Publicado
Characterization of the Culturable Gut Microbiota of Two Colombian Main Malaria Vectors
Caracterización de la microbiota intestinal cultivable de dos vectores principales de malaria de Colombia
DOI:
https://doi.org/10.15446/abc.v28n3.105865Palabras clave:
Colombia, Gut bacteria, anopheline mosquitos, malaria (en)Colombia, bacterias intestinales, mosquitos anofelinos, malaria (es)
Descargas
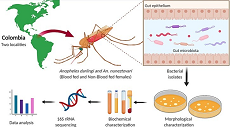
Bacteria inhabiting the gut of malaria vectors can strongly influence their biology and competence to transmit Plasmodium parasites and other pathogens. This study aimed to isolate and characterize the culturable gut bacterial microbiota in two main Colombian malaria vectors. Anopheles (Diptera: Culicidae) darlingi Root, 1926 and Anopheles (Diptera: Culicidae) nuneztovari Gabaldón, 1940, specimens were collected in two malaria-endemic regions and under two feedings status. The bacterial composition was compared according to mosquito species, geography, and feeding status. Bacterial isolates from homogenized mosquito guts were morphologically and biochemically characterized, and their taxonomy was determined by 16S rRNA sequencing. Results showed that Gram-negative bacilli, particularly of the Proteobacteria phylum, are predominant in the gut of the two-vector species regardless of geography and feeding status. At the genus level, Enterobacter, Acinetobacter, and Bacillus were common among the two-vector species and geographic sites; and some genera were locality or vector-specific. In addition, the presence of blood in the mosquito gut negatively impacted bacterial richness at the genus level. These results advanced the knowledge of mosquito-microbe interactions for these malaria vectors. In addition, the generation of a bacterial culture repertoire may allow us to investigate the potential role of some bacteria as biocontrol agents.
Las bacterias que habitan en el intestino de los vectores de la malaria pueden influir en su biología y competencia para transmitir parásitos Plasmodium y otros patógenos. Este estudio tuvo como objetivo aislar y caracterizar la microbiota bacteriana intestinal cultivable en dos vectores principales de malaria de Colombia. Los especímenes de Anopheles (Diptera: Culicidae) darlingi Root, 1926 y Anopheles (Diptera: Culicidae) nuneztovari Gabaldón, 1940, fueron colectados en dos regiones endémicas para malaria y bajo dos estados de alimentación. La composición bacteriana se comparó según la especie de mosquito, la geografía y el estado de alimentación. Las bacterias aisladas de intestinos de mosquitos homogeneizados se caracterizaron morfológica y bioquímicamente y se determinó su taxonomía mediante secuenciación de ARNr 16S. Los resultados mostraron que los bacilos Gram-negativos, particularmente del filo Proteobacteria, predominan en el intestino de las dos especies de vectores, independientemente de la geografía y el estado de alimentación. A nivel de género, Enterobacter, Acinetobacter y Bacillus fueron comunes entre las dos especies de vectores y sitios geográficos; y algunos géneros fueron específicos de la localidad o del vector. Además, la presencia de sangre en el intestino del mosquito afectó negativamente la riqueza bacteriana a nivel de género. Estos resultados aportan al avance del conocimiento sobre las interacciones mosquito-microbio para estos vectores de la malaria; sumado a esto, la generación de un repertorio de cultivos bacterianos podrá permitir investigar el papel potencial de algunas bacterias como agentes de biocontrol.
Referencias
Akorli, J., Gendrin, M., Pels, N. A. P., Yeboah-Manu D, Christophides, G. K., and Wilson, M. D. (2016). Seasonality and Locality Affect the Diversity of Anopheles gambiae and Anopheles coluzzii Midgut Microbiota from Ghana. PLoS One, 11(6), e0157529. https://doi.org/10.1371/journal.pone.0157529
Bai, L., Wang, L., Vega-Rodríguez, J., Wang, G., and Wang, S. (2019). A gut symbiotic bacterium Serratia marcescens renders mosquito resistance to Plasmodium infection through the activation of mosquito immune responses. Front Microbiol, 2(10), 1580. https://doi.org/10.3389/fmicb.2019.01580
Bascuñán, P., Niño-Garcia, J. P., Galeano-Castañeda, Y., Serre, D., and Correa, M. M. (2018). Factors shaping the gut bacterial community assembly in two main Colombian malaria vectors. Microbiome, 6(148). https://doi.org/10.1186/s40168-018-0528-y
Boissière, A., Tchioffo, M. T., Bachar, D., Abate, L., Marie, A., Nsango, S. E., Shahbazkia, H. R., Awono-Ambene, P. H., Levashina, E. A., Christen, R., and Isabelle Morlais, I. (2012). Midgut Microbiota of the Malaria Mosquito Vector Anopheles gambiae and Interactions with Plasmodium falciparum Infection. PLoS Pathog, 8. https://doi.org/10.1371/journal.ppat.1002742
Burkot, T. R., Russell, T. L., Reimer L. J, Bugoro, H., Beebe, N. W., Cooper, R. D., Sukawati, S., Collins, F. H., and Lobo, N. F. (2013). Barrier screens: a method to sample blood-fed and host-seeking exophilic mosquitoes. Malar J,12(49). https://doi.org/10.1186/1475-2875-12-49
Cienfuegos, A. V., Rosero, D. A., Naranjo, N., Luckhart, S., Conn, J. E., and Correa, M. M. (2011). Evaluation of a PCR-RFLP-ITS2 assay for discrimination of Anopheles species in northern and western Colombia. Acta Trop, 118(2),128-135. https://doi.org/10.1016/j.actatropica.2011.02.004
Cirimotich, C. M., Dong, Y., Clayton, A. M., Sandiford, S. L., Souza-Neto, J. A., Mulenga, M., and Dimopoulos. (2011). Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science. 332(6031),855-858. https://doi.org//10.1126/science.1201618
Coon, K. L, Brown, M. R., and Strand, M. R. (2016). Mosquitoes host communities of bacteria that are essential for development but vary greatly between local habitats. Mol Ecol, 25(22), 5806-5826. https://doi.org/10.1111/mec.13877
Chavshin, A. R., Oshaghi, M. A., Vatandoost, H., Pourmand, M. R., Raeisi, A., and Terenius, O. (2014). Isolation and identification of culturable bacteria from wild Anopheles culicifacies, a first step in a paratransgenesis approach. Parasit Vectors. 7(419). https://doi.org/10.1186/1756-3305-7-419
Dada, N., Jumas-Bilak, E., Manguin, S., Seidu, R., Stenström, T. A., and Overgaard, H. J. (2014). Comparative assessment of the bacterial communities associated with Aedes aegypti larvae and water from domestic water storage containers. Parasit Vectors. 7(391). https://doi.org/10.1186/1756-3305-7-391
Das, De. T., Sharma, P., Tevatiya, S., Chauhan, C., Kumari, S., Yadav, P., Singla, D., Srivastava, V., Rani, J., Hasija, Y., Pandey, K. C., Kajla, M., and Dixit, R. (2022). Bidirectional Microbiome-Gut-Brain-Axis Communication Influences Metabolic Switch-Associated Responses in the Mosquito Anopheles culicifacies. Cells, 11(11), 1798. https://doi.org/10.3390/cells11111798
de Carvalho, D. D., Costa, F. T. M., Duran, N., and Haun, M. (2006). Cytotoxic activity of violacein in human colon cancer cells. Toxicol in Vitro, 20(8),1514-1521. https://doi.org/10.1016/j.tiv.2006.06.007
Dehghan, H., Mosa-Kazemi, S. H., Yakhchali, B. Maleki-Ravasan, N., Vatandoost, H., and Oshaghi, M. A. (2022). Evaluation of anti-malaria potency of wild and genetically modified Enterobacter cloacae expressing effector proteins in Anopheles stephensi. Parasites Vectors. 15(63). https://doi.org/10.1186/s13071-022-05183-0
Dennison, N. J., Saraiva, R. G., Cirimotich, C. M., Mlambo, G., Mongodin, E. F., and Dimopoulos, G. (2016). Functional genomic analyses of Enterobacter, Anopheles and Plasmodium reciprocal interactions that impact vector competence. Malar J. 15(425). https://doi.org/10.1186/s12936-016-1468-2
Dinparast, N., Jazayeri, H., Raz, A., Favia, G., Ricci, I., and Zakeri, S. (2011).Identification of the midgut microbiota of A. stephensi and A. maculipennis for their application as a paratransgenic tool against malaria. PLoS One. 6, e28484. https://doi.org/10.1371/journal.pone.0028484
Eappen, A. G., Smith, R. C., and Jacobs-Lorena, M. (2013). Enterobacter-Activated Mosquito Immune Responses to Plasmodium Involve Activation of SRPN6 in Anopheles stephensi. PLoS One. 8, e62937. https://doi.org/10.1371/journal.pone.0062937
Ezemuoka, L. C., Akorli, E. A., Aboagye-Antwi, F., and Akorli, J. (2020). Mosquito midgut Enterobacter cloacae and Serratia marcescens affect the fitness of adult female Anopheles gambiae s.l. PLoS One. 15, e0238931. https://doi.org/10.1371/journal.pone.0238931
Favia, G., Ricci, I., Damiani, C., Raddadi, N., Crotti, E., Marzorati, M., Rizzi, A., Urso, R., Brusetti, L., Borin, S., Mora, D., Scuppa, P., Luciano Pasqualini, L., Clementi, E., Genchi, M., Silvia Corona, S., Negri, I., Grandi, G., Alma, A., Kramer, L., Esposito, F., Bandi, C., Sacchi, L., and Daffonchio, D. (2007). Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. PNAS, 104(21), 9047-9051. https://doi.org/10.1073/pnas.0610451104
Ferreira, C. V., Bos, C. L., Versteeg, H. H., Justo, G. Z., Durán, N., and Peppelenbosch, M. P. (2004). Molecular mechanism of violacein-mediated human leukemia cell death. Blood, 104(5),1459-1464. https://doi.org/10.1182/blood-2004-02-0594
Gaio, A. D. O., Gusmão, D. S., Santos, A. V., Berbert-Molina, M. A., Pimenta, P. F. P., and Lemos, F. J, A. (2011). Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (diptera: culicidae) (L.). Parasit Vectors, 4(105). https://doi.org/10.1186/1756-3305-4-105
Galeano-Castañeda, Y., Bascuñán, P., Serre, D., and Correa, M. M. (2020). Trans-stadial fate of the gut bacterial microbiota in Anopheles albimanus. Acta Trop, 201, 105204. https://doi.org/10.1016/j.actatropica.2019.105204
Galeano, Y., Urrea-Aguirre, P., Piedrahita, S., Bascuñán, P., and Correa, M. M. (2019). Composition and structure of the culturable gut bacterial communities in Anopheles albimanus from Colombia. PLoS One, 14, e0225833. https://doi.org/10.1371/journal.pone.0225833
Gnambani, E. J., Bilgo, E., Sanou, A., Dabiré, R. K., and Diabaté, A. (2020). Infection of highly insecticide-resistant malaria vector Anopheles coluzzii with entomopathogenic bacteria Chromobacterium violaceum reduces its survival, blood feeding propensity and fecundity. Malar J, 19(352). https://doi.org/10.1186/s12936-020-03420-4
Gendrin, M., Rodgers, F. H, Yerbanga, R. S, Ouédraogo, J. B, Basáñez, M. G, Cohuet, A., and Christophides, G. K. (2015). Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat Commun, 6, 5921. https://doi.org/10.1038/ncomms6921
Gonzalez-Ceron, L., Santillan, F., Rodriguez, M. H, Mendez, D., and Hernandez-Avila, J. E. (2003). Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J Med Entomol,40(3),371-374. https://doi.org/10.1603/0022-2585-40.3.371
González, R., and Carrejo, N. S. (2018). Introducción al estudio taxónomico de Anopheles de Colombia: Claves y notas de distribución. (2 Ed.) Cali:Programa Editorial Universidad del Valle. https://doi.org/10.25100/peu.309
INS. (2021). Boletín Epidemiológico Semanal: Semana epidemiológica 52. Instituto Nacional de Salud. Retrieved 28 January 2022. Available in: https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2021_Boletin_epidemiologico_semana_52.pdf.
Jupatanakul, N., Pengon, J., Selisana, S. M. G., Choksawangkarn, W., Jaito, N., Saeung, A, Bunyong, R., Posayapisit, N., Thammatinna, K., Kalpongnukul, N., Aupalee, K., Pisitkun, T., and Kamchonwongpaisan, S. (2020). Serratia marcescens secretes proteases and chitinases with larvicidal activity against Anopheles dirus. Acta Trop, 212, 105686. https://doi.org/10.1016/j.actatropica.2020.105686
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Alex, Cooper., A, Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P, and Drummond, A. (2012). Geneious Basic: an integrated and extendable desktop software platform for organizing and analyzing sequence data. Bioinformatics. 28(12), 1647-1649. https://doi.org/10.1093/bioinformatics/bts199
Lane D. (1991). 16S/23S rRNA sequencing Nucleic Acid Techniques in Bacterial Systematics. John Wiley & Sons, p. 115-175.
Maslunka, C., Gifford, B., Tucci, J., Gürtler, V., y Seviour, R. J. (2014). Insertions or deletions (Indels) in the rrn 16S-23S rRNA gene internal transcribed spacer region (ITS) compromise the typing and identification of strains within the Acinetobacter calcoaceticus-baumannii (Acb) complex and closely related members. PLoS One, 9, e105390. https://doi.org/10.1371/journal.pone.0105390
Minard, G., Tran, F. H., Raharimalala, F. N, Hellard, E., Ravelonandro, P., Mavingui, P., Valiente, C. (2013). Prevalence, genomic and metabolic profiles of Acinetobacter and Asaia associated with field-caught Aedes albopictus from Madagascar. FEMS Microbiol Ecol,83(1), 63-73. https://doi.org/10.1111/j.1574-6941.2012.01455.x
Minard, G., Tran, F. H., Van, V. T., Goubert, C., Bellet, C., Lambert, G., Huynh, K. L., Thi, H. T., Mavingui, P., y Valiente, C. (2015). French invasive Asian tiger mosquito populations harbor reduced bacterial microbiota and genetic diversity compared to Vietnamese autochthonous relatives. Front Microbiol, 6. https://doi.org/10.3389/fmicb.2015.00970
Müller, G. C., Beier, J. C., Traore, S. F., Toure, M. B., Traore, M. M., Bah, S., Seydou, D., and Schlein Y. (2010). Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar J,9(262). https://doi.org/10.1186/1475-2875-9-262
Muturi, E. J., Kim, C. H., Bara, J., Bach, E. M., and Siddappaji, M. H. (2016). Culex pipiens and Culex restuans mosquitoes harbor distinct microbiota dominated by few bacterial taxa. Parasit Vectors. 9(18). https://doi.org/10.1186/s13071-016-1299-6
Nartey, R., Owusu-Dabo, E., Kruppa, T., Baffour-Awuah, S., Annan, A., Oppong, S., Becker, N., and Obiri-Danso, K. (2013). Use of Bacillus thuringiensis var israelensis as a viable option in an Integrated Malaria Vector Control Programme in the Kumasi Metropolis, Ghana. Parasit Vectors. 6(116). https://doi.org/10.1186/1756-3305
Orjuela, L. I., Morales, J. A., Ahumada, M. L., Rios, J. F., González, J. J., Yañez, J., Rosales, A., Cabarcas, D. M., Venegas, J., Yasnot, M. F., Quiñones, M. L. (2018). Insecticide resistance and its intensity in populations of malaria vectors in Colombia. Biomed Res Int. 12. https://doi.org/10.1155/2018/9163543
Ramirez, J. L., Short, S. M., Bahia, A. C., Saraiva, R. G., Dong, Y., Kang, S., Tripathi, A., Mlambo, G., and Dimopoulos, G. (2014). Chromobacterium Csp_P Reduces Malaria and Dengue Infection in Vector Mosquitoes and Has Entomopathogenic and In Vitro Anti-pathogen Activities. PLoS Pathog,10, e1004398. https://doi.org/10.1371/journal.ppat.1004398
Rani, A., Sharma, A., Rajagopal, R., Adak, T., and Bhatnagar, R. K. (2009). Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and fieldcollected Anopheles stephensi-an Asian malarial vector. BMC Microbiol,9(96).https://doi.org/10.1186/1471-2180-9-96
Rosero, D. A., Gutiérrez, L. A., Cienfuegos, A. V., Jaramillo, L. M., and Correa, M. M. (2010). Optimización de un procedimiento de extracción de ADN para mosquitos anofelinos. Rev. Colomb. Entomol, 36(2), 260-263. https://doi.org/10.25100/socolen.v36i2.9156
Shaw, W. R., Marcenac, P., Childs, L. M., Buckee, C. O., Baldini, F., Sawadogo, S. P., Dabiré, R, K., Diabaté, A., and Catteruccia, F. (2016). Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nat Commun, 7(11772). https://doi.org/10.1038/ncomms11772
Singh, A., Allam, M., Kwenda, S., Khumalo, Z. T. H., Ismail, A., and Oliver, S. V. (2022). The dynamic gut microbiota of zoophilic members of the Anopheles gambiae complex (Diptera: Culicidae). Sci Rep, 12(1495). https://doi.org/10.1038/s41598-022-05437-y
Singh, B., Bobogare, A., Cox-Singh, J., Snounou, G., Abdullah, M. S, and Rahman, H. A. (1999). A genusand species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg, 60(4), 687-692. https://doi.org/10.4269/ajtmh.1999.60.687
Tchioffo, M. T., Boissière, A., Churcher, T. S., Abate, L., Gimonneau, G., Nsango, S. E., Awono-Ambéné, P, H., Christen, R., Berry, A., and Morlais. I. (2013). Modulation of Malaria Infection in Anopheles gambiae Mosquitoes Exposed to Natural Midgut Bacteria. PLoS One, 8, e81663. https://doi.org/10.1371/journal.pone.0081663
Terbot, J. W., Nikbakhtzadeh, M. R., and Foster, W. A. (2015). Evaluation of Bacillus thuringiensis israelensis as a Control Agent for Adult Anopheles gambiae. J. Am. Mosq Control. Assoc, 31(3),258-261. https://doi.org/10.2987/moco-31-03-258-261.1
Villegas, L. M., and Pimenta, P. F. (2014). Metagenomics, paratransgenesis and the Anopheles microbiome: a portrait of the geographical distribution of the anopheline microbiota based on a meta-analysis of reported taxa. Mem. Inst. Oswaldo. Cruz. 109(5), 672-684. https://doi.org/10.1590/0074-0276140194
WHO. World malaria report 2021. World Health Organization [serial online] 2021 December. Available in https://www.who.int/teams/global-malariaprogramme/reports/world-malaria-report-2021
Wilke, A. B. B., and Marrelli, M. T. (2015). Paratransgenesis: a promising new strategy for mosquito vector control. Parasit. Vectors. 8(342). https://doi.org/10.1186/s13071-015-0959-2
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Paola Muñoz-Laiton, Juan C. Hernandez-Valencia, Juan Pablo Isaza, Margarita M. Correa. (2025). Metatranscriptomic profiling of the bacterial and fungal microbiota of field-collected Anopheles darlingi from Colombia: Community composition and functional insights. Acta Tropica, 270, p.107795. https://doi.org/10.1016/j.actatropica.2025.107795.
2. Hossein Kamel Urmia, Mona Koosha, Bagher Yakhchali, Seyed Hassan Moosa-Kazemi, Mohammad Ali Oshaghi, Pablo Tortosa. (2025). Environmental persistence and transmission dynamics of Serratia AS1 in mosquito habitats: advancing paratransgenesis for malaria control . Applied and Environmental Microbiology, 91(12) https://doi.org/10.1128/aem.01840-25.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).




















