Introduction
Catheterization is a common procedure that consists of the insertion of a latex, polyurethane, or silicone tube into the bladder to drain its contents.1 Urinary catheters can be indwelling or placed intermittently depending on the indications and the patient’s condition. Intermittent catheters are inserted every 6 to 8 hours,2 while indwelling catheters are inserted for a period of time greater than 24 hours, and are usually connected to a collection bag.3 Indwelling catheterization is typically used in patients with pathologies such as prostatic hyperplasia, neurogenic bladder, urinary retention, severe urinary incontinence, as well as critically ill patients4 and those with pressure ulcers in the sacral region, or with contaminated perineal lesions associated with incontinence.5
Urinary catheterization is a minor procedure undertaken in up to 25% of all hospitalized patients.6 It carries substantial risks and causes high morbidity and mortality secondary to bacteremia, as well as longer hospital stays and higher resource consumption.7 Catheter-associated urinary tract infection (CAUTI) accounts for up to 30% of health-care associated infections.7 In Latin America, CAUTI is the third leading cause of nosocomial infections and its incidence is estimated at 8.9 cases per
1 000 days of exposure to this device.6 In Colombia, the estimated prevalence is 12% to 45%, which makes it one of the top five infections reported in the country.6 In Bogotá, a prevalence of 16.1% was reported for the 2012-2013 period, with an incidence rate of 3.9 cases per 1 000 days of exposure.6
Since urinary catheterization carries substantial risks, multiple interventions have been described with the aim of reducing the occurrence of infectious processes. Non-pharmacological interventions include staff training in catheter insertion and care.8 Access to guidelines and algorithms allows standardizing interventions that avoid variability among healthcare staff and guide the timely removal of unnecessary catheters.7,9 Hand washing before and after catheter insertion and manipulation reduces non-saprophytic microflora without affecting the saprophytic microflora of the skin.5,7 Using an aseptic technique, sterile equipment and supplies during the preparation of the catheter insertion area and during the insertion, using barrier measures such as sterile gowns and gloves;10 and the use of antiseptics for cleaning the urinary meatus help reduce microbial load and the entrainment of microorganisms.5,11
In addition, the lubricant applied prior to insertion contributes to bladder neck relaxation, facilitating the passage of the catheter, and also prevents urethral trauma, false passages and pain.10 Inserting a catheter of the smallest possible size can minimize urethral trauma and lead to a more effective drainage,5,12 while using a closed drainage system makes it more difficult for microorganisms to colonize the urethral meatus intraluminally.13
Regarding pharmacological interventions, silicone catheters are recommended for patients requiring a long-term urinary catheter and in those with frequent obstruction of the device.7 Antimicrobial-coated catheters are used in patients with a CAUTI that does not decrease with the application of primary strategies. Finally, the consumption of blueberries,14 lactobacilli15 and Chinese herbal medicines14 has also been proposed to prevent urinary infections.
Since the use of catheters in clinical practice is heterogenous and considering the frequency of adverse events and the appearance of infections associated with their insertion and use, this systematic review seeks to assess the effects of non-pharmacological interventions aimed at reducing the probability of CAUTI in the adult population. This will help develop policies designed to standardize the care of adult patients with urinary catheterization.
Materials and methods
The report was developed following the recommendations suggested by the Cochrane Handbook (CHB)16 and in accordance with the PRISMA statement.17 Review methods were established before conducting the literature search, which is detailed at http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017051553. Ethical approval was not required because this is a secondary study.
An attempt was made to identify as many relevant randomized controlled trials (RCTs) as possible, regardless of their language of publication. To this end, the Information Specialist of The Cochrane STI Group was contacted to conduct a complete search strategy, which was constructed using controlled vocabulary and text terms. The search was conducted in the MEDLINE, EMBASE, and LILACS databases. Grey literature was also consulted through the references listed in the included studies. The search was updated to September 30, 2016 (available at: https://www.crd.york.ac.uk/PROSPEROFILES/51553_STRATEGY_20161121.pdf) and citations were exported to EndNote version X6 (Thomson Reuters, New York, NY, USA). All published RCTs were included with no language restrictions.
The participants in the trials were non-immunocompromised men and non-pregnant women that required an indwelling urinary catheter as part of their inpatient or outpatient medical treatment. Indwelling urinary catheters are as those inserted for at least 24 hours in the urinary tract. The intervention of interest was the use of any non-pharmacological intervention during catheter insertion versus the use of placebo, or pharmacological interventions, or no intervention.
The primary outcomes were symptomatic urinary infection, time elapsed until the first episode of urinary infection, recurrent infection, bacteremia, asymptomatic bacteriuria, and major adverse effects associated with the intervention. The main secondary outcomes were satisfaction of participants, quality of life, mild adverse events, and cost-effectiveness of the intervention.
First, two authors (XSM and CFGA) selected the studies individually, and then, through consensus, they made the final selection of studies to be included in the systematic review. In addition, the other two authors (JAG and JLMV) assessed the risk of bias of the included RCTs using the tool suggested in the CHB:16 sequence generation and allocation concealment, blinding of participants, incomplete outcome data, selective reporting, and other risks of bias. Disagreement was resolved by consensus among all authors. All domains were assessed as low, high, or unclear risk of bias. The GRADE system was used for rating the quality of the evidence.
Results are presented as risk ratios (RR) with 95% confidence intervals (CI) and mean differences. I2 statistic and Chi2 test values were used to assess statistical heterogeneity, which was considered relevant if the I2 statistic was greater than 40% and if there was a low p-value (less than 0.10) in the Chi2 test for consistency. Statistical analyses were performed using Rev Man,18 with fixed-effect meta-analysis for combining data, unless there was substantial heterogeneity, while a random-effects model was implemented if there was clinical or significant statistical heterogeneity.
Results
The searches yielded 311 references, and 214 were screened after removing duplicates. Of those, the full texts of 30 references were reviewed. A total of 8 studies met the inclusion criteria;19-26 12 papers were excluded because they were not RCTs, 6 trials implemented a different intervention and finally, 4 studies recruited a different kind of population. The following PRISMA diagram illustrates the selection process (Figure 1). For excluded RCTs and the rationale for exclusion, see Appendix A.
The selected RCTs were published from 1985 to 2012 and recruited participants from Sweden, the United Kingdom, Hong Kong, New Zealand, the USA and Belgium; 2 of those studies were funded by the industry.21,25 Retrieved studies involved 8 718 men and women with an age range between 20 and 95 years, who required long-term catheterization (>24 hours) during their hospital stay due to general,20,24 cardiac,22 orthopedic25 or elective urologic surgery;26 3 studies did not include information in this regard.19,21,23 The studies excluded patients with current or previous urinary tract infection, recent exposure to antibiotics, history of diabetes or pelvic radiotherapy, or with a recent illness (Table 1).
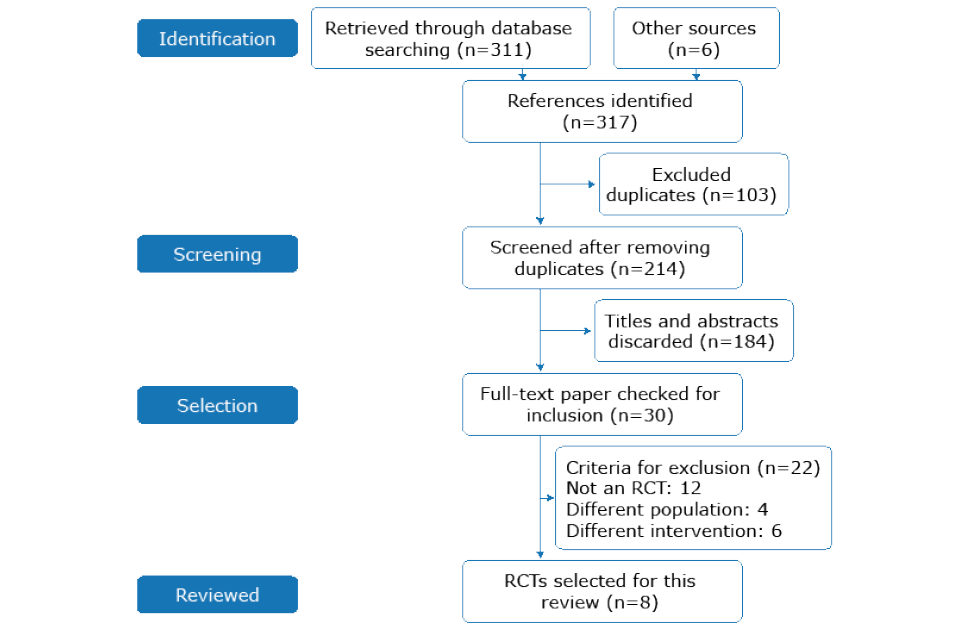
Figure 1. PRISMA flow chart.
Source: Own elaboration.
The intervention most commonly implemented was silicone-coated latex catheter in 5 studies;21,23-26 2 studies described sterile catheterization as the intervention;19,20 and the remaining trials used non-coated silicone catheters.22 The comparator in 6 studies was non-coated silicone urinary catheter21-26 and in the other 2 it was clean/non-sterile catheterization technique.19,20
Sterile catheterization is the process of cleaning the urethral meatus utilizing an antiseptic aqueous solution and avoiding contact with the practitioner’s gloves. The catheter is inserted following a non-touch technique and using forceps after lubrication with sterile lignocaine gel. On the other hand, participants assigned to non-sterile catheterization used sterile water and non-sterile gloves.19,20
Table 1. Main characteristics of the studies selected.
|
Author |
Carappetti et al.19 |
Cheung et al.20 |
Liedberg & Lundberg et al.21 |
Nacey et al.22 |
Pickard et al.23 |
Riley et al.24 |
Stenzelius et al.25 |
Verleyen et al.26 |
|
Year |
1994 |
2008 |
1990 |
1985 |
2012 |
1995 |
2011 |
1999 |
|
Study design |
RCT |
RCT |
RCT |
RCT |
RCT |
RCT |
RCT |
RCT |
|
Title |
Randomized study of sterile versus non-sterile urethral catheterisation |
Water versus antiseptic periurethral cleansing before catheterization among home care patients: a randomized controlled trial |
Silver alloy coated catheters reduce catheter-associated bacteriuria |
Catheter induced urethritis: a comparison between latex and silicone catheters in a prospective clinical trial |
Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial |
A large randomized clinical trial of a silver-impregnated urinary catheter: lack of efficacy and staphylococcal superinfection |
Noble metal alloy-coated latex versus silicone Foley catheter in short-term catheterization: a randomized controlled study |
Clinical application of the Bardex IC Foley catheter |
|
Country |
England |
Hong Kong |
Sweden |
New Zealand |
United Kingdom |
Salt Lake City |
Sweden |
Belgium |
|
Population |
Elective surgery |
Elective Cardiac surgery |
Surgery or Internal Medicine |
Elective orthopedic surgery |
Elective urologic surgery |
|||
|
Age |
22-91 |
mean 80.8 |
48-52 |
20-73 |
mean 59 |
mean 61.4 |
20-95 |
-- |
|
Number of participants |
156 |
20 |
120 |
100 |
6 394 |
1 309 |
439 |
180 |
|
Intervention |
Sterile catheterisation |
Sterile catheterization |
Silver coated latex catheter |
Silicone catheter |
Silver alloy-coated latex catheter |
Silver coated silicone catheter |
Noble metal alloy coated latex catheter |
Silver-coated catheter |
|
Comparison |
Clean/non-sterile catheterisation |
Sterile water |
Teflonised latex catheter |
Latex catheter |
PTFE-coated latex catheter |
Silicone elastomer-coated latex catheter |
Non-coated silicone catheter |
Latex catheters |
|
Primary outcomes |
Bacteriuria |
Symptomatic bacteriuria |
Bacteriuria |
Urethritis |
Symptomatic CAUTI |
Bacteriuria |
Bacteriuria |
Bacteriuria |
|
Secondary outcomes |
Costs |
- Microbiologically confirmed symptomatic CAUTI - Quality of life - Catheter-related symptoms |
Catheter-related symptoms |
Catheter-related symptoms |
Time to develop bacteriuria |
RCT: Randomized controlled trial; PTFE: Polytetrafluoroethylene; CAUTI: Catheter-Associated Urinary Tract Infection. Source: Own elaboration.
The included RCTs assessed at least one predetermined outcome, with some minor differences in the definition of the results between papers. A total of 7 studies reported bacteriuria as the primary outcome19-21,23-26 using as threshold 105 colony-forming units (CFU) per milliliter (mL), except for one study24 that defined a lower threshold (>1.000 CFU/mL). Three studies reported symptomatic urinary infection —defined as penile discomfort and purulent urethral discharge22 reported by the patient or the caregiver—, bacterial colonization in urine,20 or the presence of symptoms accompanied by antibiotic prescription.23
Urine specimens were collected at the time of catheterization,19-21,25 at the time of catheter removal,21,24,26 or within 7 to 14 days20,23,25 after catheterization. The secondary outcomes reported by the trials were quality of life (EuroQol scale; EQ-5D),23 mild adverse events,24-26 and costs of the intervention.19 For this study, data for primary outcomes (time elapsed until the first episode, recurrent urinary tract infection, bacteremia, or significant side effects), or the secondary outcome (patient satisfaction) were not collected. Finally, follow-up of participants ranged between 3 and 14 days,19,21,25,26 6 weeks,23 or 6 months.22
According to the GRADE system, publications bias should be assessed using a funnel plot and asymmetry statistical tests if 10 or more studies are included in a systematic review or a meta-analysis; therefore, since only 8 studies were included in this review, a funnel plot was not required to assess publication bias. The RCTs included (n=8) had limitations regarding the use of risk of bias tools, which are detailed in Figure 2 and Appendix B. In this regard, 4 trials19,22,23,25 implemented a valid sequence generation method and 3 established an adequate allocation concealment process (Figure 2),19,22,23,25 making selection bias unclear.
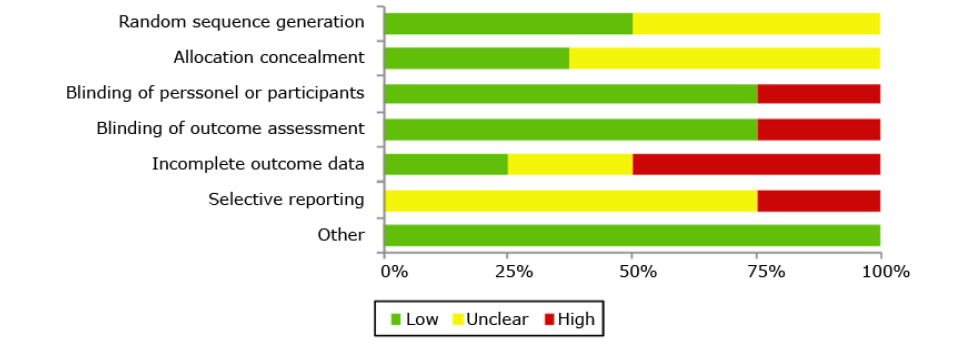
Figure 2. Risk of bias assessment of the included randomized clinical trials.
Source: Own elaboration.
Regarding blinding, 5 studies19-21,24,26 did not report the method implemented. However, the studies were considered to be at low risk of detection and performance bias since the results were objectively appraised (i.e., culture) and, therefore, the lack of blinding is unlikely to affect confidence in the results. One study22 was masked to the allocated intervention because of the similarity of the interventions, making performance and detection bias unlikely. Finally, 23,25 the participants of 2 trials were not masked to the intervention because of the distinctive appearance of the catheters; based on the subjective nature of some outcomes (i.e., mild adverse events of the intervention), these RCTs were considered as having high risk of performance and detection bias.
With respect to possible attrition bias, 2 RCTs22,24 appropriately mentioned the exclusions (<20%) and the reasons were balanced between the arms, making incomplete outcome data bias unlikely. For 6 studies, trial protocols were not available and were assessed as having unclear risk of bias.19-22,24,26 Finally, all RCTs were at low risk of other potential sources of bias. Table 2 presents a detailed description of the quality of the evidence.
Table 2. Quality of the evidence regarding non-pharmacological interventions at the time of insertion of an indwelling catheter for reducing urinary tract infection in non-immunocompromised adults.
|
Outcomes |
Absolute effects* (95% CI) |
Relative effect |
№ of participants |
Quality of the evidence |
|
|
Risk without any intervention |
Any non-pharmacological intervention |
||||
|
Symptomatic urinary infection |
136/1000 |
123/1000 |
RR 0.90 |
4762 |
VERY LOW *, †,‡ |
|
Asymptomatic bacteriuria |
175/1000 |
117/1000 |
RR 0.67 |
5810 |
LOW *,** |
|
Mild adverse events after the intervention |
193/1000 |
162/1000 |
RR 0.84 |
4157 |
LOW *,†† |
|
Quality of life |
MD - 0.01 (-0.03-0.01) |
- |
3672 |
LOW ‡,‡‡ |
|
RR: Relative risk.
* Two trials have high risk of detection, attrition and reporting bias.
† Heterogeneity I2=63%.
‡ CI overlaps the line of no difference and failed to exclude appreciable benefit or harm.
** Relevant heterogeneity I2= 71%.
†† Heterogeneity I2=0%.
‡‡ High risk for detection, attrition, and selective reporting.
Source: Own elaboration.
Low-quality evidence showed that, compared to the control group, the use of non-pharmacological intervention does not seem to decrease the frequency of symptomatic urinary infections20,22,23,25 (RR 0.90, 95%CI: 0.61-1.35; 4 762 participants, 4 RCTs; I2 statistic: 63%), or improve quality-of-life scores (MD –0.01 EQ-5D scale; 95%CI: -0.03 - 0.01, 1 RCT) (Figure 3). However, there was evidence of differences between groups in terms of asymptomatic bacteriuria episodes19-21,23-26 (RR 0.67, 95%CI: 0.48-0.94; 5 810 participants,
7 studies; I2 statistic: 71%) (Figure 4) and the rate of mild adverse events23,25 (RR 0.84, 95%CI: 0.74-0.96; 4 157 participants, 2 trials; I2 statistic: 0%) (Figure 5).
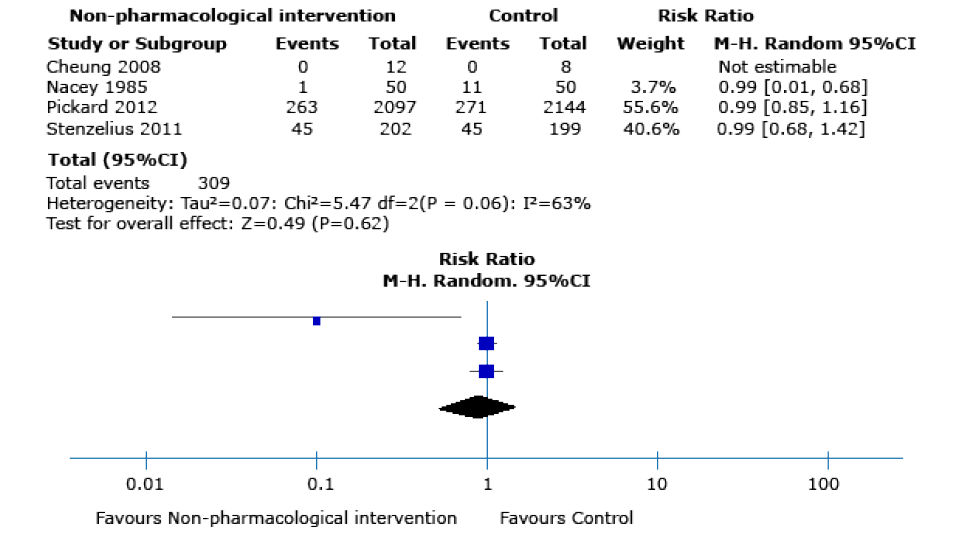
Figure 3. Symptomatic urinary infection as an outcome after performing any of the non-pharmacological interventions during catheter insertion.
Source: Own elaboration.
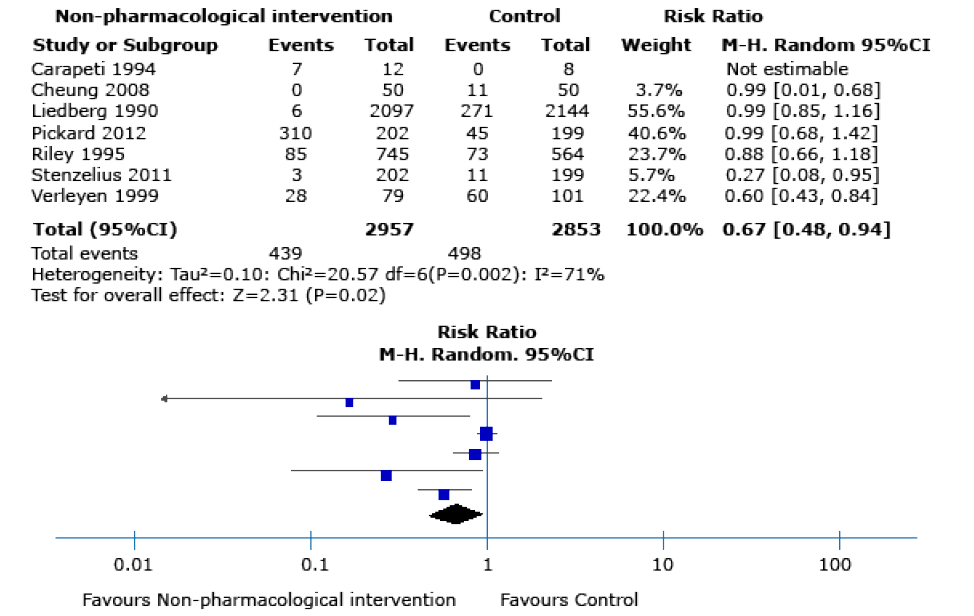
Figure 4. Asymptomatic bacteriuria as an outcome after performing any of the non-pharmacological interventions during catheter insertion.
Source: Own elaboration.
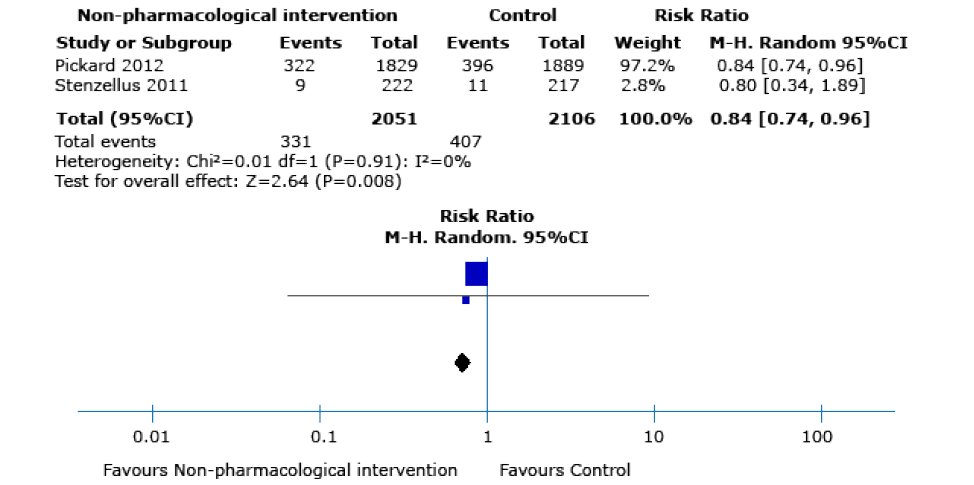
Figure 5. Mild adverse events as outcomes after performing any of the non-pharmacological interventions during catheter insertion.
Source: Own elaboration.
Carappetti et al. 19 measured resource and capital expenditure associated with the implementation of sterile interventions compared to clean catheterization. The recruited participants underwent preoperative urethral catheterization and the direct costs were estimated based on the supplies utilized: gloves, sterile gown, catheter pack, lignocaine gel, vaginal gel, sterile water, 10-milliliter syringes, catheter bag, Foley catheter, scrub solution, and skin preparation. Compared with clean catheterization, sterile technique doubled care-associated costs, as the total cost per participant was close to GBP 7.49 versus GBP 3.06, respectively, in 1994. This study did not assess indirect or long-term intervention-related costs.
To explore heterogeneity, a subgroup analysis was performed for the asymptomatic bacteriuria outcome. The tests for subgroup effect were not significantly different when the source of heterogeneity was explored (p=0.54, data not shown). Subgroup analyses did not explain the variability in the summary effect measures for the asymptomatic bacteriuria outcome, so these findings should be interpreted with caution. The outcomes symptomatic urinary infection, time elapsed until the first episode of urinary tract infection and major adverse effects derived from the intervention were not analyzed because of the sparse information provided by the RCTs included in the present review.
Discussion
This systematic review retrieved low-quality evidence to support the implementation of non-pharmacological interventions at the time of urinary catheter insertion to reduce the risk of infection in non-immunocompromised adults with indwelling catheterization. Regardless of the comparison, non-pharmacological interventions seem to reduce the frequency of asymptomatic bacteriuria episodes and the rate of mild adverse events.
One of the strengths of this systematic review is that its methodology was planned, developed and published at PROSPERO before conducting it, and all the methods that were established at that time were followed while doing the review, namely, a comprehensive literature search without language or date restrictions, two reviewers in charge of the selection of studies, data extraction and bias risk assessment using the tool suggested in the CHB;16 evidence ranking by means of the GRADE approach; and the use of subgroup analyses and methods for statistical analysis.
One of the weaknesses of the present review is that the quality of the evidence found was very low according to the GRADE system; therefore, further research is highly likely to change the conclusions presented here. On the other hand, the RCTs included were heterogenous and publication bias was not assessed using a funnel plot due to the recommendation of the GRADE system regarding the detection of this type of bias when less than 10 studies are included in a meta-analysis or a systematic review.
There were no other systematic reviews evaluating the impact of non-pharmacological interventions for catheter insertion in cases of urinary tract infection that require long-term catheterization. Consistent with this review, a Cochrane review concludes that the use of silver-coated catheters reduces the frequency of asymptomatic bacteriuria, but the studies reviewed there only assessed short-term catheterization.27
Conclusion
Very low-quality evidence shows that non-pharmacological interventions at the time of urinary catheter insertion in non-immunocompromised adults could reduce the frequency of asymptomatic bacteriuria episodes and mild adverse events, without reducing the rate of symptomatic urinary infections or improving quality-of-life scores.
Conflicts of interest
None stated by the authors.
Funding
This study was financed through a HERMES grant (code 33595) awarded by the Faculty of Nursing of Universidad Nacional de Colombia, Bogotá Campus, as stated in Resolution 073 of 2016 (Act 11, May 12, 2016).
Acknowledgements
None stated by the authors.
References
- Mazzo A, Bardivia CB, Jorge BM, Souza Júnior VD, Fumincelli L, Mendes IAC. Cateterismo urinário permanente: prática clínica. Enfermeria Global. 2015;14(2):50-9. http://doi.org/c9br.
- Jiménez-Mayorga I, Soto-Sánchez M, Vergara-Carrasco L, Cordero-Morales J, Rubio-Hidalgo L, Coll-Carreño R, et al. Protocolo de sondaje vesical. Bibl Lascasas. 2010 [cited 2017 Jan 20];6(1). Available from: https://bit.ly/2YUoyRs.
- Cifuentes M. Prácticas para la prevención de infecciones asociadas a la atención en salud: Norma de prevención de infección urinaria asociada al catéter urinario permanente (ITU/CUP). Santiago de Chile: Hospital Clínico Universidad De Chile; 2011.
- Mizerek E, Wolf L. To Foley or Not To Foley: Emergency Nurses' Perceptions of Clinical Decision Making in the Use of Urinary Catheters in the Emergency Department. J Emerg Nurs. 2015;41(4):329-34. http://doi.org/f7h4t7.
- Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-26. http://doi.org/fbp5qc.
- Colombia. Instituto Nacional de Salud. Protocolo de Vigilancia en Salud Pública: Infecciones asociadas a dispositivos. Bogotá D.C.: Ministerio de Salud; 2016.
- Álvarez CA, Cortés JA, Gómez CH, Fernández JA, Sossa MP, Beltrán F, et al. Guías de práctica clínica para la prevención de infecciones intrahospitalarias asociadas al uso de dispositivos médicos. Revista Infectio. 2010;14(4):292-308.
- Macal-Arriaza MR. Investigación acción sobre técnica de colocación y manejo del catéter vesical en el hospital de Chiquimula [tesis]. Chiquimula: Universidad de San Carlos de Guatemala; 2014.
- Gómez J, Muñoz R, Baños V, Gómez G. Tratamiento de las infecciones urinarias adquiridas en la comunidad: perspectivas actuales y enfoque clínico del paciente. Rev Esp Quimioter. 2005;18(4):319-27.
- Diez BL, Ossa-Montoya R. Cateterismo Uretral: un tema para la reflexión. Invest Educ Enferm. 2005;23(2):118-36.
- Organización Panamericana de la Salud (OPS). Guía para la prevención y el control de las infecciones en servicios de salud, dirigida a estudiantes de las carreras de ciencias de la salud. La Paz: OPS; 2007 [cited 2019 Jul 12]. Available from: https://bit.ly/2YCuY8p.
- Márquez-Rivero PA, Álvarez-Pacheco I, Márquez-Rivero A. Protocolo basado en la evidencia de los cuidados de los catéteres urinarios en unidades de cuidados intensivos. Enfermería Intensiva. 2012;23(4):171-78. http://doi.org/f2jwpm.
- Martínez JA, Cobos-Trigueros N, Mensa J. Infección urinaria asociada a catéteres urinarios. In: Pigrau C, editor. Infección del tracto urinario. España: Salvat; 2013. p. 121-36.
- Flower A, Wang LQ, Lewith G, Liu JP, Li Q. Chinese herbal medicine for treating recurrent urinary tract infections in women. Cochrane Database Syst Rev. 2015;(6):CD010446. http://doi.org/c778.
- Schwenger EM, Tejani AM, Loewen PS. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database Syst Rev. 2015;(12):CD008772. http://doi.org/c78b.
- Higgins JT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. London: The Cochrane Collaboration; 2011.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-9. http://doi.org/bpq5.
- Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration; 2014.
- Carappetti E, Andrews SM, Bentley PG. Randomised study of sterile versus non-sterile uretrhal catheterisation. Ann R Coll Surg Engl. 1996;78(1):59-60.
- Cheung K, Leung P, Wong YC, To OK, Yeung YF, Chang MW, et al. Water versus antiseptic periuretrhal cleansing before catheterization among home care patients: a randomized controlled trial. Am J Infect control. 2008;36(5):375-80. http://doi.org/fqcc64.
- Liedberg H, Lundberg T. Silver Alloy Coated Catheters Reduce Catheter-associated Bacteriuria. Br J Urol. 1990;65(4):379-81. http://doi.org/d8mqm5.
- Nacey JN, Tulloch AG, Ferguson AF. Catheter-induced urethritis: a Comparison Between Latex and Silicone Catheters in a Prospective Clinical Trial. Br J Urol. 1985;57(3):325-8. http://doi.org/fq3pz6.
- Pickard R, Lam T, MacLennan G, Starr K, Kilonzo M, McPherson G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380(9857):1927-35. http://doi.org/f2fgq5.
- Riley DK, Classen DC, Stevens LE, Burke JP. A large randomized clinical trial of a silver-impregnated urinary catheter: Lack of efficacy and staphylococcal superinfection. Am J Med. 1995;98(4):349-56. http://doi.org/dn8j4h.
- Stenzelius K, Persson S, Olsson UB, Stjarneblad M. Noble metal alloy-coated latex versus silicone Foley catheter in short-term catheterization: a randomized controlled study. Scand J Urol Nephrol. 2011;45(4):258-64. http://doi.org/dvkd3v.
- Verleyen P, de Ridder D, van Poppel, Baert L. Clinical Application of the Bardex IC Foley Catheter. Eur Urol. 1999;36(3):240-6. http://doi.org/dgskts.
- Lamb TB, Omar MI, Fisher E, Gillies K, MacLennan S. Types of indwelling urethral catheters for short-term catheterisation in hospitalised adults. Cochrane Database Syst Rev. 2014;23;(9): CD004013. http://doi.org/f8mcww.
Supplemental Digital Content
Appendix A. Characteristics of the excluded studies.
|
Cooper FP, Alexander CE, Sinha S, Omar MI. Policies for replacing long-term indwelling urinary catheters in adults. Cochrane Database Syst Rev. 2014;(5):CD011115. http://doi.org/c9gx. |
Non-randomized clinical trial. |
|
Meddings J, Rogers MAM, Krein SL, Fakih MG, Olmsted RN, Saint A. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-89. http://doi.org/f5vn4j. |
Different intervention. Interventions to reduce urinary catheter use. |
|
Boettcher S, Brandt AS, Roth S, Mathers MJ, Lazica DA. Urinary retention: benefit of gradual bladder decompression - myth or truth? A randomized controlled trial. Urol Int. 2013;91(2):140-4. |
Different interventions. Gradual decompression to prevent urinary retention. |
|
Schumm K, Lam TB. Types of urethral catheters for management of short-term voiding problems in hospitalized adults. Cochrane Database Syst Rev. 2008;16(2):CD004013. http://doi.org/dg2bbk. |
Non-randomized clinical trial. |
|
Cardenas DD, Hoffman JM. Hydrophilic catheters versus non-coated catheters for reducing the incidence of urinary tract infections: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90(10):1668-71. |
Different population. Self-intermittent catheterization. |
|
Al-Farsi S, Oliva M, Davidson R, Richardson SE, Ratnapalan S. Periurethral cleaning prior to urinary catheterization in children: sterile water versus 10% povidone-iodine. Clin Pediatr (Phila). 2009;48(6):656-60. |
Different population. Children. |
|
Tenke P, Kovacs B, Bjerklund-Johansen TE, Matsumoto T, Tambyah PA, Naber KG. European and Asian guidelines on management and prevention of catheter-associated urinary tract infections. Int J Antimicrob Agents. 2008;31(Suppl 1):S68-78. http://doi.org/dkrrz2. |
Non-randomized clinical trial. |
|
Jahn P, Preuss M, Kernig A, Seifert-Hüehmer A, Langer G. Types of indwelling urinary catheters for long-term bladder drainage in adults. Cochrane Database Syst Rev. 2007;18(3):CD004997. http://doi.org/dq9w7t. |
Non-randomized clinical trial. |
|
Leone M, Perrin AS, Granier I, Visintini P, Blasco V, Antonini F, et al. A randomized trial of catheter change and short course of antibiotics for asymptomatic bacteriuria in catheterized ICU patients. Intensive Care Med. 2007;33(4):726-9. http://doi.org/drdxw3. |
Different population. Participants with urinary tract infection. |
|
Johnson JR, Kuskowski MA, Wilt TJ. Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann Intern Med. 2006;144(2):116-26. |
Non-randomized clinical trial. |
|
Leone M, Garnier F, Avidan M, Martin C. Catheter-associated urinary tract infections in intensive care units. Microbes Infect. 2004;6(11):1026-32. http://doi.org/ddg3wt. |
Non-randomized clinical trial. |
|
Webster J, Hood RH, Burridge CA, Doidge ML, Phillips KM, George N. Water or antiseptic for periurethral cleaning before urinary catheterization: a randomized controlled trial. Am J Infect Control. 2001;29(6):389-94. |
Different population. Pregnant women. |
|
Huth TS, Burke JP, Larsen RA, Classen DC, Stevens LE. Clinical trial of junction seals for the prevention of urinary catheter-associated bacteriuria. Arch Intern Med. 1992;152(4):807-12. |
Different intervention. Junction seals to prevent infection. |
|
Johnson JR, Roberts PL, Olsen RJ, Moyer KA, Stamm WE. Prevention of catheter-associated urinary tract infection with a silver oxide-coated urinary catheter: clinical and microbiologic correlates. J Infect Dis. 1990;162(5):1145-50. http://doi.org/cjqs9h. |
Non-randomized clinical trial. |
|
Cai T, Caola I, Tessarolo F, Piccoli F, D’Elia C, Caciagli P, et al. Solidago, orthosiphon, birch and cranberry extracts can decrease microbial colonization and biofilm development in indwelling urinary catheter: a microbiologic and ultrastructural pilot study. World J Urol. 2014;32(4):1007-14. http://doi.org/f6bwvd. |
Different intervention. CISTIMEV PLUS after urinary catheter insertion. |
|
Lam TB, Omar MI, Fisher E, Gillies K, MacLennan S. Types of indwelling urethral catheters for short-term catheterisation in hospitalised adults. Cochrane Database Syst Rev. 2014;23(9):CD004013. http://doi.org/f8mcww. |
Non-randomized clinical trial. |
|
Wilde MH, Fader M, Ostaszkiewicz J, Prieto J, Moore K. Urinary bag decontamination for long-term use: a systematic review. J Wound Ostomy Continence Nurs. 2013;40(3):299-308. |
Non-randomized clinical trial. |
|
Chung YC, Chen HH, Yeh ML. Vinegar for decreasing catheter-associated bacteriuria in long-term catheterized patients: a randomized controlled trial. Biol Res Nurs. 2012;14(3):294-301. http://doi.org/cgfkk2. |
Different intervention. Rice vinegar after urinary catheter insertion. |
|
Ching TY, Seto WH. Evaluating the efficacy of the infection control liaison nurse in the hospital. J Adv Nurs. 1990;15(10):1128-31. http://doi.org/brjnwf. |
Cluster randomized clinical trial. |
|
Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Efficacy of daily meatal care regimens. Am J Med. 1981;70(3):655-8. http://doi.org/fvx23z. |
Different intervention. Daily meatal care regimens after urinary catheter insertion. |
Supplemental Digital Content
Appendix B. Summary of risk of bias according to the authors of this review for each included study
|
Random sequence generation (selection bias)
|
Four trials19,22,23,25 adequately reported the random sequence generation method by using a computer-generated randomization list or flipping a coin, making selection bias at entry unlikely. The remaining trials20,21,24 did not report the random sequence generation method, making the risk of selection bias at entry unclear. |
||||||||||||||||||
|
Allocation concealment (selection bias)
|
Three studies adequately implemented an allocation concealment method by using sequentially numbered sealed envelopes,25 a central randomization,23 or flipping an unbiased coin,19 making selection bias at entry unlikely. The other 5 trials20-22,24,26 did not report the allocation concealment method used, so the risk of bias for this domain was deemed unclear. |
||||||||||||||||||
|
Blinding (performance and detection bias)
|
Five studies,19-21,24,26 did not report the implemented methodology to blind study participants, outcome assessor and personnel from knowing what intervention was given to the participant. However, the studies were considered to be at low risk for performance or detection bias because the outcomes were objectively assessed (i.e., culture), so the lack of blinding would be unlikely to affect results. One study22 was masked to the allocated intervention because of the similarities of each catheter, making performance and detection bias unlikely. Finally, in two trials,23,25 participants, clinicians and trial team were not masked to the allocated intervention because of the distinctive characteristics of each intervention and based on the subjective nature of some outcomes (i.e., mild adverse events of the intervention). This study was considered to be at high risk of performance and detection bias. |
||||||||||||||||||
|
Incomplete outcome data (attrition bias)
|
Two trials22,24 appropriately reported the attrition and exclusions at each stage; the reasons were balanced across groups and the level of missing data was not more than 20%, making attrition bias unlikely. Four studies21,23,25,26 had a discontinuation rate and follow-up loss greater than 20%, so they were appraised as high risk of attrition bias. Finally, 2 trials19,20 did not have enough information to permit judgment of “yes” or “no” (rated as unclear risk of bias). |
||||||||||||||||||
|
Selective reporting (reporting bias)
|
Some outcomes were not pre-specified in the protocol stage in two of the trials included,23,25 but they were reported in the manuscript. These trials were considered to be at high risk of selective reporting bias. For 6 studies,19-22,24,26 the trial protocol was not available, and it is unclear whether the published reports presented all the expected outcomes, including those that were pre-specified. The report had insufficient information to permit judgment of “Yes” or “No” (rated as unclear risk of bias). |
||||||||||||||||||
|
Other potential sources of bias
|
All retrieved trials seemed to be free from other sources of bias and were rated as low risk of bias for this domain. |
||||||||||||||||||
|
|||||||||||||||||||