Publicado
EFECTO DEL GLIFOSATO SOBRE LA MICROBIOTA Y LA ACTIVIDAD ENZIMÁTICA EN RIZÓSFERA DE PLANTAS RIPARIAS
Effect of Glyphosate on Microbiota and Enzymatic Activity in Rhizosphere of Riparian Plants
DOI:
https://doi.org/10.15446/abc.v29n1.108336Palabras clave:
Faena fuerte, humedales costeros, mesocosmos, sitio RAMSAR, Soconusco (es)Faena fuerte, coastal wetlands, mesocosms, RAMSAR site, Soconusco (en)
Descargas
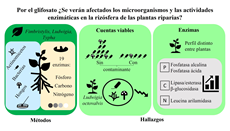
El glifosato es un herbicida foliar detectado en suelo, sedimento y agua, que ocasiona daños no visibles en organismos no blanco, pudiendo afectar la diversidad, estructura y funcionamiento de las comunidades microbianas asociadas a la vegetación riparia que provee de servicios ecosistémicos. El objetivo del presente trabajo fue 1) determinar las cuentas viables de microorganismos y 2) analizar cómo se afectan las actividades enzimáticas asociadas al metabolismo del carbono, fósforo y nitrógeno en la rizósfera de plantas riparias (Fimbristylis dichotoma, Ludwigia octovalvis y Typha domingensis) expuestas a glifosato. Para mantener el micro-hábitat en la rizósfera, se colectaron plantas con el mismo suelo donde habitaban. A las plantas se les aplicó 50 mg de glifosato ácido equivalente (ae)/L a nivel de suelo, y se mantuvieron por 15 días. Después, a partir de muestras de rizósfera, se aislaron y cuantificaron actinomicetos, bacterias totales (incluyendo actinomicetos) y hongos, y se analizó la actividad de 19 enzimas relacionadas con el metabolismo de P, C y N. Por la presencia del herbicida, se encontró que fueron afectadas negativamente 1) las células bacterianas principalmente, en comparación con actinomicetos y hongos, y 2) las poblaciones microbianas aisladas de la rizósfera de L. octovalvis en comparación con F. dichotoma y T. domingensis. Las determinaciones de actividades enzimáticas mostraron que el metabolismo del fósforo y carbono fueron estimulados positivamente por el glifosato. La información obtenida permite identificar la respuesta de la diversidad microbiana cultivable y la diversidad funcional de rizósfera de plantas de importancia ecológica.
Glyphosate is a foliar herbicide detected in soil, sediment, and water, causing non-visible damage to non-target organisms, potentially affecting the diversity, structure, and functioning of microbial communities associated with riparian vegetation that provide ecosystem services. The objective of the present work was 1) to determine the viable counts of microorganisms and 2) to analyze how the enzymatic activities associated with the metabolism of carbon, phosphorus, and nitrogen are affected in the riparian plants’ rhizosphere (Fimbristylis dichotoma, Ludwigia octovalvis, and Typha domingensis) exposed to glyphosate. The plants were collected with the same soil in which they lived to maintain the micro-habitat of the rhizosphere. Zero or fifty mg of glyphosate acid equivalent (ae)/L was applied to the plants at the ground level for 15 days. Actinomycetes, total bacteria (including actinomycetes), and fungi were then isolated and quantified, and the activity of 19 enzymes (metabolism of P, C, and N) were analyzed from rhizosphere samples. In the presence of the herbicide, it was found that 1) bacteria was most negatively affected compared to actinomycetes and fungi, and 2) microbial populations isolated from L. octovalvis were lesser than those from T. domingensis and F. dichotoma. Rhizosphere enzymatic activities showed that phosphorus and carbon metabolism were stimulated by glyphosate. The information obtained from this work allows us to identify the response of cultivable microbial diversity and functional diversity of the rhizosphere of ecologically important plants.
Referencias
Acosta-Martínez, V. and Tabatabai, M. A. (2001). Arylamidase activity in soils: effect of trace elements and relationships to soil properties and activities of amidohydrolases. Soil Biol Chem, 33(1), 17-23. https://doi.org/10.1016/S0038-0717(00)00109-7
Adetunji, A. T., Lewu, F. B., Mulidzi, R. and Ncube, B. (2017). The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: a review. J Soil Sci Plant Nutr, 17(3), 794-807. http://dx.doi.org/10.4067/S0718-95162017000300018
Álvarez, H. M. and Steinbüchel, A. (2002). Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol, 60, 367-376. https://doi.org/10.1007/s00253-002-1135-0
Barillot, C. D. C., Sarde, C. O., Bert, V., Tarnaud, E. and Cochet, N. (2013). A standardized method for the sampling of rhizosphere and rhizoplan soil bacteria associated to a herbaceous root system. Ann Microbiol, 63, 471-476. http://dx.doi.org/10.1007/s13213-012-0491-y
Battaglin, W. A., Meyer, M. T., Kuivila, K. M. and Dietze, J. E. (2014). Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. JAWRA, 50(2), 275-290. https://doi.org/10.1111/jawr.12159
Bilyera, N., Zhang, X., Duddek, P., Fan, L., Banfield, C. C., Schlüter, S., Carminati, A., Kaestner, A., Ahmed, M. A., Kuzyakov, Y., Dippold, A. M., Spielvogel, S. and Razavi, B. S. (2021). Maize genotype-specific exudation strategies: An adaptive mechanism to increase microbial activity in the rhizosphere. Soil Biol Biochem, 162, 108426. https://doi.org/10.1016/j.soilbio.2021.108426
Bois, P., Huguenot, D., Jézéquel, K., Lollier, M., Cornu, J. Y. and Lebeau, T. (2013). Herbicide mitigation in microcosms simulating stormwater basins subject to polluted water inputs. Water Res, 47(3), 1123-1135. https://doi.org/10.1016/j.watres.2012.11.029
Dosskey, M. G., Vidon, P., Gurwick, N. P., Allan, C. J., Duval, T. P. and Lowrance, R. (2010). The role of riparian vegetation in protecting and improving chemical water quality in streams. JAWRA, 46(2), 261-277. https://dx.doi.org/10.1111/j.1752-1688.2010.00419.x
Fang, Z., Li, J., Wang, Q., Fang, W., Peng, H., Zhang, X. and Xiao, Y. (2014). A novel esterase from a marine metagenomic library exhibiting salt tolerance ability. J Microbiol Biotechnol, 24(6), 771-780. https://doi.org/10.4014/jmb.1311.11071
Fei, Y. Y., Gai, J. Y. and Zhao, T. J. (2013). Identification of regulated genes conferring resistance to high concentrations of glyphosate in a new strain of Enterobacter. FEMS Microbiol Lett, 349(2), 135-143. https://doi.org/10.1111/1574-6968.12306
Franco-Correa, M. (2008). Evaluación de caracteres PGPR en actinomicetos e interacciones de 519 estas rizobacterias con hongos formadores de micorrizas. [Tesis de doctorado, Universidad de Granada]. https://digibug.ugr.es/handle/10481/2110
Funke, T., Han, H., Healy-Fried, M. L., Fischer, M. and Sch nbrunn, E. (2006). Molecular basis for the herbicide resistance of roundup ready crops. PNAS, 103(35), 13010-13015. https://doi.org/10.1073/pnas.0603638103
Giaccio, G. C. M., Laterra, P., Aparicio, V. C. and Costa, J. L. (2016). Glyphosate retention in grassland riparian areas is reduced by the invasion of exotic trees. Revista Internacional de Botánica Experimental, 85, 108-116. https://doi.org/10.32604/phyton.2016.85.108
Giaccio, G. C. M., Saez, J. M., Estévez, M. C., Salinas, B., Corral, R. A., De Gerónimo, E., Aparicio, V. and Álvarez, A. (2023). Developing a glyphosate-bioremediation strategy using plants and actinobacteria: potential improvement of a riparian environment. J Hazard Mater, 446, 130675. https://doi.org/10.1016/j.jhazmat.2022.130675
Gianfreda, L. (2015). Enzymes of importance to rhizosphere processes. J Soil Sci Plant Nutr, 15(2), 283-3062015. http://dx.doi.org/10.4067/S0718-95162015005000022
Gunarathna, S., Gunawardana, B., Jayaweera, M., Manatunge, J. and Zoysa, K. (2018). Glyphosate and AMPA of agricultural soil, surface water, groundwater and sediments in areas prevalent with chronic kidney disease of unknown etiology, Sri Lanka. J Environ Sci Health B, 53(11), 729-737. https://doi.org/10.1080/03601234.2018.1480157
Haase, S., Philippot, L., Neumann, G., Marhan, S. and Kandeler, E. (2008). Local response of bacterial densities and enzyme activities to elevated atmospheric CO2 and different N supply in the rhizosphere of Phaseolus vulgaris L. Soil Biol Biochem, 40, 1225-1234. https://doi.org/10.1016/j.soilbio.2007.12.025
Helander, M., Saloniemi, I. and Saikkonen, K. (2012). Glyphosate in northern ecosystems. Trends Plant Sci, 17(10), 569-574. https://doi.org/10.1016/j.tplants.2012.05.008
Hénault-Ethier, L., Lucotte, M., Moingt, M., Paquet, S., Maccario, S., Smedbol, E., Gomes, M. P., Lepage, L., Juneau, P. and Labrecque, M. (2017). Herbaceous or Salix miyabeana ‘SX64’ narrow buffer strips as a means to minimize glyphosate and aminomethylphosphonic acid leaching from row crop fields. Sci Total Environ, 598, 1177-1186. http://dx.doi.org/10.1016/j.scitotenv.2017.04.104
Hernández-Pérez, H. A. y Giles-Gómez, M. (2021). Métodos microbiológicos para el análisis de alimentos. Universidad Nacional Autónoma de México.
Hol, W. H. G., de Boer, W., de Hollander, M., Kuramae, E. E., Meisner, A. and van der Putten, W. H. (2015). Context dependency and saturating effects of loss of rare soil microbes on plant productivity. Front Plant Sci, 6, 485. https://dx.doi.org/10.3389/fpls.2015.00485
Kremer, R. J. K., Means, N. E. and Kim, S. (2005). Glyphosate affects soybean root exudation and rhizosphere microorganims. Int J Environ Anal Chem, 85(15), 1165-1174. https://doi.org/10.1080/03067310500273146
Kremer, R. J. and Means, N.E. (2009). Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur J Agron, 31(3), 153-161. https://doi.org/10.1016/j.eja.2009.06.004
Labrada, R. yParker, C. (1996). El control de malezas en el contexto del manejo integrado de plagas. En R. Labrada, J. C. Caseley y C. Parker, (Ed.), Manejo de malezas para países en desarrollo. Organización de las Naciones Unidas para la Agricultura y la Alimentación (FAO). http://www.fao.org/docrep/T1147S/t1147s05.htm
Leino, L., Tall, T., Helander, M., Saloniemi, I., Saikkonen, K., Ruuskanen, S. and Puigbó, P. (2021). Classification of the glyphosate target enzyme (5-enolpyruvylshikimate-3- phosphate synthase) for assessing sensitivity of organisms to the herbicide. J Hazard Mater, 408, 124556. https://doi.org/10.1016/j.jhazmat.2020.124556
Li, J., Wang, C., Liang, W. and Liu, S. (2021). Rhizosphere microbiome: the emerging barrier in plant-pathogen interactions. Front Microbiol, 12, 772420. https://doi.org/10.3389/fmicb.2021.772420
López-Chávez, M. Y., Alvarez-Legorreta, T., Infante-Mata, D., Dunn, M. F. and Guillén-Navarro, K. (2021). Glyphosateremediation potential of selected plant species in artificial wetlands. Sci Total Environ, 781, 146812. https://doi.org/10.1016/j.scitotenv.2021.146812
Madigan, M. T., Martinko, J. M., Stahl, D. A. and Clark, D. P. (2012). Brock Biology of Microorganisms. Editorial Pearson.
Mesnage, R. and Antoniou, M. N. (2020). Computational modelling provides insight into the effects of glyphosate on the shikimate pathway in the human gut microbiome. CRTOX, 1, 25-33. https://doi.org/10.1016/j.crtox.2020.04.001
Mijangos, I., Becerril, J. M., Albizu, I., Epelde, L. and Garbisu, C. (2009). Effects of glyphosate on rhizosphere soil microbial communities under two different plant compositions by cultivation-dependent and -independent methodologies. Soil Biol Biochem, 41(3), 505-513. https://doi.org/10.1016/j.soilbio.2008.12.009
Mohanram, S. and Kumar, P. (2019). Rhizosphere microbiome: revisiting the synergy of plant-microbe interactions. Ann Microbiol, 69, 307–320. https://doi.org/10.1007/s13213-019-01448-9
Newman, M. M., Hoilett, N., Lorenz, N., Dick, R. P., Liles, M. R., Ramsier, C. and Kloepper, J. (2016a). Glyphosate effects on soil rhizosphere-associated bacterial communities. Sci Total Environ, 543, 155-160, https://doi.org/10.1016/j.scitotenv.2015.11.008
Newman, M. M., Lorenz, N., Hoilett, N., Lee, N. R., Dick, R. P., Liles, M. R., Ramsier, C. and Kloepper, J. W. (2016b). Changes in rhizosphere bacterial gene expression following glyphosate treatment. Sci Total Environ, 553, 32-41. https://doi.org/10.1016/j.scitotenv.2016.02.078
Ordoñez-Arévalo, B., Guillén-Navarro, K., Huerta, E., Cuevas, R. and Calixto-Romo, M. A. (2018). Enzymatic dynamics into the Eisenia fetida (Savigny, 1826) gut during vermicomposting of coffee husk and market waste in a tropical environment. Environ Sci Pollut Res, 25, 1576–1586. https://doi.org/10.1007/s11356-017-0572-3
Pérez, D. J., Okada, E., Menone, M. L. and Costa, J. L. (2017). Can an aquatic macrophyte bioaccumulate glyphosate?Development of a new method of glyphosate extraction in Ludwigia peploides and watershed scale validation. Chemosphere, 185, 975-982. https://doi.org/10.1016/j.chemosphere.2017.07.093
Rainio, M. J., Ruuskanen, S., Helander, M., Saikkonen, K., Saloniemi, I. y Puigbò, P. (2021). Adaptation of bacteria to glyphosate: a microevolutionary perspective of the enzyme 5-enolpyruvylshikimate-3-phosphate synthase. Environ Microbiol Rep, 13(3), 309-316. https://doi.org/10.1111/1758-2229.12931
Ramírez-Muñoz, F. (2017) Mecanismo de resistencia de Paspalum paniculatum L. (Poaceae) al herbicida glifosato. [Tesis de doctorado, Instituto Tecnológico de Costa Rica]. https://repositoriotec.tec.ac.cr/handle/2238/10069
Ruiz-Toledo, J., Castro, R., Rivero-Pérez, N., Bello-Mendoza, R. and Sánchez, D. (2014). Occurrence of glyphosate in water bodies derived from intensive agriculture in a tropical region of southern Mexico. BECT, 93, 289-293. https://doi.org/10.1007/s00128-014-1328-0
Sanaullah, M., Razavi, B. S., Blagodatskaya, E. V. and Kuzyakov, Y. (2016). Spatial distribution and catalytic mechanisms of β-glucosidase activity at the root-soil interface. Biol Fertil Soils, 52, 505–514. https://doi.org/10.1007/s00374-016-1094-8
Schafer, J. R., Hallett, S. G. and Johnson, W. G. (2014). Rhizosphere microbial community dynamics in glyphosate-treated susceptible and resistant biotypes of giant ragweed (Ambrosia trifida). Weed Sci, 62(2), 370–381. https://doi.org/10.1614/WS-D-13-00164.1
Sesin, V., Davy, C.M., Dorken, M. E., Gilbert, J. M. and Freeland, J. R. (2019). Variation in glyphosate effects and accumulation in emergent macrophytes. Manag Biol Invasions, 11, (in press).
Singh, S. K., Wu, X., Shao, C. and Zhang, H. (2022). Microbial enhancement of plant nutrient acquisition. Stress Biol, 2(3). https://doi.org/10.1007/s44154-021-00027-w
Tall, T. and Puigbò, P. (2022). Rethinking the intrinsic sensitivity of fungi to glyphosate. Bio Tech, 11(3), 28. https://doi.org/10.3390/biotech11030028
Thilo, J. S. K., Batsukh, S., Bauer, E., Hirota, B., Weiss, B., Wierz, J. C., Fukatsu, T., Kaltenpoth, M. and Engl, T. (2021). Inhibition of a nutritional endosymbiont by glyphosate abolishes mutualistic benefit on cuticle synthesis in Oryzaephilus surinamensis. Commun Biol, 4(554). https://doi.org/10.1038/s42003-021-02057-6
Wang, S., Seiwert, B., Kästner, M., Miltner, A., Schäffer, A., Reemtsma, T., Yang, Q. and Nowak, K. M. (2016). (Bio) degradation of glyphosate in water-sediment microcosms - A stable isotope co-labeling approach. Water Res, 99, 91-100. https://doi.org/10.1016/j.watres.2016.04.041
Yi, S.-Y., Wu, G.-B., Lin, Y.-J., Hu, N. and Liu, Z.-D. (2015). Characterization of a new type of glyphosate-tolerant 5-enolpyruvylshikimate-3-phosphate synthase from Isoptericola variabilis. J Mol Cat B Enzym, 111, 1-8. https://doi.org/10.1016/j.molcatb.2014.11.009
Yu, Y., Gui, Y., Li, Z., Jiang, C., Guo, J. and Niu, D. (2022). Induced systemic resistance for improving plant immunity by beneficial microbes. Plants, 11(3), 386. https://doi.org/10.3390/plants11030386
Zhang, Q., Zhou, H., Li, Z., Zhu, J., Zhou, C. and Zhao, M. (2016). Effects of glyphosate at environmentally relevant concentrations on the growth of and microcystin production by Microcystis aeruginosa. Environ Monit Assess, 188(632). https://doi.org/10.1007/s10661-016-5627-2
Zucko, J., Dunlap, W. C., Shick, J. M., Cullum, J., Cercelet, F., Amin, B., Hammen, L., Lau, T., Williams, J., Hranueli, D. and Long, P. F. (2010). Global genome analysis of the shikimic acid pathway reveals greater gene loss in host-associated than in free-living bacteria. BMC Genom, 11(628). https://dx.doi.org/10.1186/1471-2164-11-628
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. José Vera, Yessenia Sarango, Mónica Villamar, Jhonny Ortiz, Jaime Sevilla-Carrasco, Josue Duarte, Leonel Lucas. (2025). Effect of herbicides on the growth of beneficial microorganisms in rhizospheric soil. Revista de la Facultad de Agronomía, Universidad del Zulia, 42(2), p.e254222. https://doi.org/10.47280/RevFacAgron(LUZ).v42.n2.VI.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).



















