro Gabriel Franco-Maz2,3 Carlos Manuel Zapata-Acevedo4,5
Carlos Manuel Zapata-Acevedo4,5