Introduction
With the emergence of MERS-CoV in 2012 and SARS-CoV in December 2019, global attention has focused on the coronavirus family, since these are major pathogens that cause respiratory tract infections of variable severity.1 The current public health crisis began in late 2019 with an unexplained increase in cases of pneumonia of unknown origin in Wuhan, China, which quickly spread to other cities and countries. Subsequently, in January 2020, SARS-CoV-2 was identified as the causative microorganism of this new disease, which was named Coronavirus Disease 2019 (COVID-19), and declared as a public health emergency of international concern on January 30 and as a pandemic on March 11, 2020, by the World Health Organization (WHO) due to its high pathogenic potential and rapid spread.2 Since its detection in China in December 2019 until March 9, 2021, 116 736 437 confirmed cases of COVID-19 were reported globally, of whom 2 593 285 died.3
At present, there is increasing research reporting that coronavirus infections do not only affect the respiratory tract. In this regard, it has been pointed out that central nervous system involvement may take place in susceptible individuals and contribute to the increased morbidity and mortality of severe COVID-19. Accordingly, it has been established that this disease not only leads to respiratory involvement, but can also affect the nervous system.4,5
As reported by Losy et al.,4 the first retrospective study on neurological symptoms in COVID-19, conducted in China by Mao et al., found that 36.4% of the 214 COVID-19 patients evaluated had neurological manifestations such as dizziness, headache, altered consciousness, impaired smell and taste, stroke, seizures, ataxia, and musculoskeletal injury, among others. Furthermore, other studies have reported the occurrence of anosmia, seizures, acute ischemic stroke, viral meningoencephalitis, acute necrotizing encephalopathy, acute flaccid paralysis, post/para-infectious syndromes, and corticospinal weakness in patients with severe COVID-19.6,7
Although the pathophysiological mechanisms underlying the involvement of the nervous system during SARS-CoV-2 infection have not been fully elucidated, evidence suggests that this disease could be a multifactorial phenomenon in which processes such as direct involvement, autoimmune factors, inflammation (cytokine storm), anterograde or retrograde axonal transport, pharmacological side effects, metabolic alterations, and neuropathy in the critically ill patient, among others, stand out.4,8-10
Taking into account the above and based on the need to offer patients with COVID-19 a more comprehensive approach to establish a diagnosis and timely treatment, the objective of this literature review was to collect and synthesize scientific evidence published within six months after the declaration of the COVID-19 pandemic on neurological manifestations in patients infected with SARS-CoV-2, as well as their variations and frequency in specific populations.
Materials and methods
A literature review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11 The search was performed in PubMed/Medline using the following search strategy: study types: any type describing neurologic manifestations in patients with COVID-19; publication period: March 11 to August 31, 2020; publication language: English; search terms and equation: ((“COVID-19”) AND “Neurologic Manifestations”) (MeSH terms).
The search, identification and selection process is described in detail below (Results section). All authors contributed to the review of the articles identified in the literature search, led by DLMA and EPCM, specialists in neurology and child neurology, respectively. It should be noted that during the title and abstract review stage, studies that did not address the topic of interest of the review were discarded. Moreover, during the full-text reading stage, studies were excluded if it was not possible to access the full text using institutional resources, as well as the following study types: narrative and systematic reviews, meta-analyses, and reflection articles. It is also necessary to point out that the review protocol was not registered.
The following data were extracted from the analysis of the articles included in the review: main neurological manifestation, secondary complications reported, sociodemographic variables of the patients, presence of comorbidities, and COVID-19 severity (presence of symptoms, requirement for hospitalization, etc.)
Data collected from individual case reports and case series were grouped by reported neurological manifestation and variables of interest to this study (age, sex, presence of comorbidities, and COVID-19 severity), and summarized in a table (available in the Results section). In said table, qualitative variables are presented as relative frequencies and medians with their respective interquartile ranges for qualitative and quantitative variables, respectively.
Results
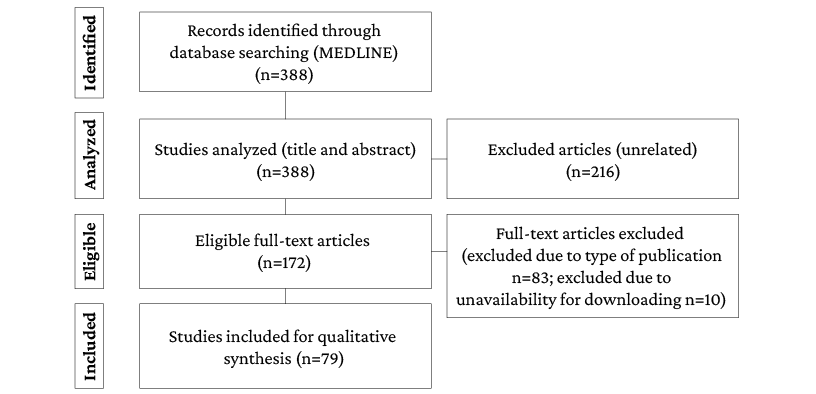
The initial search yielded 388 records, of which 216 were excluded during the title and abstract review stage because they did not address the topic of interest for the study: 10 were excluded during the full-text reading stage because of full text unavailability and 83 were excluded because of the type of study, resulting in the inclusion of 79 studies for full analysis. The search and article selection flowchart is presented in Figure 1.
Figure 1. Article search and selection flowchart.
Source: Own elaboration.
Out of the 79 included studies, 50.63% were case reports, 18.99% were case series, and 30.38% were analytical studies, the majority of which were cross-sectional studies (n=20), followed by cohort studies (n=2), and case-control studies (n=2). The most frequently reported COVID-19-associated neurological manifestation was impaired sense of smell and/or taste (43.04%), followed by peripheral neuropathy (20.25%), seizures (8.86%), encephalitis (7.59%), and delirium (5.06%). Other manifestations less frequently described were headache, myositis, stroke, and transverse myelitis (Table 1). In addition, most of the studies were published in the United States (24.05), Italy (13.92%), and Spain and the United Kingdom (8.86% each).
Demographic characteristics, presence of comorbidities, and COVID-19 severity according to neurological manifestation described in individual case reports and case series are presented in Table 2.
Table 1. Distribution of the most common neurological manifestations reported in COVID-19 patients by publication type.
|
Publication type
|
|
Case report (individual) (n=40)
|
Case series (n=15)
|
Cross-sectional study (n=20)
|
Cohort study
(n=2)
|
Case-control studies (n=2)
|
Total
|
|
Neurological manifestation
|
Smell and/or taste impairment
|
8
|
6
|
18
|
0
|
2
|
34
|
|
Peripheral neuropathy
|
11
|
5
|
0
|
0
|
0
|
16
|
|
Seizures
|
5
|
2
|
0
|
0
|
0
|
7
|
|
Encephalitis
|
6
|
0
|
0
|
0
|
0
|
6
|
|
Delirium
|
2
|
1
|
0
|
1
|
0
|
4
|
|
Other *
|
8
|
1
|
2
|
1
|
0
|
12
|
* Case report consisting of three cases with stroke; series of two cases of vasovagal syncope; two individual case reports of rhabdomyolysis; two individual case reports of visual impairment; an individual case report of ataxia, headache, and decreased consciousness.
Source: Own elaboration.
Table 2. Summary of individual cases 6,12-50 and case series.51-65
|
Impaired sense of smell and/or taste
|
Peripheral neuropathy
|
Seizures
|
Delirium
|
Encephalitis
|
Other *
|
|
Number of cases
|
27
|
20
|
10
|
8
|
6
|
15
|
|
Women
|
63%
|
68%
|
50%
|
25%
|
83%
|
46%
|
|
Age in years (IQR)
|
38 (27-53)
|
49 (35-66)
|
66.5 (47.7-76)
|
74,5 (68-83)
|
49 (29-67.5)
|
58 (38-68)
|
|
Presence of comorbidities
|
18%
|
50%
|
50%
|
75%
|
50%
|
47%
|
|
COVID-19 severity
Mild
Moderate
Severe
|
80%
8%
12%
|
62%
63%
25%
|
60%
30%
10%
|
50%
25%
25%
|
33%
17%
50%
|
67%
27%
6%
|
* Other: case report of three stroke cases; series of two cases of vasovagal syncope; two individual case reports of rhabdomyolysis; two individual case reports of visual impairment; an individual case report of ataxia, headache, and decreased consciousness. wIQR: interquartile range.
Source: Own elaboration.
In addition, Table 3 presents the objective, sample, population characteristics, severity of SARS-CoV-2 infection, primary and secondary neurological manifestations, and findings of all analytical studies (descriptive, cross-sectional, case-control, and cohort) on neurological manifestations in patients with COVID-19.
Table 3. Summary of descriptive, cross-sectional, case-control, and cohort studies on neurological manifestations in COVID-19 patients.66-89
|
Reference
|
Objective
|
n
|
Population characteristics
|
Severity of SARS-CoV-2 infection
|
Primary neurological manifestation
|
Additional neurological manifestations
|
Findings
|
|
Vaira .66 †
|
To monitor olfactory and gustatory dysfunctions in COVID-19 patients and determine their association with disease severity
|
106
|
Age: 49.6 (IQR: 43-55.2)
Female: 50%
Comorbidities:
- None: 69.8%
- One: 18.9%
- Two: 6.6%
- More than two: 4.7%
|
Hospitalized: 28.3%
|
Initial assessment (T0):
- Olfactory dysfunction: 67%.
- Gustatory dysfunction: 71%.
Tenth day of evaluation (T1):
- Anosmia: 15.1%
- Severe hyposmia: 27.4%
- Normal taste: 43.4%
20-day follow-up (T2):
- Normal smell: 44.3%
- Severe olfactory disorder: 19.7%
- Normal taste: 52.8%
|
NR
|
- Direct proportional relationship between the severity of olfactory dysfunction and fever in T2 (0.05).
- Direct proportional relationship between the severity of gustatory dysfunction and fever in both T1 and T2 (0.01 and 0.001).
- Direct proportional relationship between involvement severity based on gustatory scores and oxygen saturation. (0.001).
- Significant correlation between the severity of olfactory dysfunction and the need for hospitalization. (OR=3.7; 0.005).
|
|
Fjaeldstad67 †
|
To establish the temporal dynamics of improvement and recovery from sudden olfactory and gustatory loss in patients with suspected or confirmed diagnosis of COVID-19
|
109
|
Age (average): 39.4
Women: 72.48%
|
Outpatients: 100%
|
- Loss of smell and taste: 87.15%
- Loss of smell only: 4.59%
- Loss of taste only: 8.26%
|
Trigeminal deficit (alterations of other oral sensations such as burning, cooling, or tingling): 24.77%
|
- 44% of the patients completely recovered from the loss of smell, while 28% had no improvement.
- 50% of the patients experienced complete recovery of taste loss, while 20% had no improvement.
- Patients who experienced improvement in olfactory function were younger. (p=0.0248).
|
|
Somekh et al.68 †
|
To evaluate differences in smell and taste sensations in children and adults and to determine their correlation with angiotensin-converting enzyme 2 expression
|
73
|
Age groups:
- Children (5-17 years): 31
- Adults (≥18 years): 42
|
Outpatients: 100%
|
Smell or taste impairment: 51% (children: 25.8%; adults: 71.4%)
|
NR
|
- Smell or taste impairment was less frequent in children (risk ratio: 0.39, 95%CI: 0.23-0.65; p=0.00014)
- Smell or taste impairment was more severe in older adults than in younger adults (18-25 years old; 0.038).
- Relationship between the degree of sensory loss and the relative expression of angiotensin-converting enzyme 2 (Pearson’s Coefficient: 0.95; 0.05).
|
|
Jalessi 69†
|
To find out the frequency of olfactory impairment and its outcome in hospitalized COVID-19 patients
|
92
|
Age (mean): 52.94±13.25 years old
Female: 32.6%.
Frequent comorbidities:
- Diabetes mellitus: 23.9%.
- High blood pressure: 22.8%
- Allergies: 17.4%
- Heart disease: 15%
|
- Inpatients: 100%
- Mild pneumonia: 78.3%
- Moderate pneumonia: 21.7%
|
Olfactory impairment: 23.91%
|
Headache: 9.8%
|
- No significant association was found between sinonasal symptoms and olfactory loss, suggesting that mechanisms other than upper respiratory tract involvement are responsible for olfactory loss.
- A significant difference was found between pneumonia severity and age (p=0.002).
|
|
Kosugi .70 * †
|
To characterize patients with sudden olfactory impairment during the COVID-19 pandemic and their recovery
|
253 (79.2% tested positive for COVID-19)
|
Patients with positive COVID-19 test:
- Age (median): 36 (IQR: 31-44)
- Female: 53.1%
- Comorbidities: 32.4%
Patients with negative COVID-19 test:
- Age (median): 35.5 (IQR: 30.25-45.25)
- Females: 68.4%
- Comorbidities: 39.5%
|
NR
|
Olfactory dysfunction self-reporting: 100%
|
Headache:
- Patients with a positive COVID-19 test: 52%
- Patients with a negative COVID-19 test: 52%
|
- Patients with COVID-19 had a lower rate of olfactory dysfunction recovery than those without COVID-19 (52.6% vs. 70.3%; 0.05).
- COVID-19 patients had a longer recovery time than those without COVID-19 (15 days vs. 10 days; 0.0006).
- Patients with COVID-19 and sudden hyposmia had a higher recovery rate than those with COVID-19 and anosmia (0.04).
|
|
Chary . 71 †
|
To determine the prevalence of olfactory and taste dysfunction in patients with COVID-19, as well as short-term recovery.
|
115
|
Age (mean): 47 (range: 20-83 years)
Female: 70%
|
Hospitalization: 24%.
ICU: 4%.
|
- Olfactory or taste disorders: 70%
- Anosmia and hypogeusia: 33%
- Anosmia and ageusia: 32%
- Isolated anosmia: 15%
|
Headache: 54%
|
Olfactory or gustatory disorders were more prevalent in young patients (0.003) and in women (0.027).
- There was a significant difference in the need for hospitalization (0.016) and ICU (0.043) between patients with olfactory and taste disorders and those without these disorders.
- On day 15, 64% of patients achieved complete recovery and 33% incomplete recovery.
- The median recovery time was 15 days (range: 4-27 days) from the onset of olfactory or taste impairment symptoms.
|
|
Haehner .72 †
|
To investigate the frequency of olfactory loss in an outpatient population attending a coronavirus testing center during a 2-week period
|
500
With positive COVID-19 test: 34
|
Age (median): 41.3 years (range: 18-86 years)
Female: 54.6%
|
Outpatient: 100%
|
64.7% of COVID-19 patients reported olfactory and gustatory loss
|
NR
|
- COVID-19 patients with olfactory loss were significantly younger than those without smell loss (p=0.04) and had less severe symptoms.
- Excluding patients with blocked nose, the symptom “sudden smell loss” had a high specificity (90%) and a sensitivity of 65%, with a positive predictive value of 63% and a negative predictive value of 97% for COVID-19.
- Patients without COVID-19 experienced nasal obstruction more frequently than those with COVID-19 (p<0.001).
|
|
Dell’Era .73 †
|
To determine the prevalence and severity of smell and taste disorders in patients with COVID-19. Secondarily, to investigate the time of onset of these symptoms and their recovery time
|
355
|
Age (median): 50 years (IQR: 40- 59.5).
Female: 45.9%.
Comorbidities:
-Cardiac: 11.8%.
-Respiratory: 11.3%
-Allergic or nasosinusal: 10.7%
|
Asymptomatic or with mild or moderate symptoms: 100%
|
- Smell and/or taste disorders: 70%
- Smell impairment: 66.76%
- Taste impairment: 65.35%
|
NR
|
- Smell impairment prior to diagnosis of COVID-19 occurred in 54% of cases.
- Smell impairment was the first symptom of COVID-19 in 8.7% of the cases.
- 49.5% of patients with complete smell loss fully recovered after 14 days.
- The median recovery time was 10 days.
|
|
Boscolo-Rizzo . † 74
|
To estimate the prevalence of smell and taste impairment in people with a high risk of SARS-CoV-2 infection
|
296 people in contact with COVID-19 patients (positive COVID-19 test: 18.3%; negative COVID-19 test: 22.6%; no test: 59.1%)
|
NR
|
NR
|
Taste or smell impairment in contacts with positive COVID-19 test: 34 contacts, 63% (95%CI: 48.7-75.7).
Taste or smell impairment in contacts without COVID-19 test: 39 contacts, 22.3% (95%CI: 16.4-29.2).
|
NR
|
Contacts who tested positive for COVID-19 had significantly more symptoms (fever, cough, respiratory problems, smell or taste impairment, and other symptoms) than those with a negative COVID-19 test or no test.
|
|
Lechien .75 †
|
To investigate the frequency of olfactory dysfunction in patients with mild COVID-19 as reported in a questionnaire and evaluated with an objective psychophysical test (SNOT-22 and Sniffin’ Sticks test)
|
86
|
Age (mean): 41.7±11.8 years old
Female: 65.1%
Most frequent comorbidities:
- Gastroesophageal reflux disease: 10.5%
- Asthma: 5.8%
- Rhinitis: 5.8%
|
Mild
|
Reported:
- Anosmia: 61.4%
Objective:
- Anosmia: 47.7%
- Hypophosmia: 14%
|
Headache: 60%
|
- 38% of the patients who reported olfactory disorders had a normal result in the objective test. The prevalence of olfactory disorders may be overestimated in epidemiological studies based on subjective reports.
There was no significant association between the objective test results and the occurrence or severity of nasal obstruction or postnasal drip.
|
|
Vaira 76 †
|
To determine the frequency olfactory and gustatory function impairment in COVID-19 patients
|
345
(161 patients in quarantine at home and 184 hospitalized patients)
|
Age (mean): 48.5 ± 12.8 years (range: 23-88 years)
Female: 57.7%.
|
- Asymptomatic: 2.9%
- Mild: 48.7%.
- Moderate: 40.6%
- Severe: 7.8%
|
Chemosensitive disorders: 256, 74.2% (olfactory impairment: 94.14%; taste impairment: 60.54%).
Duration of chemosensitive symptoms:
- ≤7 days: 74.6%
- >7 days: 25.4%
Days from COVID-19 symptom onset: 14.8±7.4 (range: 2-35 days)
|
NR
|
- There was no significant correlation between the presence or severity of chemosensitive disorders and severity of COVID-19.
- Patients who reported a duration of olfactory and gustatory symptoms greater than 7 days had an increased risk of developing a moderate (relative risk: 1.12) or severe COVID-19 (relative risk: 2.33).
|
|
Coelho 77 * †
|
To describe the presentation, progression, testing status, and prognosis in patients with recent acute changes in smell or taste, regardless of the presence of COVID-19
|
220 (42.3% were positive for COVID-19 or the diagnosis was made by a physician)
|
Age (mean): 42.8±13.5 years.
Female: 78.2%.
Most frequent comorbidities:
- Seasonal allergies: 34.5%.
- Cardiovascular disease: 12.3%
- Chronic respiratory conditions (asthma, chronic obstructive pulmonary disease): 8.2%
- History of head trauma or brain injury: 4.5%
|
NR
|
Self-report of changes in smell or taste: 100%.
Changes in smell or taste as the only or first symptom: 37% (65.1% with changes in both senses, 26.5% with changes only in smell, and 8.4% with changes only in taste)
|
Headache:
- 81.7% in patients with COVID-19 diagnosis.
- 61.4% in patients with no COVID-19 diagnosis
|
- The most common COVID-19 symptoms in these patients were fatigue or weakness, headache, muscle pain, cough, fever, nasal congestion, diarrhea, and shortness of breath.
- Patients with changes in only one sense are less likely to be tested or diagnosed than patients with other symptoms (p=0.047).
- Males (p=0.020) and patients with cardiovascular disease (p=0.041) have a higher risk of presenting changes in smell or taste as their first or only symptom.
- No significant differences were found between patients with a COVID-19 diagnosis and patients without a diagnosis in terms of symptoms nasal congestion and runny nose.
|
|
Speth.78 †
|
To determine the prevalence, severity and timing of olfactory dysfunction with respect to other sinonasal and pulmonary symptoms in patients with COVID-19
|
103
|
Age (mean): 46.8±15.9 years old
Female: 51.5%.
Most frequent comorbidities:
- Allergic rhinitis: 35%.
- Asthma: 12.6%
|
Inpatients: 22.33%.
|
- Olfactory dysfunction: 61.2% (95%CI: 51.5-70.0)
- Hyposmia: 14.6% (95%CI: 51.5-70.0)
- Anosmia: 46.6%
- Taste dysfunction: 65.0% (95%CI: 55.5-73.6)
- Hypogeusia: 25.2%
- Ageusia: 39.8%
|
NR
|
- A negative correlation was found between age and olfactory dysfunction (OR=0.96, 95%CI: 0.93-0.99; p=0.007).
- A positive correlation was found between being female and experiencing olfactory dysfunction (OR=2.46, 95%CI: 0.98-6.19; p=0.056).
|
|
Paderno.79 †
|
To determine the prevalence and rate of recovery of olfactory and gustatory dysfunction in inpatients and outpatients with COVID-19
|
508
|
Age (mean): 55±15 years
Female: 44%.
Comorbidities:
- Inpatients: 78.7%
- Outpatients: 33.3%
Most frequent comorbidities in inpatients:
- High blood pressure: 47.5%
- Obesity: 20.3%
- Diabetes mellitus: 20%
- Cardiomyopathy: 16.3%
|
- Inpatients: 58% (n=295)
- Outpatients: 42% (n=213)
|
Olfactory dysfunction:
- Total: 55.70% (95%CI: 51-60)
- Inpatients: 25.6%
- Outpatients: 25.6%
- Anosmia: 64%
- As first symptom 10%
Gustatory dysfunction:
- Total: 63.19% (95%CI: 59-67)
- Inpatients: 30.1%
- Outpatients: 33.1%
- Ageusia: 60%
- As first symptom: 11%
|
Headache:
- Inpatients: 26.8%
- Outpatients: 55.9%
|
- Comorbidities were more frequent in inpatients (0.001).
- Olfactory and gustatory dysfunction was more prevalent in outpatients, young participants, women, non-smokers, and participants without comorbidities.
|
|
Lee.80 †
|
To determine the prevalence and duration of anosmia and ageusia in COVID-19 patients
|
3 191
|
Age (median): 44.0 (IQR: 25-58)
Female: 63.6%.
Frequent comorbidities:
- High blood pressure: 9.8%
- Diabetes mellitus: 5.1%
|
- Mild: 83.9%.
- Moderate: 12.1%.
- Severe: 2.5%
- Critical: 1.3%
|
Anosmia or ageusia:
- Total: 488 (15.3%).
- Both: 254 (52.0%)
- Ageusia only: 99 (20.3%)
- Anosmia only: 135 (27.7%)
Duration of anosmia: 7 days (range: 4-11)
Duration of ageusia: 6 days (range: 3-10)
Recovery (mean): 7 days
|
NR
|
- Anosmia and ageusia were significantly more frequent in women (0.01) and young people (0.001).
- The majority of patients recovered before 3 weeks.
|
|
Hopkins .81 * †
|
To characterize patients reporting in a survey the onset of smell and taste disturbances during the COVID-19 pandemic and to determine early recovery rates
|
382 (only 3.92% had a COVID-19 test, of which 80% had a positive result).
|
- Age (median): 30-39 years
- Female: 73%
|
NR
|
- Complete loss of sense of smell: 86.4%
- Severe loss of smell: 11.5%
Follow-up at 1 week:
- Improvement: 80.1%
- No change: 17.6%
- Worsening: 1.9%
- Recovered: 11.5%
- Complete loss of smell: 17.3%
|
NR
|
Of the patients who reported other symptoms associated with COVID-19, 14.9% reported anosmia before their onset, 39.3% at the same time, and 45.8% after the onset of other symptoms.
|
|
Vaira .82 †
|
To objectively assess smell and taste dysfunction in patients with COVID-19 by means of physical-psychological tests
|
72
|
Age (mean): 49.2±13.7 (range: 26-90)
Female: 62.5%.
|
- Inpatients: 34.72%.
- Outpatients: 65.28%
|
Smell and taste disorders:
- Total: 73.6%
- Both: 41.7%
- Smell only: 14.4%
- Taste only: 12.5%
Type of olfactory disorder:
- Anosmia: 2.8%
- Hyposmia: 80.6%
- None: 16.7%
Type of taste disorder:
- Ageusia: 1.4%
- Hypogeusia: 47.2%
- None: 51.4%
|
Headache: 41.6%
|
- A significant correlation was found between being >50 years old and greater severity of taste disorder (p=0.003).
- Here was a significant correlation between the results of the taste impairment test (p=0.001) and smell impairment test (p=0.000) with the number of days since symptom onset (≤15 days vs. >15 days).
|
|
Yan .83 * †
|
To determine and compare the prevalence of chemosensory impairment in patients with and without COVID-19
|
262 (59 with a positive COVID-19
positive and 203 with a negative test)
|
Age: 83.74% ≤49 years old
Female: 49.2%
Most frequent comorbidities:
- Allergic rhinitis: 33.9%
- Other immunosuppressed state: 15.3%
- High blood pressure: 13.6%
- Diabetes: 8.5%
|
Inpatients:
- With positive COVID-19 test: 7% (n=4/58)
- With negative COVID-19 test: 7% (n=14/200)
|
Loss of smell:
- Positive COVID-19 test: 68%
- Negative COVID-19 test: 16%
Taste impairment:
- Positive COVID-19 test: 71%
- Negative COVID-19 test: 17%
|
Headache: 66.1%
|
- Smell and taste impairment was strongly associated with having COVID-19 (anosmia: aOR=10.9, 95%CI: 5.08-23.5; ageusia: aOR=10.2, 95%CI: 4.74-22.1).
- 74% of patients reporting COVID-19-associated loss of smell reported recovery with clinical resolution of the disease.
|
|
Liguori .84 †
|
To identify and quantify the occurrence of neurological symptoms in hospitalized patients with COVID-19 infection who underwent an anamnestic interview
|
103
|
Age (mean): 55±14.65
|
Inpatients
Non-severe COVID-19
|
- Sleep disturbance: 51%.
- Dysgeusia: 46.60%
- Hyposmia: 38.83%.
- Headache: 38.83
- Depression: 37.86
- Anxiety: 33.01%
- Daytime sleepiness: 33.01%.
- Fatigue: 32.04%
- Dizziness: 26. 21%
- Muscle ache: 24.27%
- Confusion: 22.33%
|
NR
|
- 91.3% of patients reported at least one neurological symptom.
- Women reported neurological symptoms more frequently than men (hyposmia: p=0.0047; dysgeusia: p=0.0028; headache: p=0.0047; dizziness: p=0.0431; paresthesia: p=0.0035; daytime sleepiness: p=0.0061; muscle ache: p=0.0033).
- Muscle pain and daytime sleepiness are more frequent in patients interviewed on the first and second day of hospitalization (p=0.004 and p=0.01, respectively).
- Sleep impairment is more frequent in the group of patients interviewed after more than 7 days of hospitalization (p=0.03).
|
|
Menni .85 * †
|
To identify the symptoms most predictive of COVID-19 in the US and English population with a COVID-19 test who reported information on a smartphone-based app
|
18 401
(positive COVID-19 test: 7 178):
-UK: 15 638
-US: 2 763
|
UK:
- Average age: 41.25±12.18 in patients with a positive COVID-19 test and 41.87±12.14 in those with a negative test
- Female: 71.88%
US:
- Mean age: 44.65±14.31 in patients with a positive COVID-19 test and 46±13.8 in those with a negative test
- Female: 78.10%
|
NR
|
Loss of smell and taste:
- UK: 64.76% in patients with a positive COVID-19 test and 22.68% in those with a negative test
- US: 67.49% in patients with a positive COVID-19 test and 17.33% in those with a negative test
|
Delirium
- UK: 17.87% in patients with a positive COVID-19 test and 13.25% in those with a negative test
- US: 23.55% in patients with a positive COVID-19 test and 23.47% in those with a negative test
|
- Loss of smell or taste occurred in a higher proportion in patients with a positive COVID-19 test (65.03% vs. 21.71%; OR=6.74, 95%CI: 6.31-7.21, p<0.0001).
- According to the multivariate model, it was predicted that 140 312 (17.42%) of the participants were likely to have COVID-19.
|
|
Carignan .86 ‡
|
To confirm whether anosmia and dysgeusia are specific symptoms in patients with a positive COVID-19 test
|
268 (134 with a positive COVID-19 test and 134 controls, matched for age and sex)
|
Age (median): 57.1 (IQR 41.2-64.5)
Female: 47.8%
|
Inpatients: 2 positive for COVID-19 and 1 control
|
Anosmia:
- COVID-19 patients: 51.5%.
- Control patients: 4.5%
Dysgeusia:
- COVID-19 patients: 63.4%
- Control patients: 6.7%
Anosmia and dysgeusia:
- COVID-19 patients: 64.9%
- Control patients: 8.2%
|
Headache:
- COVID-19 patients: 64.9%
- Control patients: 46.3%
Muscle pain:
- COVID-19 patients: 56.7%
- Control patients: 21.6%
|
Associations were found between the following symptoms and having COVID-19:
Crude analysis:
- Dysgeusia: OR=16.2 (95%CI: 6.6-40.0).
- Anosmia: OR=32.5 (95%CI: 8.0-132.7).
- Dysgeusia+anosmia: OR=20 (95%CI: 7.3-54.6).
- Headache: OR=2.9 (95%CI: 1.3-3.4).
Adjusted analysis:
- Anosmia, dysgeusia, or both: aOR=62.9 (95%CI: 11.0-359.7).
- Muscle pain: aOR=7.6 (95%CI: 1.9-29.9).
|
|
Tsivgoulis .87 ‡
|
To objectively assess olfactory dysfunction in hospitalized patients with COVID-19 (using two instruments: Q-SIT and SNOT-22)
|
22 cases (patients with COVID-19) and 22 controls, matched for age and sex
|
Average age: 55±10
Female: 43%.
Most frequent comorbidities:
- High blood pressure: 57%
- Diabetes mellitus: 22%
- Atrial fibrillation: 13%
- Ischemic cardiomyopathy: 13%
|
Inpatients
(moderate COVID-19 cases with no reports of intubation requirement or deaths)
|
Some degree of olfactory dysfunction: 77% in COVID-19 patients and 36% in controls
Mean Q-SIT:
- Cases: 2 (IQR: 1-2)
- Controls: 3 (IQR: 2-3)
Nasal congestion (SNOT 22): 18.75% of patients with microsmia and anosmia (n=16)
|
NR
|
- Moderate olfactory dysfunction was found in almost three quarters of COVID-19 patients.
- Olfactory dysfunction was more prevalent in COVID cases than in control patients (0.006).
|
|
Helms.88 **
|
To describe the prevalence of delirium and other neurological symptoms in COVID-19 patients treated in an intensive care unit
|
140
|
Age (median): 62 years (IQR: 52-70)
Female: 28.6%.
Most common comorbidities:
- Cardiovascular disease: 50.0%
-Diabetes: 15%
- Hematopathology/malignancy: 15%
- Respiratory disease: 15.7%
|
Severe: 100%
|
Neurological test alteration: 84.3%.
Delirium with attention, awareness, and cognition disturbances: 84.3%.
|
NR
|
- Patients with delirium and/or abnormal neurological test result were on invasive mechanical ventilation longer than those with a normal neurological test (14 days [IQR: 10-25] vs. 9 days [IQR: 5-17]; p=0.011).
- Length of intensive care unit stay was longer in patients with delirium and/or abnormal neurological test result than in those with a normal neurological test (15 days [IQR: 11-25] vs. 10 days [IQR: 6-21]; p=0,017).
|
|
Trigo.89 **
|
To analyze which symptoms and laboratory abnormalities are associated with the presence of headache in hospitalized patients with COVID-19 and to assess whether patients with a headache have a higher adjusted in-hospital risk of mortality
|
576
|
Age (mean): 67.2±14.7
Female: 43.3%
Most common comorbidities:
- High blood pressure: 52.1%
- Cardiac disorders: 26.7%
- Pulmonary diseases: 25%
-Diabetes: 19.6%
|
Inpatients: 100%
|
Headache:23.7% (As first symptom: 26.0% [within the first 24 hours: 38.5%; within the first 48 hours: 62.5%; within the first 72 hours: 74.0%])
|
- Anosmia: 25.3%
- Myalgia 24.1%
|
- Having anosmia, muscle pain or fever and being female were associated with an increased risk of headache in COVID-19 patients.
- The presence of headache was more frequent in women (0.019) and patients with a history of headache (0.019).
- Headache was less frequent in patients with cardiac disorders (0.001) and hypertension (0.001).
- Patients with headache had a better score on the modified Rankin scale, which measures the level of dependence in activities of daily living (0.75 vs. 0.15; 0.001).
- In the multivariate analysis, patients with headache had a lower risk of death (OR=0.39, 95%CI: 0.17-0.88, 95%CI: 0.17-0.88; 0.007).
- Variables associated with headache in the multivariate analysis: anosmia, arthralgia, age, female sex, fever, myalgia, increased C-reactive protein level on admission, Rankin scale score, abnormal platelet count on admission, presence of lymphopenia on admission, elevated D-dimer level on admission, and high international normalized index during hospitalization.
|
aOR: adjusted odds ratio; CI: confidence interval; IQR: interquartile range; NR: not reported; OR: odds ratio; Q-SIT: Quick Smell Identification Test; SNOT-22: SinoNasal Outcome Test 22; UK: United Kingdom; US: United States of America.
* Data collection via internet or mobile applications.
† Cross-sectional study.
‡ Case-control study.
** Cohort study.
Source: Own elaboration.
Discussion
The articles retrieved describe the neurological manifestations that were associated with COVID-19 during a 6-month period following the WHO declaration of the pandemic.2 The main findings of these studies are presented below by type of neurological manifestation.
Impaired sense of smell and taste
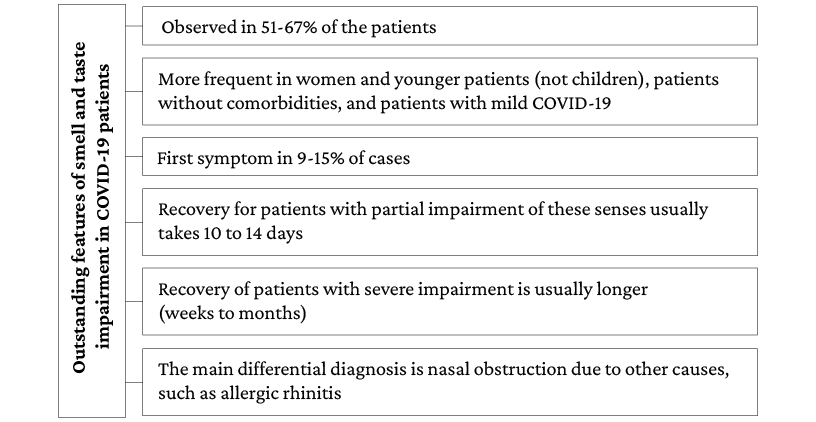
Most of the publications included in the present review report an impaired sense of smell and taste (43%). Moreover, evidence suggests that between 51% and 67% of patients with mild COVID-19 report impairment of these senses. However, only one study80 conducted in South Korea in 3 191 patients reported the prevalence of acute anosmia or ageusia in 15.3% (n=488/3 191) of patients in the early stages of COVID-19 and 15.7% (n=367/2 342) of patients with asymptomatic-to-mild disease severity. It should be noted that the main limitation of this research is that it studied the complete impairment of these senses, omitting the review of partial alterations.
On the other hand, a lower prevalence of these disorders was observed in patients with moderate and severe COVID-19 (23%-40%, respectively), since most of the analytical studies reviewed reported that the presence of these neurological manifestations was significantly more frequent in young people, women, patients without comorbidities, and patients with mild COVID-19. Furthermore, the only publication suggesting that there might be a direct association between disease severity and olfactory impairment is the study by Vaira et al.,66 in which a statistically significant correlation was found between the severity of olfactory or gustatory impairment and the presence of fever, altered oxygen saturation, and requirement for hospitalization in patients with severe SARS-CoV-2 infection.
Studies using standardized tools for measuring smell and taste impairment (such as the SinoNasal Outcome Test 22 [SNOT-22] and the Quick Smell Identification Test [Q-SIT]) confirm that COVID-19 patients experience these impairments more frequently than the general population.75,82,87 Likewise, these studies suggest that the duration of sensory impairment is proportional to its severity. These papers also report that smell and taste disorders cannot be objectively confirmed in 26-51% of the patients who report them.
Regarding the usefulness of smell and taste impairment as a marker for COVID-19, three aspects stand out. First, case-control studies clearly showed that these disorders are significantly more frequent in patients with COVID-19 than in the general population.86,87 Second, individuals who report the presence of smell and taste impairment are more likely to have COVID-19 if they also have other symptoms of infection; for example, Menni et al.85 report that smell and taste impairment, fatigue, persistent cough, and loss of appetite were significantly associated with SARS-CoV-2 infection. Thirdly, it has been reported that the main differential diagnosis in cases of sudden smell and taste impairment is nasal obstruction due to other causes, such as allergic rhinitis.72
Concerning the onset and duration of symptoms, data are quite heterogeneous. The reviewed publications report that between 9% and 15% of patients presented anosmia as the first symptom of the disease.73,81 Also, most patients report partial smell and taste impairment and a recovery time of 10 to 14 days, although some cases may require up to 1 month to recover.71,73,80
It is noteworthy that recovery seems to be related to the degree of smell and taste disorder, since patients with anosmia took longer to recover than those with hyposmia.66,70,76,82 Likewise, a prolonged duration of these impairments could be associated with the severity of COVID-19; however, the correlation between the severity of smell and taste impairment and the severity of COVID-19 has not been clearly established.66 The most relevant aspects of this disorder are presented in Figure 2.
Figure 2. Main findings on olfactory and gustatory impairment in COVID-19 patients.
Source: Own elaboration.
Peripheral nerve involvement
Sixteen articles (case studies and case series) were retrieved, reporting 20 COVID-19 patients with neuropathies, polyneuropathies, and polyradiculopathies. In general, these patients were younger than 60 years of age (only 7 were older) and had few comorbidities since only three of them had hypertension and one had paranoid schizophrenia (a 48-year-old man). Additionally, more than half of these patients had mild COVID-19, while only 3 had severe COVID-19, and no participant died. This may be related to the moment of neuropathy onset because, in most cases, the neuropathy occurred after the acute stage of the disease.
Regarding the type of neuropathy, most cases corresponded to Guillain-Barré syndrome (GBS) or one of its variants, and its onset occurred between 6 and 21 days after the onset of signs and symptoms; it was also reported that these neurological manifestations usually occurred after the onset of respiratory and systemic symptoms of COVID-19. This is consistent with reports in similar studies.90
Other types of neuropathy found were facial paralysis, hearing impairment, and supranuclear ophthalmoplegia. In general, the prognosis of patients with peripheral nerve involvement was good and chronic symptoms were reported in only one case. This case involved a 72-year-old man who had had mild COVID-19 (which manifested only with diarrhea) and whose comorbidities included hypertension, coronary artery disease, and alcoholism. This patient presented a very severe form of GBS and dysautonomia and had an unfavorable progression, requiring tracheostomy and percutaneous endoscopic gastrostomy tube placement.28
Headache
While only one of the retrieved analytical articles focuses on investigating the association between headache and COVID-19, 11 publications report data on this symptom (Table 3).69-71,75,77,79,82-84,86,89 In 8 of them, the prevalence of headache in patients with COVID-19 exceeds 50% and even reaches 82%. Importantly, most of these patients had mild SARS-CoV-2 infection. In fact, prevalence data below 50% is for patients with moderate and severe disease.
Despite the above, headache is considered a nonspecific symptom and is not included in the items considered in the diagnostic algorithms for COVID-19. In general terms, this symptom seems to be more frequent in women and in patients with a history of primary headache.89
Distribution of neurological manifestations at different age ranges
The behavior of neurological manifestations in patients with SARS-CoV-2 infection had a different distribution across extremes of age. One of the conditions presenting in elderly patients is delirium. In the case reports and case series in which this disorder was reported,30,31,57,62 all patients (n=8) were older than 65 years and, at the same time, they had more comorbidities than the patients described in the other case reports included in the present review. In addition, half of these patients had mild COVID-19 (n=4), but it is striking that 3 of these participants had dementia and the other had paranoid schizophrenia. These findings confirm that patients with dementia, even if they have mild COVID-19, are at increased risk of developing delirium. Moreover, case reports reporting data on symptoms of delirium or mental confusion indicate that these symptoms appeared within the first four days from the onset of disease symptoms,31,62 suggesting that delirium may be an early neurological manifestation in older adults.
On the other hand, it was found that the presence of delirium is a marker for poor prognosis in older adults with severe COVID-19. In this regard, in the study by Helms et al.,88 patients with delirium required invasive mechanical ventilation for a longer time and had a longer stay in the intensive care unit (ICU) than those without delirium and with a normal neurological examination.
At the other extreme of age, very few articles reported data on pediatric patients, which is a limitation for the representativeness of all population groups. On this point, only four of the publications reviewed explicitly refer to children; actually, being of legal age is one of the inclusion criteria in most of the studies reviewed. In particular, two of the four articles report smell and taste impairment. On the one hand, in the study by Somekh et al.,68 in which 31 of the patients were between 5 and 17 years of age, it was found that these symptoms were less frequent in this age group (children: 26%; adults: 71%). On the other hand, in the United Kingdom, Mak et al.,55 described three cases of adolescents between 14 and 17 years of age with COVID-19 who presented loss of smell and/or taste.
The other two articles are individual case reports. The first reports the case of a previously healthy 11-year-old boy who presented status epilepticus and was subsequently diagnosed with encephalitis based on the findings of cerebrospinal fluid analysis. Based on what was reported, the patient tested positive for COVID-19 as well as for rhinovirus/enterovirus on nasal swab test, although no evidence of the latter was found in this fluid, and finally, the child recovered completely in 6 days without the need for treatment.41
The second reports a case of COVID-19-associated rhabdomyolysis in a 16-year-old patient with autism spectrum disorder, attention deficit hyperactivity disorder, morbid obesity, obstructive sleep apnea, and eczema. According to this report, the patient, who tested positive for COVID-19, presented with myalgia, fever, mild shortness of breath, and dark-colored urine, and his creatine phosphokinase level was 427 656U/L, leading to a diagnosis of rhabdomyolysis. Finally, the child progressed satisfactorily during hospitalization and was discharged after 12 days with creatine phosphokinase levels of 6526U/L.45
Severe neurological manifestations
In addition to severe forms of GBS and cases of delirium that were associated with extubation difficulties and longer ICU stay, other severe neurological manifestations associated with COVID-19 have been described. Although the case of a pediatric patient with encephalitis was mentioned above, inflammatory involvement of the central nervous system was also reported in five other patients in the publications reviewed. Of these, a case of acute disseminated encephalomyelitis following SARS-CoV-2 infection in a 64-year-old woman with hypertension and vitiligo stands out.42 Also, 3 patients between 35 and 59 years of age (2 with severe COVID-19 and 1 with mild COVID-19) were reported to have encephalitis in the second week after symptom onset.37-39 It is noteworthy that only 1 of these 3 patients had comorbidities and, in fact, was the only one who died.
Likewise, a series of three cases of patients aged 33, 77 and 55 years with mild COVID-19 from the United States, who were admitted to the emergency department due to clinical symptoms of stroke, was retrieved. In these three cases, internal carotid artery thrombosis was documented.60
In addition, epileptic seizures associated with SARS-CoV-2 infection were reported in 6 patients, of whom 1 had severe infection and 2 had a previous diagnosis of epilepsy.32,33,35,36,64 In 5 of these patients, the onset of seizures was focal, while in the other one it was described as generalized tonic-clonic. In turn, 4 of the 6 patients presented status epilepticus, of which 2 had de novo status epilepticus, 1 had sequelae of herpetic encephalitis, and the other had thrombosis of the venous sinuses requiring craniotomy. Besides epileptiform activity, electroencephalogram monitoring reported encephalopathic tracing and graphoelements, such as periodic lateralized epileptiform discharges.
Other non-epileptic paroxysmal disorders were also described in some patients. The occurrence of vasovagal syncope was reported in 5 patients, most of whom were over 65 years of age,34,65 and only 1 of them reported the presence of convulsive epileptic seizures associated with vasovagal syncope, as well as autonomic dysfunction.34 Finally, a series of three patients with generalized non-epileptic myoclonus, similar to the startle reflex, was interpreted as a para-infectious immune-mediated disorder.63 None of the patients with vasovagal syncope or myoclonus had severe COVID-19.
Regarding the limitations of this literature review, two aspects should be mentioned that could have affected the inclusion of all the articles published on this topic in the period analyzed.
First, there is an underreporting of clinical manifestations that may be interpreted as general manifestations, but which, in other contexts, may be considered neurological. Such is the case of symptoms like myalgias, which are not the main objective of any of the articles retrieved, but are reported in at least 12 of the patients described, of which only 3 presented rhabdomyolysis.16,17,23,35,37,44-47,50,63,64 In addition, some of the descriptive articles reviewed report prevalence figures of myalgia in patients with COVID-19 between 25% and 57%,84,86,89 which is similar to those described in patients with headache, as mentioned above.
Second, some articles may not have been identified because, although they address this topic, they do not meet the parameters of the proposed search strategy, for example, those that do not include the selected keywords. Despite the above, the results obtained in this literature review allow us to establish an overview of the neurological manifestations in patients with COVID-19 infection.
Conclusions
In the six months following the declaration of the COVID-19 pandemic, multiple articles were published reporting data on the spectrum of neurological manifestations associated with SARS-CoV-2 infection. The search, review and analysis of these studies provided a general overview and led to the conclusion that, although the main involvement of COVID-19 is pulmonary, the nervous system can also be affected directly or indirectly. While taste and smell impairment is the most studied and reported neurological manifestation, a wide number of such manifestations were found to be associated with the disease.
Some of these neurological manifestations, such as headache and myalgia, are underreported because they are considered general symptoms and signs of the disease. However, other more specific manifestations such as epileptic seizures, peripheral nerve inflammation, encephalitis, delirium, and stroke have been described. Most of them do not seem to be related to the patient’s previous health condition or to the severity of COVID-19. Furthermore, other neurological manifestations such as delirium and epileptic seizures may be more frequent in patients with a history of dementia and epilepsy, respectively.
Finally, it has been reported that the prognosis of patients with these neurological manifestations is usually favorable, except in those with conditions that cause sequelae, such as GBS or stroke. Future research is expected to better characterize the mechanisms underlying nervous system involvement in patients with COVID-19.
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgments
None stated by the authors.
References
1.Mann R, Perisetti A, Gajendran M, Gandhi Z, Umapathy C, Goyal H. Clinical Characteristics, Diagnosis, and Treatment of Major Coronavirus Outbreaks. Front Med (Lausanne). 2020;7:581521. https://doi.org/jvgx.
2.World Health Organization (WHO). Rolling updates on coronavirus disease (COVID-19). Geneva: WHO; 2020 [cited 2021 Mar 23]. Available from: https://bit.ly/3Dh8GMI.
3.Pan American Health Organization (PAHO) & World Health Organization (WHO). Epidemiological Update: Coronavirus disease (COVID-19) (11 March 2021). Washington D.C.: PAHO & WHO; 2021 [cited 2021 Mar 23]. Available from: https://bit.ly/3LDy7fH.
4.Losy J. SARS-CoV-2 Infection: Symptoms of the Nervous System and Implications for Therapy in Neurological Disorders. Neurol Ther. 2020;10(1):31-42. https://doi.org/gj9rcf.
5.Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID-19: a review article. Neurol Sci. 2020;41(7):1667-71. https://doi.org/gjvhfk.
6.Melley LE, Bress E, Polan E. Hypogeusia as the initial presenting symptom of COVID-19. BMJ Case Rep. 2020;13(5):e236080. https://doi.org/ggx4f6.
7.Khateb M, Bosak N, Muqary M. Coronaviruses and Central Nervous System Manifestations. Front Neurol. 2020;11:715. https://doi.org/gg42pn.
8.Yavarpour-Bali H, Ghasemi-Kasman M. Update on neurological manifestations of COVID-19. Life Sci. 2020;257:118063. https://doi.org/gg4rkv.
9.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18-22. https://doi.org/ggq7s2.
10.Zhou Z, Kang H, Li S, Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;267(8):2179-84. https://doi.org/ggx2n3.
11.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/gcsk56.
12.Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020;58(3):299-301. https://doi.org/gg3qmm.
13.Hjelmesæth J, Skaare D. Loss of smell or taste as the only symptom of COVID-19. Tidsskr Nor Laegeforen. 2020;140(7). https://doi.org/ggvc2t.
14.Laurendon T, Radulesco T, Mugnier J, Gérault M, Chagnaud C, El Ahmadi AA, et al. Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology. 2020;95(5):224-5. https://doi.org/ggx38n.
15.Lee JM, Lee SJ. Olfactory and Gustatory Dysfunction in a COVID-19 Patient with Ankylosing Spondylitis Treated with Etanercept: Case Report. J Korean Med Sci. 2020;34(21):e201. https://doi.org/jvj6.
16.Mermelstein S. Acute anosmia from COVID-19 infection. Pract Neurol. 2020;20(4):343-4. https://doi.org/jvj7.
17.Ollarves-Carrero MF, Rodriguez-Morales AG, Bonilla-Aldana DK, Rodriguez-Morales AJ. Anosmia in a healthcare worker with COVID-19 in Madrid, Spain. Travel Med Infect Dis. 2020;35:101666. https://doi.org/jvj8.
18.Pissurno N, Lichs GGC, Santos E, Druzian AF, Oliveira S, Paniago AMM. Anosmia in the course of COVID-19: A case report. Medicine (Baltimore). 2020;99(31):e21280. https://doi.org/gg7pbf.
19.Degen C, Lenarz T, Willenborg K. Acute Profound Sensorineural Hearing Loss After COVID-19 Pneumonia. Mayo Clin Proc. 2020;95(8):1801-3. https://doi.org/gnrtdg.
20.Figueiredo R, Falcão V, Pinto MJ, Ramalho C. Peripheral facial paralysis as presenting symptom of COVID-19 in a pregnant woman. BMJ Case Rep. 2020;13(8). https://doi.org/jvj9.
21.Homma Y, Watanabe M, Inoue K, Moritaka T. Coronavirus Disease-19 Pneumonia with Facial Nerve Palsy and Olfactory Disturbance. Intern Med. 2020;59(14):1773-5. https://doi.org/gg4725.
22.Juliao Caamaño DS, Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARS-CoV-2. J Clin Neurosci. 2020;77:230-2. https://doi.org/ggx34t.
23.Lantos JE, Strauss SB, Lin E. COVID-19-Associated Miller Fisher Syndrome: MRI Findings. AJNR Am J Neuroradiol. 2020;41(7):1184-6. https://doi.org/gj9rmd.
24.Pérez-Álvarez AI, Suárez-Cuervo C, Fernández-Menéndez S. SARS-CoV-2 infection associated with diplopia and anti-acetylcholine receptor antibodies. Neurologia. 2020;35(4):264-5. https://doi.org/jz99.
25.Pfefferkorn T, Dabitz R, von Wernitz-Keibel T, Aufenanger J, Nowak-Machen M, Janssen H. Acute polyradiculoneuritis with locked-in syndrome in a patient with Covid-19. J Neurol.2020;267(7):1883-4. https://doi.org/ggx4c8.
26.Rana S, Lima AA, Chandra R, Valeriano J, Desai T, Freiberg W, et al. Novel Coronavirus (COVID-19)-Associated Guillain-Barré Syndrome: Case Report. J Clin Neuromuscul Dis. 2020;21(4):240-2. https://doi.org/ggx39r.
27.Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: A case report and review of recent literature. J Peripher Nerv Syst. 2020;25(2):204-7. https://doi.org/ggvzx9.
28.Su XW, Palka SV, Rao RR, Chen FS, Brackney CR, Cambi F. SARS-CoV-2-associated Guillain-Barré syndrome with dysautonomia. Muscle Nerve. 2020;62(2):E48-9. https://doi.org/ggx38c.
29.Tiet MY, AlShaikh N. Guillain-Barré syndrome associated with COVID-19 infection: a case from the UK. BMJ Case Rep. 2020;13(7):e236536. https://doi.org/gg4sgn.
30.Alkeridy WA, Almaghlouth I, Alrashed R, Alayed K, Binkhamis K, Alsharidi A, et al. A Unique Presentation of Delirium in a Patient with Otherwise Asymptomatic COVID-19. J Am Geriatr Soc. 2020;68(7):1382-4. https://doi.org/ggvqnz.
31.Payne S, Jankowski A, Shutes-David A, Ritchey K, Tsuang DW. Mild COVID-19 Disease Course With Protracted Delirium in a Cognitively Impaired Patient Over the Age of 85 Years. Prim Care Companion CNS Disord. 2020;22(4). https://doi.org/j2rw.
32.Fasano A, Cavallieri F, Canali E, Valzania F. First motor seizure as presenting symptom of SARS-CoV-2 infection. Neurol Sci. 2020;41(7):1651-3. https://doi.org/ggx33x.
33.Kadono Y, Nakamura Y, Ogawa Y, Yamamoto S, Kajikawa R, Nakajima Y, et al. A case of COVID-19 infection presenting with a seizure following severe brain edema. Seizure. 2020;80:53-5. https://doi.org/gmnjkm.
34.Logmin K, Karam M, Schichel T, Harmel J, Wojtecki L. Non-epileptic seizures in autonomic dysfunction as the initial symptom of COVID-19. J Neurol. 2020;267(9):2490-1. https://doi.org/ggx4b7.
35.Lyons S, O’Kelly B, Woods S, Rowan C, Brady D, Sheehan G, et al. Seizure with CSF lymphocytosis as a presenting feature of COVID-19 in an otherwise healthy young man. Seizure. 2020;80:113-4. https://doi.org/ghc7pw.
36.Vollono C, Rollo E, Romozzi M, Frisullo G, Servidei S, Borghetti A, et al. Focal status epilepticus as unique clinical feature of COVID-19: A case report. Seizure. 2020;78:109-12. https://doi.org/ggv3xt.
37.Afshar H, Yassin Z, Kalantari S, Aloosh O, Lotfi T, Moghaddasi M, et al. Evolution and resolution of brain involvement associated with SARS- CoV2 infection: A close Clinical - Paraclinical follow up study of a case. Mult Scler Relat Disord. 2020;43:102216. https://doi.org/gmzxrt.
38.Dixon L, Varley J, Gontsarova A, Mallon D, Tona F, Muir D, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e789. https://doi.org/ggxz65.
39.Efe IE, Aydin OU, Alabulut A, Celik O, Aydin K. COVID-19-Associated Encephalitis Mimicking Glial Tumor. World Neurosurg. 2020;140:46-8. https://doi.org/gh77f8.
40.Farhadian S, Glick LR, Vogels CBF, Thomas J, Chiarella J, Casanovas-Massana A, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20(1):248. https://doi.org/j2rx.
41.McAbee GN, Brosgol Y, Pavlakis S, Agha R, Gaffoor M. Encephalitis Associated with COVID-19 Infection in an 11-Year-Old Child. Pediatr Neurol. 2020;109:94. https://doi.org/jzhz.
42.Novi G, Rossi T, Pedemonte E, Saitta L, Rolla C, Roccatagliata L, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e797. https://doi.org/ghc6ks.
43.Pattanakuhar S, Tangvinit C, Kovindha A. A Patient With Acute Cervical Cord Injury and COVID-19: A First Case Report. Am J Phys Med Rehabil. 2020;99(8):674-6. https://doi.org/gg7fm3.
44.Borku Uysal B, Ikitimur H, Yavuzer S, Islamoglu MS, Cengiz M. Case Report: A COVID-19 Patient Presenting with Mild Rhabdomyolysis. Am J Trop Med Hyg. 2020;103(2):847-50. https://doi.org/j2rz.
45.Gefen AM, Palumbo N, Nathan SK, Singer PS, Castellanos-Reyes LJ, Sethna CB. Pediatric COVID-19-associated rhabdomyolysis: a case report. Pediatr Nephrol. 2020;35(8):1517-20. https://doi.org/ggxdnh.
46.Zhang H, Charmchi Z, Seidman RJ, Anziska Y, Velayudhan V, Perk J. COVID-19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve. 2020;62(3):E57-60. https://doi.org/ghzj3t.
47.Diezma-Martín AM, Morales-Casado MI, García-Alvarado N, Vadillo Bermejo A, López-Ariztegui N, Sepúlveda Berrocal MA. Temblor y ataxia en COVID-19. Neurología. 2020;35(6):409-10. https://doi.org/j2r2.
48.Kaya Y, Kara S, Akinci C, Kocaman AS. Transient cortical blindness in COVID-19 pneumonia; a PRES-like syndrome: Case report. J Neurol Sci. 2020;413:116858. https://doi.org/ggx3zn.
49.Selvaraj V, Sacchetti D, Finn A, Dapaah-Afriyie K. Acute Vision Loss in a Patient with COVID-19. R I Med J (2013). 2020;103(6):37-8.
50.Cebrián J, Gonzalez-Martinez A, García-Blanco MJ, Celdrán-Vivancos D, Palacios EL, Reig-Roselló G, et al. Headache and impaired consciousness level associated with SARS-CoV-2 in CSF: A case report. Neurology. 2020;95(6):266-8. https://doi.org/gnp983.
51.Alamri A, Oriez C, Bouilloud F, Dupuy O, Ben Hamou A. Sudden onset anosmia and dysgeusia in two patients: An early sign of SARS-CoV-2 infection. Presse Med. 2020;49(1):104027. https://doi.org/j2r3.
52.Chen C, Chen M, Cheng C, Chi Y, Hu Z, Liu Y, et al. A special symptom of olfactory dysfunction in coronavirus disease 2019: report of three cases. J Neurovirol. 2020;26(3):456-8. https://doi.org/ggx35q.
53.Gilani S, Roditi R, Naraghi M. COVID-19 and anosmia in Tehran, Iran. Med Hypotheses. 2020;141:109757. https://doi.org/ggtxs7.
54.Kirschenbaum D, Imbach LL, Ulrich S, Rushing EJ, Keller E, Reimann RR, et al. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet. 2020;396(10245):166. https://doi.org/gg42gg.
55.Mak PQ, Chung KS, Wong JS, Shek CC, Kwan MY. Anosmia and Ageusia: Not an Uncommon Presentation of COVID-19 Infection in Children and Adolescents. Pediatr Infect Dis J. 2020;39(8):e199-e200. https://doi.org/jxtz.
56.Pallanti S. Importance of SARS-CoV-2 anosmia: From phenomenology to neurobiology. Compr Psychiatry. 2020;100:152184. https://doi.org/ggx224.
57.Beach SR, Praschan NC, Hogan C, Dotson S, Merideth F, Kontos N, et al. Delirium in COVID-19: A case series and exploration of potential mechanisms for central nervous system involvement. Gen Hosp Psychiatry. 2020;65:47-53. https://doi.org/ggxwqf.
58.Bigaut K, Mallaret M, Baloglu S, Nemoz B, Morand P, Baicry F, et al. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(5). https://doi.org/ggx4ps.
59.Dinkin M, Gao V, Kahan J, Bobker S, Simonetto M, Wechsler P, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020;95(5):221-3. https://doi.org/ggx33s.
60.Fara MG, Stein LK, Skliut M, Morgello S, Fifi JT, Dhamoon MS. Macrothrombosis and stroke in patients with mild Covid-19 infection. J Thromb Haemost. 2020;18(8):2031-3. https://doi.org/gj4t2t.
61.Kilic O, Kalcioglu MT, Cag Y, Tuysuz O, Pektas E, Caskurlu H, et al. Could sudden sensorineural hearing loss be the sole manifestation of COVID-19? An investigation into SARS-CoV-2 in the etiology of sudden sensorineural hearing loss. Int J Infect Dis. 2020;97:208-11. https://doi.org/gmg6tp.
62.Hosseini AA, Shetty AK, Sprigg N, Auer DP, Constantinescu CS. Delirium as a presenting feature in COVID-19: Neuroinvasive infection or autoimmune encephalopathy? Brain Behav Immun. 2020;88:68-70. https://doi.org/j2r4.
63.Rábano-Suárez P, Bermejo-Guerrero L, Méndez-Guerrero A, Parra-Serrano J, Toledo-Alfocea D, Sánchez-Tejerina D, et al. Generalized myoclonus in COVID-19. Neurology. 2020;95(6):e767-72. https://doi.org/ggxdr6.
64.Somani S, Pati S, Gaston T, Chitlangia A, Agnihotri S. De Novo Status Epilepticus in patients with COVID-19. Ann Clin Transl Neurol. 2020;7(7):1240-4. https://doi.org/ggx4ds.
65.Birlutiu V, Birlutiu RM, Feiereisz AI. SARS-CoV-2 infection associated with micturition syncope: Our experience with 4 case reports. Medicine (Baltimore). 2020;99(31):e21512. https://doi.org/gg7pdk.
66.Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Soma D, Giovanditto F, et al. Do olfactory and gustatory psychophysical scores have prognostic value in COVID-19 patients? A prospective study of 106 patients. J Otolaryngol Head Neck Surg. 2020;49(1):56. https://doi.org/gg7n9n.
67.Fjaeldstad AW. Prolonged complaints of chemosensory loss after COVID-19. Dan Med J. 2020;67(8):A05200340.
68.Somekh I, Yakub Hanna H, Heller E, Bibi H, Somekh E. Age-Dependent Sensory Impairment in COVID-19 Infection and its Correlation with ACE2 Expression. Pediatr Infect Dis J. 2020;39(9):e270-2. https://doi.org/gg42sj.
69.Jalessi M, Barati M, Rohani M, Amini E, Ourang A, Azad Z, et al. Frequency and outcome of olfactory impairment and sinonasal involvement in hospitalized patients with COVID-19. Neurol Sci. 2020;41(9):2331-8. https://doi.org/gg4r3q.
70.Kosugi EM, Lavinsky J, Romano FR, Fornazieri MA, Luz-Matsumoto GR, Lessa MM, et al. Incomplete and late recovery of sudden olfactory dysfunction in COVID-19. Braz J Otorhinolaryngol. 2020;86(4):490-6. https://doi.org/jv4z.
71.Chary E, Carsuzaa F, Trijolet JP, Capitaine AL, Roncato-Saberan M, Fouet K, et al. Prevalence and Recovery From Olfactory and Gustatory Dysfunctions in Covid-19 Infection: A Prospective Multicenter Study. Am J Rhinol Allergy. 2020;34(5):686-93. https://doi.org/gkhsxj.
72.Haehner A, Draf J, Dräger S, de With K, Hummel T. Predictive Value of Sudden Olfactory Loss in the Diagnosis of COVID-19. ORL J Otorhinolaryngol Relat Spec. 2020;82(4):175-80. https://doi.org/grpgrf.
73.Dell’Era V, Farri F, Garzaro G, Gatto M, Aluffi Valletti P, Garzaro M. Smell and taste disorders during COVID-19 outbreak: Cross-sectional study on 355 patients. Head Neck. 2020;42(7):1591-6. https://doi.org/ghc7tf.
74.Boscolo-Rizzo P, Borsetto D, Spinato G, Fabbris C, Menegaldo A, Gaudioso P, et al. New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2-positive subjects. Eur Arch Otorhinolaryngol. 2020;277(9):2637-40. https://doi.org/ggx2rr.
75.Lechien JR, Cabaraux P, Chiesa-Estomba CM, Khalife M, Hans S, Calvo-Henriquez C, et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 2020;42(7):1583-90. https://doi.org/ggx2bd.
76.Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 2020;42(7):1560-9. https://doi.org/ggx2bq.
77.Coelho DH, Kons ZA, Costanzo RM, Reiter ER. Subjective Changes in Smell and Taste During the COVID-19 Pandemic: A National Survey-Preliminary Results. Otolaryngol Head Neck Surg. 2020;163(2):302-6. D https://doi.org/ggx22x.
78.Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: Prevalence, Severity, Timing, and Associated Characteristics. Otolaryngol Head Neck Surg. 2020;163(1):114-20. https://doi.org/ggwtft.
79.Paderno A, Schreiber A, Grammatica A, Raffetti E, Tomasoni M, Gualtieri T, et al. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol. 2020;10(8):955-62. https://doi.org/ggx25g.
80.Lee Y, Min P, Lee S, Kim SW. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J Korean Med Sci. 2020;35(18):e174. https://doi.org/ggvwx9.
81.Hopkins C, Surda P, Whitehead E, Kumar BN. Early recovery following new onset anosmia during the COVID-19 pandemic - an observational cohort study. J Otolaryngol Head Neck Surg. 2020;49(1):26. https://doi.org/ggv23m.
82.Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck. 2020;42(6):1252-8. https://doi.org/ggtx2t.
83.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806-13. https://doi.org/ggr22r.
84.Liguori C, Pierantozzi M, Spanetta M, Sarmati L, Cesta N, Iannetta M, et al. Subjective neurological symptoms frequently occur in patients with SARS-CoV-2 infection. Brain Behav Immun. 2020;88:11-6. https://doi.org/ggx24h.
85.Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(7):1037-40. https://doi.org/ggvzp2.
86.Carignan A, Valiquette L, Grenier C, Musonera JB, Nkengurutse D, Marcil-Héguy A, et al. Anosmia and dysgeusia associated with SARS-CoV-2 infection: an age-matched case-control study. Cmaj. 2020;192(26):E702-7. https://doi.org/ggxz6c.
87.Tsivgoulis G, Fragkou PC, Delides A, Karofylakis E, Dimopoulou D, Sfikakis PP, et al. Quantitative evaluation of olfactory dysfunction in hospitalized patients with Coronavirus [2] (COVID-19). J Neurol. 2020;267(8):2193-5. https://doi.org/ggx2qr.
88.Helms J, Kremer S, Merdji H, Schenck M, Severac F, Clere-Jehl R, et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24(1):491. https://doi.org/gg7t6f.
89.Trigo J, García-Azorín D, Planchuelo-Gómez A, Martínez-Pías E, Talavera B, Hernández-Pérez I, et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: a retrospective cohort study. J Headache Pain. 2020;21(1):94. https://doi.org/gg6n4x.
90.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767-83. https://doi.org/d259.
 Carlos Mario Echeverria-Palacio2,3
Carlos Mario Echeverria-Palacio2,3 Sofía Albornoz1
Sofía Albornoz1 Juan Argothy1
Juan Argothy1 Lilia Ballesteros-Egurrola1
Lilia Ballesteros-Egurrola1 María Camila Ocampo1
María Camila Ocampo1 Juan Felipe Vera1
Juan Felipe Vera1