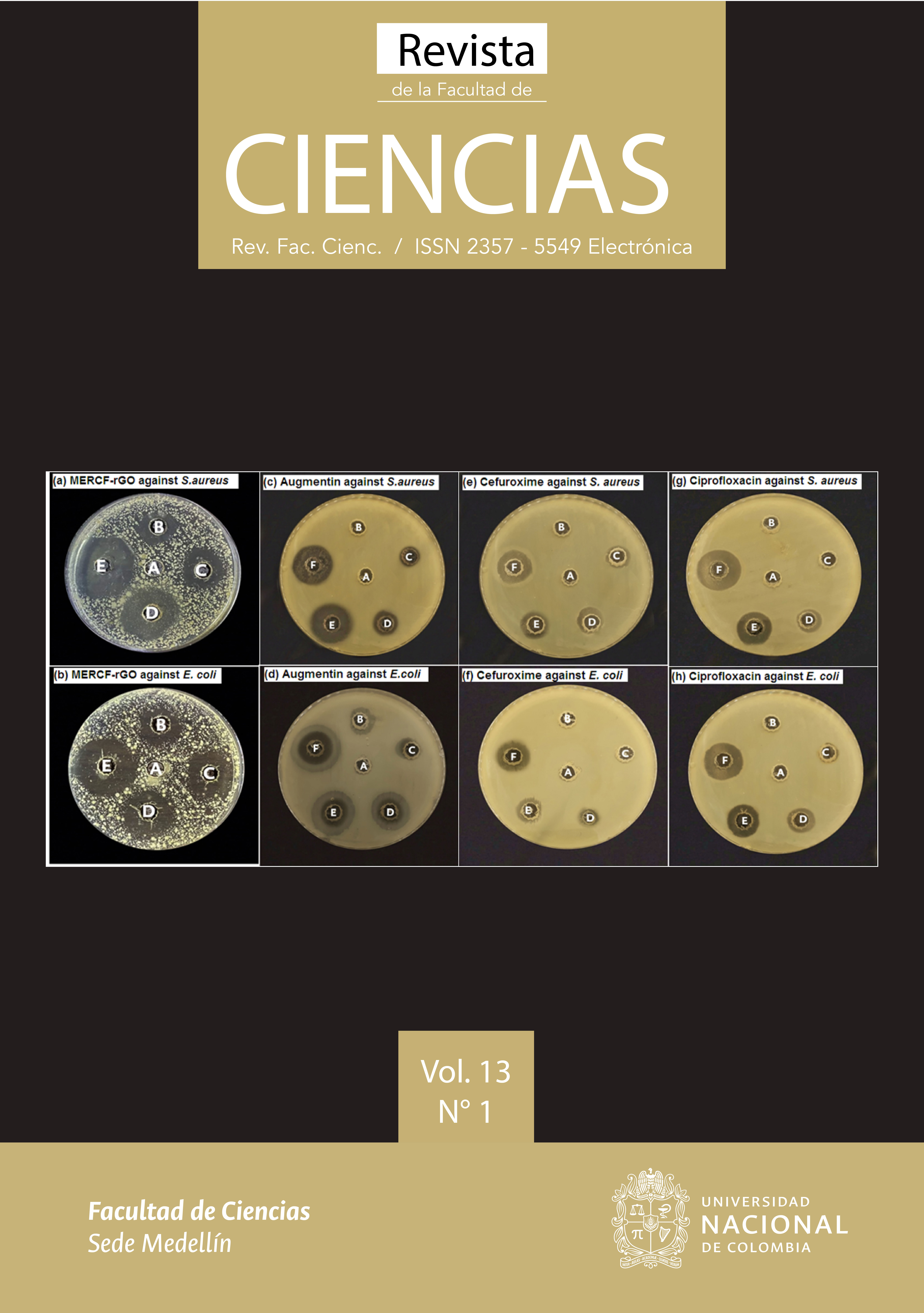
MODELOS DE INTERACCIONES ATÓMICAS EN MECÁNICA MOLECULAR
MODELS OF ATOMIC INTERACTIONS IN MOLECULAR MECHANICS
DOI:
https://doi.org/10.15446/rev.fac.cienc.v13n1.109657Palabras clave:
mecánica molecular, modelos matemáticos, interacciones atómicas (es)molecular mechanics, mathematical models, atomic interactions (en)
Descargas
Referencias
Allinger, N. L. (1976). Calculation of molecular structure and energy by force-field methods. In: Advances in physical organic chemistry. Gold, V.; Bethell, D. eds. Academic Press. 1-82. DOI: https://doi.org/10.1016/S0065-3160(08)60212-9
Allured, V. S., Kelly, C. M. & Landis, C. R. (1991). SHAPES empirical force field: new treatment of angular potentials and its application to square-planar transition-metal complexes. J. Am. Chem. Soc. 113 1-12. DOI: https://doi.org/10.1021/ja00001a001
Anandakrishnan, R., Baker, C., Izadi, S. & Onufriev, A. V. (2013). Point charges optimally placed to represent the multipole expansion of charge distributions. PLOS ONE, 8, e67715. DOI: https://doi.org/10.1371/journal.pone.0067715
Anisimov, V. M., Lamoreux, G., Vorobyov, I. V., Huang, N., Roux, B. & MacKerell, A. D. (2005). Determination of electrostatic parameters for a polarizable force field based on the classical Drude oscillator. J. Chem. Theory Comput., 1, 153-168. DOI: https://doi.org/10.1021/ct049930p
Applequist, J. (1985). A multipole interaction theory of electric polarization of atomic molecular assemblies. J. Chem. Phys., 83, 809-826. DOI: https://doi.org/10.1063/1.449496
Applequist, J., Carl, J. R. & Fung, K. K. (1972). An atom dipole interaction model for molecular polarizability. Application to polyatomic molecules and determination of atom polarizabilities. J. Am. Chem. Soc., 94, 2952-2960. DOI: https://doi.org/10.1021/ja00764a010
Aranha, M. P., Spooner, C., Demerdash, O., Czejdo, B., Smith & J. C., Mitchell, J. C. (2020). Prediction of peptide binding to MHC using machine learning with sequence and structure-based feature sets. Biochim. Biophys. Acta, Gen. Subj., 1864, 129535. DOI: https://doi.org/10.1016/j.bbagen.2020.129535
Artemova, S., Jaillet, L. & Redon, S. (2016). Automatic molecular structure perception for the universal force field. J. Comput. Chem., 37, 1191-1205. DOI: https://doi.org/10.1002/jcc.24309
Bader, R. F. W. (1994). Atoms in molecules: a quantum theory. Oxford University Press. 438 p.
Bayly, C I., Ciepak, P., Cornell, W. D. & Kollman, P. A. (1993). A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem., 97, 10269-10280. DOI: https://doi.org/10.1021/j100142a004
Berendsen, H. J. C., Grigera, J. R. & Straatsma, T. P. (1987). The missing term in effective pair potentials. J. Phys. Chem., 91, 6269-6271. DOI: https://doi.org/10.1021/j100308a038
Berthelot, D. (1898). On the gas mixture. Comptes Rendus Acad. Sci., 126, 1703-1706.
Bondi, A. (1964). Van der Waals volumes and radii. J. Phys. Chem., 68, 441-451. DOI: https://doi.org/10.1021/j100785a001
Born, M. & Mayer, J. E. (1932). On the lattice theory of ion crystals. Z. Physik, 75, 1-18. DOI: https://doi.org/10.1007/BF01340511
Bowen, J. P. & Allinger, N. L. (1991). Molecular mechanics: the art and science of parametrization. In: Reviews in Computational Chemistry. Lipkowitz, K. B., Boyd, D. B. eds. Wiley-VCH Inc. 81-97. DOI: https://doi.org/10.1002/9780470125793.ch3
Buckingham, R. A. (1938), The classical equation of state of gaseous helium, neon and argon. Proc. R. Soc. Lond. A, 168, 264-283. DOI: https://doi.org/10.1098/rspa.1938.0173
Burden, R. L. & Faires, J. D. (2011). Numerical analysis. Brooks/Cole, Cengage Learning. Boston, USA. 872 p.
Cardamone, S., Hughes, T. J. & Popelier, P. L. A. (2014). Multipolar electrostatics. Phys. Chem. Chem. Phys., 16, 10367-10387. DOI: https://doi.org/10.1039/c3cp54829e
Chen, J., Hundertmark, D. & Martínez, T. J. (2008). A unified theoretical framework for fluctuating-charge models in atom-space and bond-space. J. Chem. Phys., 129, 214113. DOI: https://doi.org/10.1063/1.3021400
Chen, T., Li, M.& Liu, J. (2018). $pi-pi$ stacking interaction: a nondestructive and facile means in material engineering for bioapplications. Cryst. Growth Des., 18, 2765-2783. DOI: https://doi.org/10.1021/acs.cgd.7b01503
Chen, J. & Martínez, T. J. (2007). QTPIE: charge transfer with polarization current equalization. A fluctuating charge model with correct asymptotics. Chem. Phys. Lett., 438, 315-320. DOI: https://doi.org/10.1016/j.cplett.2007.02.065
Chen, J. & Martínez, T. J. (2009). The dissociation catastrophe in fluctuating charge models and its implications for the concept of atomic electronegativity. In: Progress in theoretical chemistry and physics. Piecuch, P., Maruani, J., Delgado-Barrio, G., Wilson S. eds. Springer. 397-415. DOI: https://doi.org/10.1007/978-90-481-2596-8_19
Choi, H., Kang, H. & Park, H. (2009). New angle-dependent potential energy function for backbone-backbone hydrogen bond protein-protein interactions. J. Comput. Chem., 31, 897-903. DOI: https://doi.org/10.1002/jcc.21378
Cohen, E. R., Cvitas, T., Frey, J. G., Holmstr" o m, B., Kuchitsu, K., Marquardt, R., Mills, I., Pavese, F., Quack, M., Stohner, J., Strauss, H. L., Takami, M. & Thor, A. J. (2008). Quantities, units and symbols in physical chemistry. IUPAC Green Book. IUPAC & RSC Publishing. 233 p.
Coulomb, C. A. (1884). First memoir on electricity and magnetism. Construction & use of an electrical balance, based on the property of the metal wires, to have a torsion reaction force proportional to the twist angle. In: Collection de Mémoires relatifs a la Physique. La Société Franc c aise de Physique. Paris. 107-115.
Cox, S. R. & Williams, D. E. (1981). Representation of the molecular electrostatic potential by a net atomic charge model. J. Comput. Chem., 2, 304-323. DOI: https://doi.org/10.1002/jcc.540020312
Dahiyat, B., Gordon, D. B. & Mayo, S. L. (1997). Automated desing of the surface points of protein helices. Protein Sci., 6, 1333-1337. DOI: https://doi.org/10.1002/pro.5560060622
Dans, P. D., Zeida, A., Machado, M. R. & Pantano, S. (2010). A coarse grained model for atomic-detailed DNA simulations with explicit electrostatics. J. Chem. Theory Comput., 6, 1711-1725. DOI: https://doi.org/10.1021/ct900653p
Darré, L., Machado, M. R., Brandner, A. F., González, H. C., Ferreira, S. & Panatano, S. (2015). SIRAH: a structurally unbiased coarse-grained force field for proteins with aqueous solvation and long-range electrostatics. J. Chem. Theory Comput., 11, 723-739. DOI: https://doi.org/10.1021/ct5007746
Demerdash, O. N. A. & Mitchell, J. C. (2013). Using physical potentials and learned models to distinguish native binding interfaces from de novo designed interfaces that do not bind. Proteins, 81, 1919-1930. DOI: https://doi.org/10.1002/prot.24337
Denning, E. J., Priyakumar, U. D., Nilsson, L. & MacKerell Jr., A. D. (2011). Impact of 2'-hydroxyl sampling on the conformational properties of RNA: update of the CHARMM all-atom additive force field for RNA. J. Comput. Chem., 32, 1929-1943. DOI: https://doi.org/10.1002/jcc.21777
Dickson, C. J., Walker, R. C. & Gould, I. R. (2022). Lipid21: complex lipid membrane simulations with AMBER. J. Chem. Theory Comput., 18, 1726-1736. DOI: https://doi.org/10.1021/acs.jctc.1c01217
Dykstra, C. E. (1988). Efficient calculation of electrically based intermolecular potentials of weakly bonded clusters. J. Comput. Chem., 9, 476-487. DOI: https://doi.org/10.1002/jcc.540090506
Dykstra, C. E. (1993). Electrostatic interaction potentials in molecular force fields. Chem. Rev., 93, 2339-2353. DOI: https://doi.org/10.1021/cr00023a001
Ermer O. (1976). Calculation of molecular properties using force fields. Applications in organic chemistry. In: Bonding Forces. Springer. Heidelberg. 161-211. DOI: https://doi.org/10.1007/3-540-07671-9_3
Fuji, H., Qi, F., Qu, L., Takaesu, Y. & Hoshino, T. (2017). Prediction of ligand binding affinity to target proteins by molecular mechanics theoretical calculation. Chem. Phar. Bull., 65, 461-468. DOI: https://doi.org/10.1248/cpb.c16-00913
Gao, J., Habibollazadeh, D. & Shao, L. (1995). A polarizable intermolecular potential function for simulation of liquid alcohols. J. Phys. Chem., 99, 16460. DOI: https://doi.org/10.1021/j100044a039
Gasteiger, J. & Marsili, M. (1980a). Iterative partial equalization of orbital electronegativity -- A rapid access to atomic charges. Tetrahedron, 36, 3219-3228. DOI: https://doi.org/10.1016/0040-4020(80)80168-2
Gasteiger, J. & Marsili, M. (1980b). $pi$ charge distribution from molecular topology and $pi$ orbital electronegativity. Croat. Chem. Acta, 53, 601-614.
Gresh, N., Cisneros, A., Darden, T. A. & Piquemal, J-P. (2007). Anisotropic, polarizable molecular mechanics studies of inter- and intramolecular interactions and ligand-macromolecule complexes. A bottom-up strategy. J. Chem. Theory Comput., 3, 1960-1986. DOI: https://doi.org/10.1021/ct700134r
Halgren, T. A. (1996). Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem., 17, 490-519. DOI: https://doi.org/10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P
Harder, E., Anisimov, V. M., Vorobyov, I. V., Lopes, P. E. M., Noskov, S. Y., MacKerell Jr., A. D. & Roux, B. (2006). Atomic level anisotropy in the electrostatic modeling of lone pairs for a polarizable force field based on the classical Drude oscillator. J. Chem. Theory Comput., 2, 1587-1597. DOI: https://doi.org/10.1021/ct600180x
Harder, E., Damm, W., Maple, J., Wu, C., Reboul, M., Xiang, J. Y., Wang, L., Lupyan, D., Dahlgren, M. K., Knight, J. L., Kaus, J. W., Cerutti, D. S., Krilov, G., Jorgensen, W. L., Abel, R. & Friesner, R. A. (2015). OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput., 12, 281-296. DOI: https://doi.org/10.1021/acs.jctc.5b00864
Hart, K., Foloppe, N., Baker, C. M., Denning, E. J., Nilsson, L. & MacKerell Jr., A. D. (2012). Optimization of the CHARMM additive force field for DNA: improved treatment of the BI/BII conformational equilibrium. J. Chem. Theory Comput., 8, 348-362. DOI: https://doi.org/10.1021/ct200723y
Hill, T. L. (1948). Steric effects. I. Van der Waals potential energy curves. J. Chem. Phys., 16, 399-404. DOI: https://doi.org/10.1063/1.1746902
Hinze, J. & Jaffé, H. H. (1962). Electronegativity. I. Orbital electronegativity of neutral atoms. J. Am. Chem. Soc., 84, 540-546. DOI: https://doi.org/10.1021/ja00863a008
Hinze, J., Whitehead, M. A. & Jaffé, H. H. (1963). Electronegativity. II. Bond and orbital electronegativities. J. Am. Chem. Soc., 85, 148-154. DOI: https://doi.org/10.1021/ja00885a008
Hooke, R. (1678). Lectures de potentia restitutiva, or of spring, explaining the power of springing bodies. [En línea]. Carnegie Mellon University. Digital Collections. [Consultada en Julio de 2022]. Disponible en: https://digitalcollections.library.cmu.edu/node/68323
Horn, H. W., Swope, W. C., Pitera, J. W., Madura, J. D., Dick, T. J., Hura, G. L. & Head-Gordon, T. (2004). Development of an improved four-site water model for biomolecular simulations: TIP4P-Ew. J. Chem. Phys., 120, 9665-9678. DOI: https://doi.org/10.1063/1.1683075
Huang, J., Rauscher, S., Nawrocki, G., Ran, T., Feig, M., L de Groot, B., Grubm" u ller, H. & MacKerell Jr., A. D. (2017). CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods, 14, 71-73. DOI: https://doi.org/10.1038/nmeth.4067
Izadi, S., Anandakrishnan, R.& Onufriev, A. V. (2014). Building water models: a different approach. J. Phys. Chem. Lett., 5, 3863-3871. DOI: https://doi.org/10.1021/jz501780a
Izadi, S., Anandakrishnan, R. & Onufriev, A. V. (2016). Implicit solvent model for million-atom atomistic simulations: insights into the organization of 30-nm chromatin fiber. J. Chem. Theory Comput., 12, 5946-5959. DOI: https://doi.org/10.1021/acs.jctc.6b00712
Artemova, S. & Redon, S. (2017). IM-UFF: extending the universal force field for interactive molecular modeling. J. Mol. Graph. Model., 77, 350-362. DOI: https://doi.org/10.1016/j.jmgm.2017.08.023
Jensen, F. (2017). Introduction to computational chemistry. John Wiley & Sons, Ltd. Chichester, UK. 638 p.
Jones, J. E. (1924a). On the determination of molecular fields.--I. From the variation of the viscosity of a gas with temperature. Prod. R. Soc. Lond. A, 106, 441-462. DOI: https://doi.org/10.1098/rspa.1924.0081
Jones, J. E. (1924b). On the determination of molecular fields.--II. From the equation of state of a gas. Prod. R. Soc. Lond. A, 106, 463-477. DOI: https://doi.org/10.1098/rspa.1924.0082
Jorgensen, W. L., Chandrasekhar, J., Madura, J. & Klein, M. L. (1983). Comparison of simple potential functions for simulating liquid water. J. Chem. Phys., 79, 926-935. DOI: https://doi.org/10.1063/1.445869
Joung, S. & Cheatham, T. E. (2008). Determination of alkali and halide ion parameters for use in explicitly solvated biomolecular simulations. J. Phys. Chem. B, 112, 9020-9041. DOI: https://doi.org/10.1021/jp8001614
Kirschner, K. N., Yongye, A. B., Tschampel, S. M., González-Outeiriño, J., Daniels, C. R., Foley, B. L. & Woods, R. J. (2007). GLYCAM06: a generalizable biomolecular force field. Carbohydrates. J. Comput. Chem., 29, 622-655. DOI: https://doi.org/10.1002/jcc.20820
Klauda, J. B,, Venable, R. M., Freites, J. A., O'Connor, J. W., Tobias, D. J., Mondragon-Ramirez, C., Vorobyov, I., MacKerell Jr., A. D. & Pastor, R. W. (2010). Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B, 114, 7830-7843. DOI: https://doi.org/10.1021/jp101759q
Klein, F., Soñora, M., Santos, L. H., Frigini, E. N., Ballesteros-Casallas, A., Machado, M. R. & Panatano, S. (2023). The SIRAH force field: a suite for simulations of complex biological systems at the coarse-grained and multiscale levels. J. Struct. Biol., 215, 107985. DOI: https://doi.org/10.1016/j.jsb.2023.107985
Lacava, F. (2016). Classical electrodynamics. From image charges to the photon mass and magnetic monopoles. Springer International Publishing. Switzerland. 195 p. DOI: https://doi.org/10.1007/978-3-319-39474-9
Leach, A. R. (2001). Molecular modelling: principles and applications. Pearson Education Limited. England. 744 p.
Levine, I. N. (2014). Quantum chemistry. Pearson Educaction, Inc. USA. 714 p.
Lewards, E. G. (2016). Computational chemistry. Introduction to the theory and applications of molecular and quantum mechanics. Springer International Publishing. Switzerland. 739 p.
Li, Z., Song, F., Li, P. & Merz Jr., K. M. (2014). Taking into account the ion-induced dipole interaction in the nonbonded model of ions. J. Chem. Theory Comput., 10, 289-297. DOI: https://doi.org/10.1021/ct400751u
Li, Z., Song, F., Li, P. & Merz Jr., K. M. (2015). Parametrization of highly charged metal ions using the 12-6-4 LJ-type nonbonded model in explicit water. J. Chem. Phys. B, 119, 883-895. DOI: https://doi.org/10.1021/jp505875v
Li, Z., Song, F., Li, P. & Merz Jr., K. M. (2015). Systematic parametrization of monovalent ions using the nonbonded model. J. Chem. Theory Comput., 11, 1645-1657. DOI: https://doi.org/10.1021/ct500918t
Li, Z., Song, F., Li, P. & Merz Jr., K. M. (2020). Systematic parametrization of divalent metal ions for the OPC3, OPC, TIP3P-FB, and TIP4P-FB water models. J. Chem. Theory Comput., 16, 4429-4442. DOI: https://doi.org/10.1021/acs.jctc.0c00194
Li, Z., Song, F., Li, P. & Merz Jr., K. M. (2021). Parametrization of monovalent ions for the OPC3, OPC, TIP3P-FB and TIP4P-FB water models. J. Chem. Inf. Model., 61, 869-880.
Li, Z., Song, F., Li, P. & Merz Jr., K. M. (2021). Parametrization of trivalent and tetravalent metal ions for the OPC3, OPC, TIP3P-FB, and TIP4P-FB water models. J. Chem. Inf. Model., 17, 2342-2354. DOI: https://doi.org/10.1021/acs.jctc.0c01320
Lin, F.-Y., Huang, J., Pandey, P., Rupakheti, C., Li, J., Roux, B.& MacKerell Jr., A. D. (2020). Further optimization and validation of the classical Drude polarizable protein force field. J. Chem. Theory Comput., 16, 3221-3239. DOI: https://doi.org/10.1021/acs.jctc.0c00057
Lindan, P. J. D. & Gillan, M. J. (1993). Shell-model molecular dynamics simulation of superionic conduction in CaF2. J. Phys.: Condens. Matter, 5, 1019. DOI: https://doi.org/10.1088/0953-8984/5/8/005
Liu, C., Piquemal, J.-P. & Ren, P. (2019). AMOEBA+ classical potential for molecular interactions. J. Chem. Theory Comput., 15, 4122-4139. DOI: https://doi.org/10.1021/acs.jctc.9b00261
Liu, C., Piquemal, J.-P. & Ren, P. (2020). Implementation of geometry-dependent charge flux into the polarizable AMOEBA+ potential. J. Phys. Chem. Lett., 11, 419-426. DOI: https://doi.org/10.1021/acs.jpclett.9b03489
London, F. (1937). The general theory of molecular forces. Trans. Faraday Soc., 33, 8b-26. DOI: https://doi.org/10.1039/tf937330008b
Lopes, P. E. M., Huang, J., Shim, J., Luo, Y., Roux, B. & MacKerell, Jr., A. D. (2013). Polarizable force field for peptides and proteins based on the classical Drude oscillator. J. Chem. Theory Comput., 12, 5430-5449. DOI: https://doi.org/10.1021/ct400781b
Lorentz, H. A. (1880). On the relationship between the rate of light propagation and body density. Ann. Phys., 245, 641-665. DOI: https://doi.org/10.1002/andp.18802450406
Lorentz, H. A. (1881). On the application of the virial theorem in the kinetic theory of gases. Ann. Phys., 248, 127-136. DOI: https://doi.org/10.1002/andp.18812480110
Lorenz, L. (1880). On the refraction constant. Ann. Phys., 247, 70-103. DOI: https://doi.org/10.1002/andp.18802470905
Maerzke, K. A. & Siepmann, J. I. (2011). Transferable potentials for phase equilibria--coarse-grain description for linear alkanes. J. Phys. Chem. B, 115, 3452-3465. DOI: https://doi.org/10.1021/jp1063935
Mallajosyula, S. S., Guvench, O., Hatcher, E. & MacKerell, Jr., A. D. (2012). CHARMM additive all-atom force field for phosphate and sulfate linked to carbohydrates. J. Chem. Theory Comput., 8, 759-776. DOI: https://doi.org/10.1021/ct200792v
Martin, M. G. & Siepmann, J. I. (1998). Transferable potentials for phase equilibria. 1. United-atom description of n-alkanes. J. Phys. Chem. B, 102, 2569-2577. DOI: https://doi.org/10.1021/jp972543+
Mayo, S. L., Olafson, B. D. & Goddard III, W. A. (1990). DREIDING: a generic force field for molecular simulations. J. Am. Chem. Soc., 94, 8897-8909. DOI: https://doi.org/10.1021/j100389a010
Maple, J. R., Hwang, M.-J., Stockfisch, T. P., Dinur, U., Waldman, M., Ewig, C. S. & Hagler, T. (1994). Derivation of class II force fields. I. Methodology and quantum force field for the alkyl functional group and alkane molecules. J. Comput. Chem., 15, 162-182. DOI: https://doi.org/10.1002/jcc.540150207
Mauger, N., Plé, T., Lagard` e re, L., Huppert, S. & Piquemal, J.-P. (2022). Improving condensed-phase water dynamics with explicit nuclear quantum effects: the polarizable Q-AMOEBA force field. J. Comput. Chem., 15, 162-182. DOI: https://doi.org/10.1021/acs.jpcb.2c04454
Meister, J. & Schwarz, W. H. E. (1994). Principal components of ionicity. J. Phys. Chem., 98, 8245-8252. DOI: https://doi.org/10.1021/j100084a048
Momany, F. A. (1978). Determination of partial atomic charges from ab initio electrostatic potentials. Application to formamide, methanol and formic acid. J. Phys. Chem., 82, 592-601. DOI: https://doi.org/10.1021/j100494a019
Morse, P. M. (1929). Diatomic molecules according to the wave mechanics. II. Vibrational levels. Phys. Rev., 34, 57-64. DOI: https://doi.org/10.1103/PhysRev.34.57
Mulliken, R. S. (1934). A new electroaffinity scale, together with data on valence states and on valence ionization potentials and electron affinities. J. Chem. Phys., 2, 782-793. DOI: https://doi.org/10.1063/1.1749394
Mulliken, R. S. (1955). Electronic population analysis on LCAO-MO molecular wave functions. I. J. Chem. Phys., 23, 1833-1840. DOI: https://doi.org/10.1063/1.1740588
Naseem-Khan, S., Lagard` e re, L., Narth, C., Cisneros, G. A., Ren, P., Gresh, N. & Piquemal, J.-P. (2022). Development of the quantum-inspired SIBFA many-body polarizable force field: enabling condensed-phase molecular dynamics simulations. J. Chem. Theory Comput., 18, 3607-3621. DOI: https://doi.org/10.1021/acs.jctc.2c00029
Nesse, F. (2012). The ORCA program system. WIREs Comput. Mol. Sci., 2, 73-78. DOI: https://doi.org/10.1002/wcms.81
Nocedal, J. & Wright, S. J. (2006). Numerical optimization. Springer Science+Business Media, LLC. New York, USA. 664 p.
O'Boyle, N. M., Banck, M., James, C. A., Morley, C., Vandermeersch, T. & Hutchison, G. R. (2011). Open babel: an open chemical toolbox. J. Cheminformatics, 3, 33. DOI: https://doi.org/10.1186/1758-2946-3-33
Oda, A. & Irono, S. (2003). Geometry-dependent atomic charge calculations using charge equilibration method with empirical two-center Coulombic terms. J. Mol. Struct.: TEOCHEM, 634, 159-170. DOI: https://doi.org/10.1016/S0166-1280(03)00338-5
Ongari, D., Boyd, P. G., Kadioglu, O., Mace, A. K., Keshin, S. & Smit, B. (2019). Evaluating charge equilibration methods to generate electrostatic fields in nanoporous materials. J. Chem. Theory Comput., 15, 382-401. DOI: https://doi.org/10.1021/acs.jctc.8b00669
Pauli, W. (1925). On the connection between the completion of electron groups in an atom with the complex structure of spectra. Z. Physik, 31, 765-783. DOI: https://doi.org/10.1007/BF02980631
Pauling, L. (1931). The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules. J. Am. Chem. Soc., 53, 1367-1400. DOI: https://doi.org/10.1021/ja01355a027
Pearson, R. (1988). Absolute electronegativity and hardness: application to inorganic chemistry. Inorg. Chem., 27, 734-740. DOI: https://doi.org/10.1021/ic00277a030
Press, W. H., Teukolsky, S. A., Vetterling, W. T. & Flannery, B. P. (2007). Numerical recipes: the art of scientific computing. Cambridge University Press. Cambridge, UK. 1235 p.
Ramachandran, S., Lenz, T. G., Skiff, W. M. & Rappé, A. K. (1996). Toward an understanding of zeolite Y as a cracking catalyst with the use of periodic charge equilibration. J. Phys. Chem., 100, 5898-5907. DOI: https://doi.org/10.1021/jp952864q
Rappé, A. K., Bormann-Rochotte, L. M., Wiser, D. C., Hart, J. R., Pietsch, M. A., Casewit, C. J. & Skiff, W. M. (2007). APT a next genereration QM-based reactive force field. Mol. Phys., 105, 301-324. DOI: https://doi.org/10.1080/00268970701201106
Rappé, A. K., Casewit, C. J., Cowell, K. S., Goddard III, W. A. & Skiff, W. M. (1992). UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc., 114, 10024-10035. DOI: https://doi.org/10.1021/ja00051a040
Rappé, A. K. & Goddard III, W. A. (1991). Charge equilibration for molecular dynamics simulations. J. Phys. Chem., 95, 3358-3363. DOI: https://doi.org/10.1021/j100161a070
Rappé, A. K., Pietsch, M. A., Wiser, D. C., Hart, J. R. & Bormann-Rochotte, L. M. (1997). RFF, conceptual development of a full periodic table force field for studying reaction potential surfaces. Mol. Eng., 7, 385-400. DOI: https://doi.org/10.1023/A:1008264127580
Ren P. & Ponder, J. W. (2003). Polarizable atomic multipole water model for molecular mechanics simulation. J. Phys. Chem. B, 107, 5933-5947. DOI: https://doi.org/10.1021/jp027815+
Ren, P., Wu, C. & Ponder, J. W. (2011). Polarizable atomic multipole-based molecular mechanics for organic molecules. J. Chem. Theory Comput., 7, 3143-3161. DOI: https://doi.org/10.1021/ct200304d
Rick, S. W. & Stuart, S. J. (2002). Potentials and algorithms for incorporating polarizability in computer simulations. In: Reviews in Computational Chemistry. Lipkowitz, K. B., Boyd, D. B. eds. John Wiley & Sons, Inc. 89-146. DOI: https://doi.org/10.1002/0471433519.ch3
Rick, S. W., Stuart, S. J. & Berne, B. J. (1994). Dynamical fluctuating charge force fields: application to liquid water. J. Chem. Phys., 101, 6141-6156. DOI: https://doi.org/10.1063/1.468398
Robertson, M. J., Tirado-Rives, J. & Jorgensen, W. L. (2015). Improved peptide and protein torsional energetics with the OPLS-AA force field. J. Chem. Theory Comput., 11, 3499-3509. DOI: https://doi.org/10.1021/acs.jctc.5b00356
Robertson, M. J., Qian, Y., Robinson, M. C., Tirado-Rives, J. & Jorgensen, W. L. (2019). Development and testing of the OPLS-AA/M force field for RNA. J. Chem. Theory Comput., 15, 2734-2742. DOI: https://doi.org/10.1021/acs.jctc.9b00054
Root, D. M., Landis, C. R. & Cleveland, T. (1993). Valence bond concepts applied to the molecular mechanics description of molecular shapes. 1. Application to nonhypervalent molecules of the P-block. J. Am. Chem. Soc., 115, 4201-4209. DOI: https://doi.org/10.1021/ja00063a043
Sanderson, R. T. (1955). Partial charges on atoms in organic compounds. Science, 121, 207-208. DOI: https://doi.org/10.1126/science.121.3137.207
Scherer, P. O. J. (2017). Computational physics: simulation of classical and quantum systems. Springer. Germany. 633 p.
Schlegel, H. B. (2011). Geometry optimization. WIREs Comput. Mol. Sci., 1, 790-809. DOI: https://doi.org/10.1002/wcms.34
Scott, W. R. P., H" u nenberg, P. H., Tironi, I. G., Mark, A. E., Billeter, S. R., Fennen, J., Torda, A. E., Huber, T., Kr" u ger, P. & Van Gunsteren, W. F. (1999). The GROMOS biomolecular simulation packacge. J. Phys. Chem. A, 103, 3596-3607. DOI: https://doi.org/10.1021/jp984217f
Sengupta, A., Li, Z., Song, L. F., Li, P. & Merz Jr., K. M. (2021). Parametrization of monovalent ions for the OPC3, OPC, TIP3P-FB, and TIP4P-FB water models. J. Chem. Inf. Model., 61, 869-880. DOI: https://doi.org/10.1021/acs.jcim.0c01390
Shah, M. S., Siepmann, J. I. & Tsapatsis, M. (2017). Transferble potentials for phase equilibria. Improved united-atom description of ethane and ethylene. AIChE J., 63, 5098-5110. DOI: https://doi.org/10.1002/aic.15816
Shi, S., Yan, L., Yang, Y., Fisher-Shaulsky, J. & Thacher, T. (2003). An extensible and systematic force field, ESFF, for molecular modeling of organic, inorganic, and organometallic systems. J. Comput. Chem., 24, 1059-1076. DOI: https://doi.org/10.1002/jcc.10171
Shi, Y., Zhang, J., Best, R., Wu, C., Ponder J. W. & Ren, P. (2013). Polarizable atomic multipole-based AMOEBA force field for proteins. J. Chem. Theory Comput., 9, 4046-4063. DOI: https://doi.org/10.1021/ct4003702
Silverman, R. A., Kolmogorov, A. N. & Fomin, S. V. (1970). Introductory real analysis. Dover Publications, Inc. New York, USA. 403 p.
Slater, J. C. (1930). Atomic shielding constants. Phys. Rev., 36, 57-64. DOI: https://doi.org/10.1103/PhysRev.36.57
Souza, P. C. T., Alessandri, R., Barnoud, J., Thallmair, S., Faustino, I., Gr" u newald, F., Patmanidis, I., Abdizadeh, H., Bruininks, B. M. H., Wassenaar, T. A., Kroon, P. C., Melcr, J., Nieto, V., Corradi, V., Khan, H. M., Doma' n ski, J., Javanainen, M., Martinez-Seara, H., Reuter, N., Best, R. B., Vattulainen, I., Monticelli, L., Periole, X., Tieleman, D. P., de Vries, A. H. & Marrink, S. J. (2021). Martini3: a general purpose force field for coarse-grained molecular dynamics. Nat. Methods, 18, 382-388. DOI: https://doi.org/10.1038/s41592-021-01098-3
Spicher, S. & Grimme, S. (2020). Robust atomistic modeling of materials, organometallic, and biochemical systems. Angew. Chem. Int. Ed., 59, 15665-15673. DOI: https://doi.org/10.1002/anie.202004239
Sun, H,, Jin, Z., Yang, C., Akkermans, R. L. C., Robertson, S. H., Spenley, N. A., Miller, S. & Todd, S. M. (2016). COMPASS II: extended coverage for polymer and drug-like molecule databases. J. Mol. Model., 22, 47. DOI: https://doi.org/10.1007/s00894-016-2909-0
Szabo, A. & Ostlund, N. S. (1996). Modern quantum chemistry. Introduction to advanced electronic structure theory. Dover Publications, Inc. Mineola, New York. 479 p.
Thakuria, R., Nath, N. K. & Saha, B. K. (2019). The nature and applications of $pi-pi$ interactions: a perspective. Cryst. Growth Des., 19, 523-528. DOI: https://doi.org/10.1021/acs.cgd.8b01630
Thole, B. T. (1981). Molecular polarizabilities calculated with a modified dipole interaction. Chem. Phys., 59, 341-350. DOI: https://doi.org/10.1016/0301-0104(81)85176-2
Tian, C., Kasavajhala, K., Belfon, K. A. A., Raguette, L., Huang, H., Migues, A. N., Bickel, J., Wang, Y., Pincay, J., Wu, Q. & Simmerling, C. (2020). ff19B: amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J. Chem. Theory Comput., 16, 528-552. DOI: https://doi.org/10.1021/acs.jctc.9b00591
Urey, H. C. & Bradley Jr., D. A. (1931). The vibrations of pentatonic tetrahedral molecules. Phys. Rev., 38, 1969-1978. DOI: https://doi.org/10.1103/PhysRev.38.1969
Van der Waals, J. D. (1873). On the continuity of the gas and liquid state [Doctoral thesis, University of Leiden]. Leiden.
Van Duijnen, P. Th. & Swart, M. (1998). Molecular and atomic polarizabilities: Thole's model revisited. J. Phys. Chem. A, 102, 2399-2407. DOI: https://doi.org/10.1021/jp980221f
Van Duin, A. C. T., Dasgupta, D., Lorant, F. & Goddard III, W. A. (2001). ReaxFF: a reactive force field for hydrocarbons. J. Phys. Chem. A, 105, 9396-9409. DOI: https://doi.org/10.1021/jp004368u
Vanommeslaeghe, K., Guvench, O. & MacKerell Jr., A. D. (2014). Molecular mechanics. Curr. Pharm. Des., 20, 3281-3292. DOI: https://doi.org/10.2174/13816128113199990600
Vanommeslaeghe, K., Hatcher, E., Acharya, C., Kundu, S., Zhong, S., Shim, J., Darian, E., Guvench, O., Lopes, P., Vorobyov, I. & MacKerell Jr., A. D. (2009). CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem., 31, 671-690. DOI: https://doi.org/10.1002/jcc.21367
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. (2004). Development and testing of a general amber force field. J. Comput. Chem., 25, 1157-1174. DOI: https://doi.org/10.1002/jcc.20035
Weels, B. A., De Bruin-Dickason, C. & Chaffe, A. L. (2015). Charge equilibration based on atomic ioinization in metal-organic frameworks. J. Phys. Chem. C, 119, 456-466. DOI: https://doi.org/10.1021/jp510415h
Westheimer, F. H. & Mayer, J. E. (1946). The theory of the racemization of optically active derivatives of diphenyl. J. Chem. Phys., 14, 733-738. DOI: https://doi.org/10.1063/1.1724095
Wilmer, C. E., Kim, K. C. & Snurr, R. Q. (2012). An extended charge equilibration method. J. Phys. Chem. Lett., 3, 2506-2511. DOI: https://doi.org/10.1021/jz3008485
Xie, W., Pu, J., Mackerell Jr., A. D. & Gao, J. (2007). Development of a polarizable intermolecular potential function (PIPF) for liquid amides and alkanes. J. Chem. Theory Comput., 3, 1878-1889. DOI: https://doi.org/10.1021/ct700146x
Xu, P., Guidez, E. B., Bertoni, C. & Gordon, M. S. (2018). Perspective: ab initio force field methods derived from quantum mechanics. J. Chem. Phys., 148, 090901. DOI: https://doi.org/10.1063/1.5009551
Yang, L., Sun, L. & Deng, W. (2020). Van der Waals function for molecular mechanics. J. Phys. Chem. A, 124, 2102-2107. DOI: https://doi.org/10.1021/acs.jpca.9b11222
Yuki, H., Tanaka, Y., Hata, M., Ishikawa, H., Neya, S. & Hoshino, T. (2007). Implementation of $pi-pi$ interactions in molecular dynamics simulation. J. Comput. Chem., 28, 1091-1099. DOI: https://doi.org/10.1002/jcc.20557
Zgarbová, M., Sponer, J. & Jurecka, P. (2021). Z-DNA as a touchstone for additive empirical force fields and refinement of the alpha/gamma DNA torsions for AMBER. J. Chem. Theory Comput., 17, 6292-6301. DOI: https://doi.org/10.1021/acs.jctc.1c00697
Zgarbová, M., Otyepka, M., Sponer, J., Mládek, A., Cheatham, T. E. & Jurecka, P. (2011). Refinement of the Cornell et al. nucleic acids force field based on reference quantum chemical calculations of glycosidic torsion profiles. J. Chem. Theory Comput., 7, 2886-2902. DOI: https://doi.org/10.1021/ct200162x
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2024 Revista de la Facultad de Ciencias

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Los autores o titulares del derecho de autor de cada artículo confieren a la Revista de la Facultad de Ciencias de la Universidad Nacional de Colombia una autorización no exclusiva, limitada y gratuita sobre el artículo que una vez evaluado y aprobado se envía para su posterior publicación ajustándose a las siguientes características:
1. Se remite la versión corregida de acuerdo con las sugerencias de los evaluadores y se aclara que el artículo mencionado se trata de un documento inédito sobre el que se tienen los derechos que se autorizan y se asume total responsabilidad por el contenido de su obra ante la Revista de la Facultad de Ciencias, la Universidad Nacional de Colombia y ante terceros.
2. La autorización conferida a la revista estará vigente a partir de la fecha en que se incluye en el volumen y número respectivo de la Revista de la Facultad de Ciencias en el Sistema Open Journal Systems y en la página principal de la revista (https://revistas.unal.edu.co/index.php/rfc/index), así como en las diferentes bases e índices de datos en que se encuentra indexada la publicación.
3. Los autores autorizan a la Revista de la Facultad de Ciencias de la Universidad Nacional de Colombia para publicar el documento en el formato en que sea requerido (impreso, digital, electrónico o cualquier otro conocido o por conocer) y autorizan a la Revista de la Facultad de Ciencias para incluir la obra en los índices y buscadores que estimen necesarios para promover su difusión.
4. Los autores aceptan que la autorización se hace a título gratuito, por lo tanto renuncian a recibir emolumento alguno por la publicación, distribución, comunicación pública y cualquier otro uso que se haga en los términos de la presente autorización.
5. Todos los contenidos de la Revista de la Facultad de Ciencias, están publicados bajo la Licencia Creative Commons Atribución – No comercial – Sin Derivar 4.0.
MODELO DE CARTA DE PRESENTACIÓN y CESIÓN DE DERECHOS DE AUTOR