SEARCHING AND PARASITISM OF Diatraea saccharalis (LEPIDOPTERA: CRAMBIDAE) BY Trichospilus diatraeae (HYMENOPTERA: EULOPHIDAE)
Búsqueda y parasitismo de Diatraea saccharalis (Lepidoptera: Crambidae) por Trichospilus diatraeae (Hymenoptera: Eulophidae)
Palabras clave:
biological control, host location, parasitoid (en)control biológico, localización hospedero, parasitoide. (es)
Descargas
The ability of Trichospilus diatraeae Cherian and Margabandhu, 1942 (Hymenoptera: Eulophidae) to search and parasitize Diatraea saccharalis (Fabricius, 1794) (Lepidoptera: Crambidae) pupae in sugarcane stalks was evaluated. To analyze the ability for search and parasitism were used stalks of sugarcane (20 cm) where it was introduced a pupa of D. saccharalis (T1); a pupa and a caterpillar (T2) or a pupa and fecal matter (T3). Each stalk was placed in a transparent plastic bottle with 21 females of T. diatraeae. These pupae were isolated, after 72 h, in glass tubes at 25 ± 2 °C, 70 ± 10 % relative humidity, 14:10 light/dark. The experiment was developed in an entirely casualized design with three treatments and 12 repetitions. Percentage of D. saccharalis pupa parasitized by T. diatraeae was 50.00 %, 83.33 % and 16.66 % in the T1, T2 and T3, respectively (X2 = 3.896, p= 0.04). The presence of D. saccharalis caterpillars favored searching and parasitism of this host.
La capacidad de Trichospilus diatraeae Cherian e Margabandhu, 1942 (Hymenoptera: Eulophidae) para buscar y parasitar las pupas de Diatraea saccharalis (Fabricius, 1794) (Lepidoptera: Crambidae) en los tallos de la caña de azúcar fue estudiada. Para analizar la habilidad de búsqueda y parasitismo fueron utilizados tallos de la caña de azúcar (20 cm) donde se introdujo una pupa de D. saccharalis (T1); pupas y orugas (T2) o pupa y residuos fecales (T3). Cada tallo fue colocado en una botella plástica transparente con 21 hembras de T. diatraeae. Esas pupas fueron individualizadas, luego de 72 h, en tubos de vidrio a 25 ± 1°C, 70 ± 10 % UR y 14 h de foto período. El experimento se desarrolló en un diseño completamente al azar, con tres tratamientos y 12 repeticiones. Los porcentajes de pupas de D. saccharalis parasitados por T. diatraeae fueron del 50,00 %, 83,33 % y 16,66 % en el T1, T2 y T3, respectivamente (X2 = 3.896, p = 0,04). La presencia de D. saccharalis en los tallos de caña de azúcar favorecieron la búsqueda y parasitismo de su hospedero.
SEARCHING AND PARASITISM OF Diatraea saccharalis (LEPIDOPTERA: CRAMBIDAE) BY Trichospilus diatraeae (HYMENOPTERA: EULOPHIDAE)
Búsqueda y parasitismo de Diatraea saccharalis (Lepidoptera: Crambidae) por Trichospilus diatraeae (Hymenoptera: Eulophidae)
ELIZANGELA LEITE VARGAS1, M.Sc.; FABRICIO FAGUNDES PEREIRA1, Ph. D.; DANIELE FABIANA GLAESER2, Ph. D.; VANESSA RODRIGUES FERREIRA CALADO1, M.Sc.; FABIANA GARCIA DE OLIVEIRA1, M.Sc; PATRIK LUIZ PASTORI3, Ph. D.
1Universidade Federal da Grande Dourados, rodovia Dourados-Ithaum, Km 12, CXP: 533, Zip Code: 79804-970, Dourados, MS, Brasil. 2Embrapa Agropecuária Oeste, CXP: 661, Zip Code: 79804-970, Dourados, MS, Brasil. 3Universidade Federal do Ceará, Departamento de Fitotecnia, Zip Code: 60.356-000, Fortaleza, CE, Brasil. Corresponding autor: Elizangela Leite Vargas, elileitevargas@gmail.com.
Presentado el 19 de diciembre de 2012, aceptado el 14 de abril de 2013, correcciones el 22 de mayo de 2013.
ABSTRACT
The ability of Trichospilus diatraeae Cherian and Margabandhu, 1942 (Hymenoptera: Eulophidae) to search and parasitize Diatraea saccharalis (Fabricius, 1794) (Lepidoptera: Crambidae) pupae in sugarcane stalks was evaluated. To analyze the ability for search and parasitism were used stalks of sugarcane (20 cm) where it was introduced a pupa of D. saccharalis (T1); a pupa and a caterpillar (T2) or a pupa and fecal matter (T3). Each stalk was placed in a transparent plastic bottle with 21 females of T. diatraeae. These pupae were isolated, after 72 h, in glass tubes at 25 ± 2 °C, 70 ± 10 % relative humidity, 14:10 light/dark. The experiment was developed in an entirely casualized design with three treatments and 12 repetitions. Percentage of D. saccharalis pupa parasitized by T. diatraeae was 50.00 %, 83.33 % and 16.66 % in the T1, T2 and T3, respectively (X2 = 3.896, p= 0.04). The presence of D. saccharalis caterpillars favored searching and parasitism of this host.
Keywords: biological control, host location, parasitoid.
RESUMEN
La capacidad de Trichospilus diatraeae Cherian e Margabandhu, 1942 (Hymenoptera: Eulophidae) para buscar y parasitar las pupas de Diatraea saccharalis (Fabricius, 1794) (Lepidoptera: Crambidae) en los tallos de la caña de azúcar fue estudiada. Para analizar la habilidad de búsqueda y parasitismo fueron utilizados tallos de la caña de azúcar (20 cm) donde se introdujo una pupa de D. saccharalis (T1); pupas y orugas (T2) o pupa y residuos fecales (T3). Cada tallo fue colocado en una botella plástica transparente con 21 hembras de T. diatraeae. Esas pupas fueron individualizadas, luego de 72 h, en tubos de vidrio a 25 ± 1°C, 70 ± 10 % UR y 14 h de foto período. El experimento se desarrolló en un diseño completamente al azar, con tres tratamientos y 12 repeticiones. Los porcentajes de pupas de D. saccharalis parasitados por T. diatraeae fueron del 50,00 %, 83,33 % y 16,66 % en el T1, T2 y T3, respectivamente (X2 = 3.896, p = 0,04). La presencia de D. saccharalis en los tallos de caña de azúcar favorecieron la búsqueda y parasitismo de su hospedero.
Palabras clave: control biológico, localización hospedero, parasitoide.
RESUMO
A capacidade de Trichospilus diatraeae Cherian e Margabandhu, 1942 (Hymenoptera: Eulophidae) para procurar e parasitar pupas de Diatraea saccharalis (Fabricius, 1794) (Lepidoptera: Crambidae) em colmos de cana-de-açúcar foi avaliada. Para analisar a capacidade de busca e parasitismo foram usados colmos de cana-de-açúcar (20 cm), onde foi introduzida uma pupa de D. saccharalis (T1); uma pupa e uma lagarta (T2) or uma pupa e resíduo fecal (T3). Cada colmo foi colocado em uma garrafa plástica transparente com 21 fêmeas de T. diatraeae. Essas pupas foram retiradas dos colmos após 72 h, e colocadas em tubos de vidro a 25 ± 2 ° C, 70 ± 10 % de umidade relativa e fotofase de 14 h. O experimento foi desenvolvido em delineamento inteiramente casualizado, com três tratamentos e 12 repetições. A porcentagem de pupas de D. saccharalis parasitadas por T. diatraeae foi de 50,00 %, 83,33 % e 16,66 % em T1, T2 e T3, respectivamente (X2= 3,896, p = 0,04). A presença de lagartas de D. saccharalis favoreceu a busca e parasitismo deste hospedeiro.
Palavras-chave: controle biológico, localização de hospedeiro, parasitoide.
INTRODUCTION
Adult female parasitoids respond to semiochemicals in the micro-habitat to find host pupae (Pinto et al., 2007; Fontana et al., 2011). These compounds are released from plants infested by herbivores and/or from fecal matter of these insects which are detected by parasitoid receptors (Fatouros et al., 2007; Dicke et al., 2009; Girling et al., 2011; Hegde et al., 2011). The efficiency of parasitoids depends on its ability to detect hosts in the field natural conditions (Silva-Torres et al., 2009), although it lay be reduced in absence of hosts at right developmental stages for parasitism (Hausmann et al., 2005). The capacity parasitoids to find endophytic pupa is important because they usually remain hidden which reduces emission of detectable volatiles (Bruinsma et al., 2009). Caterpillars may build shelters as protection that reduce its parasitism (Rodovalho et al., 2007).
Females of the parasitoid Brachymeria intermedia (Nees, 1834) (Hymenoptera: Chalcididae) can identify kairomones of Lymantria dispar (Linnaeus, 1758) (Lepidoptera: Lymantriidae) after being exposed to pupa of this host (Cardé and Lee, 1989). This indicates that searching-behaviors may depend on acquired experience of parasitoids (Hoballah and Turlings, 2005; Peñaflor et al., 2011). Contact with host before releasing may improve searching efficiency of laboratory reared parasitoids released in the field (González et al., 2011). Trichospilus diatraeae Cherian and Margabandhu, 1942 (Hymenoptera: Eulophidae) parasitizes insect pupa, mainly those of lepidopterans, and present potentially for biological control of agricultural and forest pests (Boucek, 1976; Zaché et al., 2010; Melo et al., 2011). The ability of this insect to find and parasitize D. saccharalis pupae inside sugarcane stalksare related with semiochemicals (Krugner et al., 2008), vibrations (Fischer et al., 2003), and/or chromatic and achromatic cues (Fischer et al., 2004).
Diatraea saccharalis is an important pest of sugarcane, because the intensity of the attack and cause losses in sugar and alcohol productivity (Segato et al., 2006; Dinardo-Miranda et al., 2012). Controlling the sugarcane borer with chemicals is difficult, because this insect develops in a protected location, inside the stem of the plant (Vacari et al., 2012). Therefore, biological control with parasitoids has been the most used way to combat this insect, so it is important to simulate the natural conditions before performing releases of T. diatraeae in the plantations of sugarcane, to check efficiency of parasitism in the field.
The present work evaluated capacity of T. diatraeae to search and parasitize D. saccharalis pupae in the presence of final instar caterpillars or fecal matter of this host.
MATERIALS AND METHODS
Rearing D. saccharalis
Diatraea saccharalis pupae were supplied by Empresa Agentes Biológicos BUG. This insect was reared with the following methodology: recently hatched caterpillars were maintained in glass tubes (8.5 x 2.5 cm) sealed with cotton and fed an artificial diet until pupa stage. Pupae were collected, sexed, and 20 males and 30 females were placed together for ovipositon in PVC cages (22 x 10 cm) lined with sheets of sulfite paper humidified with distilled water. This PVC cages were sealed with a voile-type fabric and elastic (Parra, 2007).
Rearing T. diatraeae
Trichospilus diatraeae adults were maintained in glass tubes (14 x 2.2 cm) sealed with cotton and fed with drops of pure honey. D. saccharalis pupae with 24 to 48 hours old were subsequently introduced into these tubes during 24 hours. Pupae were removed and individualized in glass tubes (14 x 2.2 cm) in a chamber with controlled conditions chamber (25 ± 2 °C, 70 ± 10 % relative humidity, under a 14:10 light/ dark) until emergence (Pereira et al., 2008).
Experimental Design
Sugarcane stalks were cut in 20 cm long segments. An orifice was made in each segment and a 24 hour-old D. saccharalis pupa (184.00 ± 0.01 mg) was placed in each cavity. After the fixed inside each cavity and the stalk segments placed individually in transparent plastic tubes (25 x 9 cm) with 21 T. diatraeae females for 72 hours (Chichera et al., 2012). Pupae were then removed and individualized in glass tubes (14 x 2.2 cm) in a chamber with controlled conditions (25 ± 2 °C, 70 ± 10 % relative humidity, 14:10 light/dark) to evaluate parasitism and to observe the emergence of T. diatraeae. Controls consisted of D. saccharalis pupae fixed into sugarcane stalk segments under identical conditions, but without T. diatraeae females. Treatments consisted of exposing the parasitoid to: a pupa (T1); a pupa and a caterpillar (T2) or a pupa and the fecal matter (T3) of D. saccharalis. Treatment T2 had two holes in the stalks (one at each internode) with one pupa in one hole and a fourth or fifth instar caterpillar in the other one. Treatment T3 had one pupa in an orifice into the stalk with fecal matter of D. saccharalis around its external area.
Each parcel was exposed to 21 T. diatraeae females, with 12 replications in an entirely randomized design. It was evaluated the percentage of parasitism [;(number of pupae of D. saccharalis with emergence of parasitoid + pupae without adult emergence of D. saccharalis) / (total number of pupae) x 100]; the emergence percentage [;(number of pupae of D. saccharalis to adult emergence of parasitoids) / (number of parasitized pupae) x 100] and progeny per pupa (number of parasitoid emerged per pupa of D. saccharalis). The successful of search of the parasitoid was measured by the percentage of parasitism of pupae. Percentages of T. diatraeae parasitism were analyzed with a general linear model with binomial distribution (p ≤ 0.05) using the R Statistical System software package (Ihaka and Gentleman, 1996). This analysis was performed using the original non-parametric data in percentages to facilitate interpretation. Data of emergence of T. diatraeae progeny were submitted to an analysis of variance with the test F at 5 % significance.
RESULTS
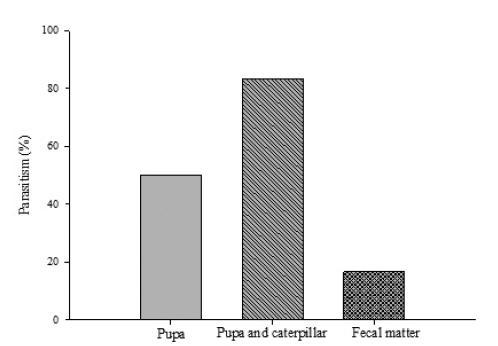
The present of final instar caterpillars or fecal matter of D. saccharalis affected the search and percentage of parasitism of females T. diatraeae. Percentage of D. saccharalis pupa parasitized by T. diatraeae was 50.00 %, 83.33 % and 16.66 % in the T1, T2 and T3, respectively (X2= 3.896, p = 0.04) (Fig. 1).
The presence of D. saccharalis caterpillars increased its shearching and parasitism of T. diatraeae.
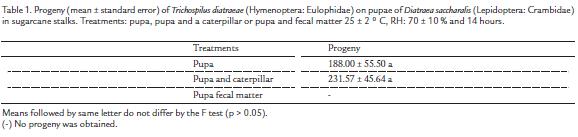
Emergence of T. diatraeae adults from D. saccharalis pupa was similar between treatments with pupa (66.00 %) or pupa and caterpillars (70.00 %) (p > 0.05). This parasitoid did not emerged in the treatment with pupa and fecal matter of D. saccharalis.
Progeny of T. diatraeae from D. saccharalis pupae was similar between treatments with pupae and caterpillars or only pupae (Table 1) of this host.
DISCUSSION
The ability of T. diatraeae to find D. saccharalis pupae in the orifices in sugarcanes talks this species is important for biological control (Chichera et al., 2012). High parasitism levels of D. saccharalis pupae indicated potential of T. diatraeae as a biological control agent of this lepidopteran.
The parasitism of T. diatraeae in pupae of D. saccharalis introduced in the stalks of sugarcane was 50 %. Chichera et al. (2012) also noted 56 % parasitism in the experiment where the pupae of D. saccharalis were introduced in stems and exposed to T. diatraeae. A total of 83.33 % of D. saccharalis pupae was parasitized in the presence of caterpillars that host, this indicates that T. diatraeae was stimulated in the presence of larvae.
Higher ability of T. diatraeae to search D. saccharalis pupae with host caterpillars present suggests that it can recognize substances released by them and that they were close to pupation. As Cotesia kariyai (Watanabe, 1937) (Hymenoptera: Braconidae) females can distinguish chemical from damage by Pseudaletia separata (Walker, 1865) (Lepidoptera: in sugarcane stalks. Treatments: pupa, pupa and a caterpillar or pupa and fecal matter 25 ± 2 ° C, RH: 70 ± 10 % and 14 hours. Treatments Progeny Pupa 188.00 ± 55.50 a Pupa and caterpillar 231.57 ± 45.64 a Pupa fecal matter - Means followed by same letter do not differ by the F test (p > 0.05). (-) No progeny was obtained. Noctuidae) caterpillars up to fourth instar, but not between later ones (Takabayashi et al., 1995) - when they are not appropriate for parasitism.
The emergence of the progeny of T. diatraeae indicates that this parasitoid can search, parasitized and develop within D. saccharalis pupae in the orifices in sugarcane stalks - again demonstrating its potential for biological control (Keasar and Steinberg, 2008; Chichera et al., 2012). The emergence of progeny favors establishment of this parasitoid in plantations (Bellows et al., 2006) and may reduce the numbers of re-introductions and costs of producing and releasing this wasp (Gichini et al., 2008).
Lower parasitism of D. saccharalis pupae by T. diatraeae with host fecal matter may be due to odors released these residues. Additionally, this fecal matter used in the experiment came from D. saccharalis caterpillars reared on artificial diet, and may have different composition in fecal matter of caterpillars fed on sugarcane plants in the natural environment. The searching behavior of the parasitoid Cotesia flavipes (Cameron, 1891) (Hymenoptera: Braconidae) is mediated by a water-soluble substance from the fecal matter of D. saccharalis caterpillars. The contact with this substance induces searching-behavior characterized by reduced locomotion and tapping the feces with its antenna (Van Leerdam et al., 1985). On the other hand, semiochemicals may not be important for Spathius agrili Yang, 2005 (Hymenoptera: Braconidae) to f ind Agrilus planipennis Fairmaire, 1888 (Coleoptera: Buprestidae) by this parasitoid females rely on host-generated vibrations to find suitable hosts (Wang et al., 2010).
Pupae and caterpillars of D. saccharalis together in sugarcane stalks favored parasitism of this pest by T. diatraeae by simulating naturally infested plants. Volatile compounds released from the sugarcane stalks due to D. saccharalis caterpillar-feeding may facilitated searching of pupae of this host by this natural enemy. Cabbage plants infested by Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae) (Girling et al., 2011) stimulated searching behavior of females of the larval parasitoid Cotesia vestalis (Haliday, 1834) (Hymenoptera: Braconidae). The parasitoid Trichogramma pretiosum Riley, 1879 (Hymenoptera: Trichogrammatidae) was attracted by volatile compounds released by corn plants damaged by caterpillars of Elasmopalpus lignosellus (Zeller, 1848) (Lepidoptera: Pyralidae) (Xavier et al., 2011).
The presence of D. saccharalis caterpillars in sugarcane stalks increased its searching and parasitism by T. diatraeae - which could be due to the perception and identification of substances released by the caterpillars or to odors from damaged sugarcane stalks. On the other hand, fecal matter from D. saccharalis reduced parasitism, which could be attributed to chemicals such us not identified by T. diatraeae. The origin and compositions of chemical substances that help this parasitoid to search host pupae need to be investigated to understand the efficiency of T. diatraeae to control D. saccharalis in the field.
In summary, T. diatraeae females searched and parasitized D. saccharalis pupae in sugarcane stalks in the laboratory. The presence of D. saccharalis caterpillars favored searching and increased parasitism of pupae of this pest in sugarcane stalks. Fecal matter from D. saccharalis caterpillars reduced parasitism by T. diatraeae.
ACKNOWLEDGMENTS
To Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
BIBLIOGRAPHY
Bellows JTS, Paine TD, Bezark LG, Ball J. Optimizing natural enemy release rates, and associated pest population decline rates, Encarsia inaron Walker (Hymenoptera: Aphelinidae) and Siphoninus phillyreae (Haliday) (Homopter a: Aleyrodidae). Biol Control. 2006; 37(1):25-31.
Bruinsma M, Posthumus MA, Mumm R, Mueller MJ, Van Loon JJA, Dicke M. Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: effects of time and dose, and comparison with induction by herbivores. J Exp Bot. 2009;60(9):2575-2587.
Boucek Z. The African and Asiatic species of Trichospilus and Cotterellia (Hymenoptera, Eulophidae). B Entomol Res. 1976;65(4):669-681.
Cardé RT, Lee H. Effect of experience on the responses of the par asitoid Brachymeria intermedia (Hymenopter a: Chalcidadae) to its host, Lymantria dispar (Lepidoptera: Lymantriidae), and kairomone. Ann Entomol Soc Am. 1989;82(5):653-657.
Chichera RA, Pereira FF, Kassab O, Barbosa RH, Pastori PL, Rossoni C. Capacidad de búsqueda y reproducción de Trichospilus diatraeae y Palmistichus elaeisis (Hymenoptera: Eulophidae) en pupas de Diatraea saccharalis (Lepidoptera: Crambidae). Interciencia. 2012;37(11):852-856.
Dicke M, Van Lon JJA, Soler R. Chemical complexity of volatiles from plants induced by multiple attacks. Nat Chem Biol. 2009;5(5):317-324.
Dinardo Miranda LL, Anjos IA, Costa VP, Fracasso JV. Resistance of sugarcane cultivars to Diatraea saccharalis. Pesq Agropec Bras. 2012;47(1):1-7.
Fatouros NE, Kiss BG, Dicke M, Hilker M. The response specificity of Trichogramma egg parasitoids towards infochemicals during host location. J Insect Behav. 2007;20(1):53-65.
Fischer S, Samietz EJ, Dorn ES. Efficiency of vibrational sounding in parasitoid host location depends on substrate density. J Comp Physiol. 2003;189(10):723-730.
Fischer S, Samietz J, Wackers FL, Dorn S. Perception of chromatic cues during host location by the pupal parasitoid Pimpla turionellae (L.) (Hymenoptera: Ichneumonidae). Environ Entomol. 2004;33(1):81-87.
Fontana A, Held M, Fantaye CA. Attractiveness of constitutive and herbivore-induced sesquiterpene blends of maize to the parasitic wasp Cotesia marginiventris (Cresson). J Chem Ecol. 2011;37(6):582-591.
Gichini G, Löhr B, Rossbash A, Nyambo B, Gathu R. Can low releases numbers lead to establishment and spread of an exotic parasitoid, Diadegma semiclausum (Hellén), in East Africa. Crop Prot. 2008;27(6):906-914.
Girling RB, Stewart-Jones A, Dherbecourt JT, Wright DJ, Poppy GM. Parasitoids select plants more heavily infested with their caterpillar hosts: a new approach to aid interpretation of plant headspace volatiles. Proc R Soc. 2011;278(1718):2646-2653.
González JM, Cusumano A, Williams HJ, Colazza S, Vinson SB. Behavioral and chemical investigations of contact kairomones released by the mud Dauber Wasp Trypoxylon politum, a host of the parasitoid Melittobia digitata. J Chem Ecol. 2011;37(6):629-639.
Hausmann C, Mattiacci L, Dorn S. Role of host feeding niches and host refuges in habitat-related behaviour of Hyssopus pallidus (Hymenoptera: Eulophidae), a larval parasitoid of the codling moth. B Entomol Res. 2005;95(5):429-436.
Hegde M, Oliveira JN, Costa JG, Bleicher E, Santana AEG, Bruce TJA, et al. Identif ication of semiochemicals released by cotton, Gossypium hirsutum, upon infestation by the cotton aphid, Aphis gossypii. J Chem Ecol. 2011; 37(7):741-750.
Hoballah ME, Turlings TCJ. The role of fresh versus old leaf damage in the attraction of parasitic wasps to herbibore-induced maize volatiles. J Chem Ecol. 2005;31(9):2003-2018.
Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5(3):299-314.
Keasar T, Steinberg S. Evaluation of the parasitoid Copidosoma koehleri for biological control of the potato tuber moth, Phthorimaea operculella, in Israeli potato fields. Biocontrol Sci Techn. 2008;18(4):325-336.
Krugner R, Johnson MW, Daane KM, Morse JG. Olfactory responses of the egg parasitoid, Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae), to host plants infested by Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae). Biol Control. 2008;47(1):8-15.
Melo RL, Pratissoli D, Polanczyk RA, Tavares M, Milanez AM, Melo DF. Ocorrência de Trichospilus diatraeae (Hym.: Eulophidae) em broca-das-cucurbitáceas, no Brasil. Hortic Bras. 2011;29(2):228-230.
Parra JRP. Técnicas de Criação de Insetos para Programa de Controle Biológico, sexta ed. Piracicaba: ESALQ/ FEALQ; 2007.
Peñaflor MFGV, Erb M, Miranda, LA, Werneburg AG, Bent JMS. Herbivore-induced plant volatiles can serve as host location cues for a generalist and a specialist egg parasitoid. J Chem Ecol. 2011;37(12):1304-1313.
Pereira FF, Zanuncio JC, Tavares MT, Pastori PL, Jacques GC, Vilela EF. New record of Trichospilus diatraeae as a parasitoid of the eucalypt defoliator Thyrinteina arnobia in Brazil. Phytoparasitica. 2008;36(3):304-306.
Pinto DM, Nerg AM, Holopainen JK. The role of ozone-reactive compouds, terpenes, and green leaf volatiles (GLVs), in the orientation of Cotesia plutelae. J Chem Ecol. 2007;33(12):2218-2228.
Rodovalho SR, Laumann RA, Diniz IV. Ecological aspects of lepidopteran caterpillar parasitoids from Caryocar brasiliense Camb. (Caryocaraceae) in a cerrado sensu stricto of Central Brazil. Biota Neotrop. 2007;7(3):239-243.
Segato SV, Pinto AS, Jediroba E, Nóbrega JCM, organizadores. Atualização em produção de cana-de-açúcar. Piracicaba: CP2; 2006.
Silva-Torres CSA, Barros R, Torres JB. Efeito da idade, fotoperíodo e disponibilidade de hospedeiro no comportamento de Oomyzus sokolowskii Kurdjumov (Hymenoptera: Eulophidae). Neotrop Entomol. 2009;38(4):512-519.
Takabayashi J, Takahashi S, Dicke M, Posthumus MA. Developmental stage of herbivore Pseudaletia separata affects production of herbivore-induced synomone by corn plants. J Chem Ecol. 1995;21(3):273-287.
Vacari AM, De Bortoli SA, Borba DF, Martins MIEG. Quality of Cotesia flavipes (Hymenoptera: Braconidae) reared at different host densities and the estimated cost of its commercial production. Biol Control. 2012;63(2):102-106.
Van Leerdam MBJ, Smith JW, Fuchs TW. Frass-mediated host-finding behavior of Cotesia flavipes, a braconid parasite of Diatraea saccharalis (Lepidoptera: Pyralidae). Ann Entomol Soc Am. 1985;78(5):646-650.
Wang XY, Yang ZQ, Gould JR, Wu H, Ma JH. Host-seeking behavior and par asitism by Spathius agrili Yang (Hymenoptera: Braconidae), a parasitoid of the emerald ash borer. Biol Control. 2010;52(1):24-29.
Xavier LMS, Laumann RA, Borges M, Magalhães DM, Vilela EF, Blassioli-Moraes MC. Trichogramma pretiosum attraction due to the Elasmopalpus lignosellus damage in maize. Pesq Agropec Bras. 2011;46(6):578-585.
Zaché B, Wilcken CF, Dacosta RR, Soliman EP. Trichospilus diatraeae Cherian & Margabandhu, 1942 (Hymenoptera: Eulophidae), a new parasitoid of Melanolophia consimilaria (Lepidoptera: Geometridae). Phytoparasitica. 2010;38(4): 355-357.

Este obra está bajo una licencia de Creative Commons Reconocimiento 3.0 Unported.
Referencias
Bellows JTS, Paine TD, Bezark LG, Ball J. Optimizing natural enemy release rates, and associated pest population decline rates, Encarsia inaron Walker (Hymenoptera: Aphelinidae) and Siphoninus phillyreae (Haliday) (Homopter a: Aleyrodidae). Biol Control. 2006; 37(1):25-31.
Bruinsma M, Posthumus MA, Mumm R, Mueller MJ, Van Loon JJA, Dicke M. Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: effects of time and dose, and comparison with induction by herbivores. J Exp Bot. 2009;60(9):2575-2587.
Boucek Z. The African and Asiatic species of Trichospilus and Cotterellia (Hymenoptera, Eulophidae). B Entomol Res. 1976;65(4):669-681.
Cardé RT, Lee H. Effect of experience on the responses of the par asitoid Brachymeria intermedia (Hymenopter a: Chalcidadae) to its host, Lymantria dispar (Lepidoptera: Lymantriidae), and kairomone. Ann Entomol Soc Am. 1989;82(5):653-657.
Chichera RA, Pereira FF, Kassab O, Barbosa RH, Pastori PL, Rossoni C. Capacidad de búsqueda y reproducción de Trichospilus diatraeae y Palmistichus elaeisis (Hymenoptera: Eulophidae) en pupas de Diatraea saccharalis (Lepidoptera: Crambidae). Interciencia. 2012;37(11):852-856.
Dicke M, Van Lon JJA, Soler R. Chemical complexity of volatiles from plants induced by multiple attacks. Nat Chem Biol. 2009;5(5):317-324.
Dinardo Miranda LL, Anjos IA, Costa VP, Fracasso JV. Resistance of sugarcane cultivars to Diatraea saccharalis. Pesq Agropec Bras. 2012;47(1):1-7.
Fatouros NE, Kiss BG, Dicke M, Hilker M. The response specificity of Trichogramma egg parasitoids towards infochemicals during host location. J Insect Behav. 2007;20(1):53-65.
Fischer S, Samietz EJ, Dorn ES. Efficiency of vibrational sounding in parasitoid host location depends on substrate density. J Comp Physiol. 2003;189(10):723-730.
Fischer S, Samietz J, Wackers FL, Dorn S. Perception of chromatic cues during host location by the pupal parasitoid Pimpla turionellae (L.) (Hymenoptera: Ichneumonidae). Environ Entomol. 2004;33(1):81-87.
Fontana A, Held M, Fantaye CA. Attractiveness of constitutive and herbivore-induced sesquiterpene blends of maize to the parasitic wasp Cotesia marginiventris (Cresson). J Chem Ecol. 2011;37(6):582-591.
Gichini G, Löhr B, Rossbash A, Nyambo B, Gathu R. Can low releases numbers lead to establishment and spread of an exotic parasitoid, Diadegma semiclausum (Hellén), in East Africa. Crop Prot. 2008;27(6):906-914.
Girling RB, Stewart-Jones A, Dherbecourt JT, Wright DJ, Poppy GM. Parasitoids select plants more heavily infested with their caterpillar hosts: a new approach to aid interpretation of plant headspace volatiles. Proc R Soc. 2011;278(1718):2646-2653.
González JM, Cusumano A, Williams HJ, Colazza S, Vinson SB. Behavioral and chemical investigations of contact kairomones released by the mud Dauber Wasp Trypoxylon politum, a host of the parasitoid Melittobia digitata. J Chem Ecol. 2011;37(6):629-639.
Hausmann C, Mattiacci L, Dorn S. Role of host feeding niches and host refuges in habitat-related behaviour of Hyssopus pallidus (Hymenoptera: Eulophidae), a larval parasitoid of the codling moth. B Entomol Res. 2005;95(5):429-436.
Hegde M, Oliveira JN, Costa JG, Bleicher E, Santana AEG, Bruce TJA, et al. Identif ication of semiochemicals released by cotton, Gossypium hirsutum, upon infestation by the cotton aphid, Aphis gossypii. J Chem Ecol. 2011; 37(7):741-750.
Hoballah ME, Turlings TCJ. The role of fresh versus old leaf damage in the attraction of parasitic wasps to herbibore-induced maize volatiles. J Chem Ecol. 2005;31(9):2003-2018.
Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5(3):299-314.
Keasar T, Steinberg S. Evaluation of the parasitoid Copidosoma koehleri for biological control of the potato tuber moth, Phthorimaea operculella, in Israeli potato fields. Biocontrol Sci Techn. 2008;18(4):325-336.
Krugner R, Johnson MW, Daane KM, Morse JG. Olfactory responses of the egg parasitoid, Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae), to host plants infested by Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae). Biol Control. 2008;47(1):8-15.
Melo RL, Pratissoli D, Polanczyk RA, Tavares M, Milanez AM, Melo DF. Ocorrência de Trichospilus diatraeae (Hym.: Eulophidae) em broca-das-cucurbitáceas, no Brasil. Hortic Bras. 2011;29(2):228-230.
Parra JRP. Técnicas de Criação de Insetos para Programa de Controle Biológico, sexta ed. Piracicaba: ESALQ/ FEALQ; 2007.
Peñaflor MFGV, Erb M, Miranda, LA, Werneburg AG, Bent JMS. Herbivore-induced plant volatiles can serve as host location cues for a generalist and a specialist egg parasitoid. J Chem Ecol. 2011;37(12):1304-1313.
Pereira FF, Zanuncio JC, Tavares MT, Pastori PL, Jacques GC, Vilela EF. New record of Trichospilus diatraeae as a parasitoid of the eucalypt defoliator Thyrinteina arnobia in Brazil. Phytoparasitica. 2008;36(3):304-306.
Pinto DM, Nerg AM, Holopainen JK. The role of ozone-reactive compouds, terpenes, and green leaf volatiles (GLVs), in the orientation of Cotesia plutelae. J Chem Ecol. 2007;33(12):2218-2228.
Rodovalho SR, Laumann RA, Diniz IV. Ecological aspects of lepidopteran caterpillar parasitoids from Caryocar brasiliense Camb. (Caryocaraceae) in a cerrado sensu stricto of Central Brazil. Biota Neotrop. 2007;7(3):239-243.
Segato SV, Pinto AS, Jediroba E, Nóbrega JCM, organizadores. Atualização em produção de cana-de-açúcar. Piracicaba: CP2; 2006.
Silva-Torres CSA, Barros R, Torres JB. Efeito da idade, fotoperíodo e disponibilidade de hospedeiro no comportamento de Oomyzus sokolowskii Kurdjumov (Hymenoptera: Eulophidae). Neotrop Entomol. 2009;38(4):512-519.
Takabayashi J, Takahashi S, Dicke M, Posthumus MA. Developmental stage of herbivore Pseudaletia separata affects production of herbivore-induced synomone by corn plants. J Chem Ecol. 1995;21(3):273-287.
Vacari AM, De Bortoli SA, Borba DF, Martins MIEG. Quality of Cotesia flavipes (Hymenoptera: Braconidae) reared at different host densities and the estimated cost of its commercial production. Biol Control. 2012;63(2):102-106.
Van Leerdam MBJ, Smith JW, Fuchs TW. Frass-mediated host-finding behavior of Cotesia flavipes, a braconid parasite of Diatraea saccharalis (Lepidoptera: Pyralidae). Ann Entomol Soc Am. 1985;78(5):646-650.
Wang XY, Yang ZQ, Gould JR, Wu H, Ma JH. Host-seeking behavior and par asitism by Spathius agrili Yang (Hymenoptera: Braconidae), a parasitoid of the emerald ash borer. Biol Control. 2010;52(1):24-29.
Xavier LMS, Laumann RA, Borges M, Magalhães DM, Vilela EF, Blassioli-Moraes MC. Trichogramma pretiosum attraction due to the Elasmopalpus lignosellus damage in maize. Pesq Agropec Bras. 2011;46(6):578-585.
Zaché B, Wilcken CF, Dacosta RR, Soliman EP. Trichospilus diatraeae Cherian & Margabandhu, 1942 (Hymenoptera: Eulophidae), a new parasitoid of Melanolophia consimilaria (Lepidoptera: Geometridae). Phytoparasitica. 2010;38(4): 355-357.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2013 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).