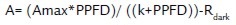PHOTOSYNTHETIC PERFORMANCE AND LEAF WATER POTENTIAL OF GULUPA (Passiflora edulis Sims, PASSIFLORACEAE) IN THE REPRODUCTIVE PHASE IN THREE LOCATIONS IN THE COLOMBIAN ANDES
Desempeño fotosintético y potencial hídrico foliar de gulupa (Passiflora edulis Sims, Passifloraceae) en estado reproductivo en tres localidades de los Andes colombianos
DOI:
https://doi.org/10.15446/abc.v20n1.42196Palabras clave:
Chlorophyll fluorescence, crop, horticulture, passion fruit, photosynthesis, plant physiology, water. (en)Agua, ecofisiología vegetal, Intercambio de gases, fluorescencia de la clorofila, fotosíntesis (es)
Descargas
Artículo de investigación
PHOTOSYNTHETIC PERFORMANCE AND LEAF WATER POTENTIAL OF GULUPA (Passiflora edulis Sims, PASSIFLORACEAE) IN THE REPRODUCTIVE PHASE IN THREE LOCATIONS IN THE COLOMBIAN ANDES
Desempeño fotosintético y potencial hídrico foliar de gulupa (Passiflora edulis Sims, Passifloraceae) en estado reproductivo en tres localidades de los Andes colombianos
Laura Victoria PEREZ MARTINEZ1, Luz Marina MELGAREJO1 .
1 Laboratorio de Fisiología y Bioquímica Vegetal. Departamento de Biología. Universidad Nacional de Colombia. Carrera 30 n. 45-03. Bogotá, Colombia.
For correspondence. lmmelgarejom@unal.edu.co
Received 20th February 2014, Returned for revision 3rd June 2014, accepted 30th July 2014.
Citation / Citar este artículo como: Perez Martinez LV, Melgarejo LM. Photosynthetic performance and leaf water potential of gulupa (Passiflora edulis Sims, Passifloraceae) in the reproductive phase in three locations in the colombian Andes. Acta biol. Colomb. 2015;20(1):183-194. doi: https://doi.org/10.15446/abc.v20n1.42196.
ABSTRACT
Gulupa, Passiflora edulis Sims (Passifloraceae), is an important fruit due to its organoleptic and nutritional characteristics and its demand in the international market; however, very few studies have been conducted for studying its Ecophysiology. Until now, this crop has spread throughout the country through empirical knowledge without data that indicate the zones that are more suitable for its cultivation. For this reason, gas exchange, chlorophyll fluorescence (photosystem II operating efficiency and maximum quantum efficiency of photosystem II photochemistry) and leaf water potential were measured in three different locations of Cundinamarca department (Chia [2610 m a.s.l., 14 °C], Granada [2230 m a.s.l., 15 °C] and Tena [2090 m a.s.l., 17 °C]), whose climatic conditions were monitored with meteorological stations to evaluate the physiologic performance in each location related to the environmental factors. The results indicate that, even though the photosynthetic capacity was similar and high in Granada and Tena, the water status of the plant, the stomatal control of water loss and recovery of photosystems during the night were more efficient in Granada (p < 0.05). In Tena, the small differences between day and night temperature, humidity, and vapor pressure deficit (VPD) would limited the night water recovery in the plants. Meanwhile, in Chia, the increase of VPD during the day and the low temperatures would decreased the water potential both during the day and during the night, as well as the recovery of photosystem II. Therefore, in conclusion the climatic conditions similar to Granada, which are 18/13 °C day/night, a VPD close to 0.5 KPa, and radiation that did not exceed 1000 μmol photons/m2s favored the good physiological performance of gulupa.
Keywords: chlorophyll fluorescence, crop, horticulture, passion fruit, photosynthesis, plant physiology, water.
RESUMEN
La gulupa, Passiflora edulis Sims (Passifloraceae) es un frutal importante debido a sus características organolépticas, nutricionales y su demanda en el mercado internacional; sin embargo, existen muy pocos estudios relacionados con su ecofisiología. Hasta el momento, el cultivo se ha extendido a través del país por medio de conocimiento empírico sin tener datos que sustenten las zonas más apropiadas para su cultivo. Por esta razón, en el presente estudio se midió el intercambio de gases, la fluorescencia de la clorofila (factor de eficiencia del fotosistema II y eficiencia cuántica fotoquímica máxima del fotosistema II) y el potencial hídrico foliar en tres localidades diferentes del departamento de Cundinamarca (Chía [2610 m s.n.m., 14 °C], Granada [2230 m s.n.m., 15 °C] y Tena [2090 m s.n.m., 17 °C]), cuyas condiciones climáticas fueron monitoreadas con estaciones meteorológicas para evaluar el desempeño fisiológico en cada localidad y relacionarlo con los factores ambientales. Los resultados indican que aunque la capacidad fotosintética fue alta y similar en Granada y Tena, el estado hídrico de la planta, el control estomático de la pérdida de agua y la recuperación de los fotosistemas durante la noche fueron más eficientes en Granada (p < 0,05). En Tena, la estrecha diferencia entre los valores día/noche de temperatura, humedad y déficit de presión de vapor (DPV) limitarían la recuperación hídrica de la planta, mientras que en Chía el aumento de DPV en el día, y las bajas temperaturas disminuirían el potencial hídrico, tanto durante el día como durante la noche, así como la recuperación del fotosistema II. Por tanto, en conclusión, condiciones climáticas cercanas a las de Granada; 18/13 °C día/noche, DPV de 0,5 KPa, y una radiación que no exceda los 1000 μmol fotones/ m2s favorecen el buen desempeño de la planta.
Palabras clave: agua, cultivo, fisiología vegetal, fluorescencia de la clorofila, fotosíntesis, fruto de la pasión, horticultura.
INTRODUCTION
Plant ecophysiology is the study of the behavior of plants in particular habitats which allow the understanding of physiological performance in the field (Lüttge and Scarano, 2004). This facilitates the determination of conditions that are helpful for the development of a crop (Solarte et al., 2010), providing decision-making tools for crop management and sowing sites (Higgins et al., 1992) because the genetic potential of the individual will only be reached if the environmental conditions are close to optimal (Rabbinge et al., 1985).
Plant ecophysiology analyze variables such as photosynthetic rate, that provide the driving force for the metabolism of sink organs (Foyer, 1987) and at the same time, are linked to the transpiration rate or water loss through the stomata. Stomata are multisensorial structures that sense environmental and physiological factors (Zeiger et al,. 1987) for determining water use efficiency, which is reflected in the water status of the plant which can be measured through the water potential. The stomatal control and water status of plants change in accordance with the climatic conditions so they vary throughout the day (Pandey et al., 2003) and throughout the phenological cycle of plants (Thomas and Winner, 2002; Kenzo et al., 2006).
The use of light by the photosynthetic apparatus is measured through chlorophyll fluorescence, allowing for the understanding of processes such as the photoinhibition caused by excess radiation that affects photosystems, causing chronic or dynamic damage (Aro et al., 1993). The evaluation of the response of horticultural crops to environmental factors such as temperature, water availability, light and carbon dioxide concentration is useful for determining the effect of suboptimal environmental conditions and for managing the crops for maximum productivity (Schaffer and Andersen, 1994).
Changes in environmental factors are more evident in field conditions where the plants can be subjected to minimal and inevitable stresses to which they must respond. Therefore, studies under uncontrolled conditions are necessary for understanding events such as the reproductive phase in plants at the agronomic level.
Gulupa, Passiflora edulis Sims, is among the fruit crops of high importance in Colombia due to the fact that it is highly sought after in the international market due to its organoleptic and physical properties, making up one of the primary export lines for fruit in Colombia (Pinzón et al., 2007). Ecophysiological studies on passiflora are scarce although the importance of the studies have been recognized (Ocampo, 2013); most of the knowledge of the crop comes from empirical observations of crops sown in different zones. Although Jiménez-Neira (2006) and Jiménez et al., (2009) report its growth between 15-20 °C and at an altitudinal range from 1400 to 2200 m a.s.l., the most suitable conditions within these ranges are unknown. Despite the fact that studies on topics such as phytopatology and postharvest physiology have increased, ecophysiological studies are scarce and most of them have been conducted under controlled conditions with passiflora hybrids. Only two photosynthetic rates have been registered in seedling phase (Turner et al,. 1996, Cruz-Aguilar, 2012) and two in adult Passiflora spp. hybrid plants with an ornamental use (Abreu et al., 2014) and in different varieties of passion fruit (Passiflora edulis fv. flavicarpa) in the reproductive phase (Gama et al., 2013).
For this reason, the present study aimed to describe and analyze the ecophysiological performance of gulupa in the reproductive phase under three environmental conditions in the field in order to produce tools that elucidate the conditions that result in better performance in the crop as linked to good agricultural practices, which will be reflected in fruit quality and yield. It is expected that climatic conditions such as high humidity and a low water deficit pressure result in a more efficient water use and a better water status in the plants, while the quantity of light of each location directly influence the photosynthetic rate.
MATERIAL AND METHODS
Study site
Experimental plots were sown with 100 gulupa plants at a distance of 6x3 m, in three different locations in the Department of Cundinamarca: Chia, located at 2610 m a.s.l., with an average temperature of 14 °C (04° 50.952' N 74° 04.343' W); Granada, located at 2230 m a.s.l. with an average temperature of 15 °C (4°30.150'N 74° 21.532'W); and Tena, located at 2090 m a.s.l. with an average temperature of 17 °C (4° 41.234' N 74° 21.592'W).
A simple trellis system with double wires was used. The plants were managed with formation and maintenance pruning and were fertilized monthly in accordance with edaphic analysis. Irrigation was carried out at field capacity in periods of low precipitation in order to ensure water availability in the soil.
Meteorological stations were installed in each of the plots (Coltein Ltda, Bogotá, Colombia) with data loggers (Coltein Ltda, Bogotá and Hobo U12-006, Onset Computer Corporation, Bourne, Massachusetts, USA) that monitored the relative humidity (%) and temperature (THR-102sensors, USA), and photosynthetically active radiation (PAR; LI 190 Bsensors, LI-COR Inc. Lincoln, Nebraska, USA) every fifteen minutes. The vapor pressure deficit was determined by the method developed by Allen et al., (2006).
Ecophysiological variables
The ecophysiological samplings were taken in the reproductive phase; in the flowering stage (flowering peak) and during fructification (fructification peak) in each of the locations during 2011. The measurements were taken in 1021 months-old plants, in completely healthy and developed young leaves in tertiary branches directed towards the two wires of the trellis system. The ambient carbon dioxide (Ca) and the internal carbon dioxide (Ci), the transpiration rate (E) and the stomatal conductance (gs) were measured with an IRGA gas analyzer (ADC LCPro+, Bioscientific Ltd, Hoddesdon, UK). The Ci/Ca index was determined to evaluate the photosynthesis stomatal limitations and the extrinsic water use efficiency was determined as photosynthetic rate/transpiration rate. The leaf water potential (Ψ) was measured with a Schöllander pump, model 1000 (PMS Instruments Co, Albany, Oregon, USA). The photosystem II operating efficiency of (ɸPSII), which estimates the efficiency at which light is absorbed by PSII and is used by the first acceptor of electrons (plastoquinone A) (Baker, 2008), was measured with a modulated fluorometer (FMS2, Hansatech, King's Lynn, UK). In order to analyze the results related to the photosynthesis variables, the photon flow density (PPFD) was measured with the PAR sensors adjusted to IRGA gas analyzer and with modulated fluorometer, allowing registering the instantaneous incident radiation in the measuring moment.
All of the above measurements were taken in ranges of two hours, between 7:00 and 18:00 for two days in the two stages of the reproductive phase. The water potential and maximum quantum efficiency of photosystem II photochemistry (Fv/Fm) were measured at 4:00. The fluorescence measurements were taken in 20 plants and, in each one, 40 leaves were measured (20 leaves in each tertiary branch exposed on the trellis wire) and the water potential and gas exchange measurements were taken in ten plants, six leaves of each plant (three leaves in each tertiary branch exposed on the trellis wire).
At the same time, HH2 soil moisture meters were installed (Delta T Devices Ltd, Cambridge, UK) in three different places in the plots of each location to measure the soil moisture content.
Photosynthetic light curves
The photosynthetic response light curves (A/PPFD) were done between 7:30 and 12:00 in three plants in each location, controlling the temperature at 25 °C and the CO2 at 380 ppm. The plants were subjected to radiations of 1200, 1000, 800, 600, 400, 300, 200, 100, 50 and 0 μmol photons/m2s consecutively and the leaves in each radiation level were left to stabilize for a minimum of three minutes. The curves were adjusted by regression to a rectangular hyperbolic model (Mielke et al., 2005; Solarte et al., 2010) of the equation:
Where A is the photosynthetic rate, Amax the maximum photosynthetic rate, PPFD the photosynthetically active radiation, and k is half of the photosynthesis saturation radiation. The light compensation point, Ic, was determined by solving the equation for zero. The quantum efficiency, фPPFD, was determined from the slope of the initial lineal portion of the A/PPFD curve (Bauerle et al., 2006).
Statistical analysis
Lilliefors test was used to test the suppositions of normality. Due to the absence of normality of the data, the non-parametric tests of Kruskal-Wallis and Friedman were applied, using hour-location as factor. The differences were considered significant if the probabilities were less than 0.05. The area under the curve was determined to find the total fixed CO2 and the total water loss through the foliar surface during the sampling days by using the disease progress curve method (AUDPC). All of the tests were carried out with the RStudio software (2012) with the R MESS (Ekstrom, 2013), MASS and Pgirmess packages (Venables and Ripley, 2002).
RESULTS
Climatic conditions
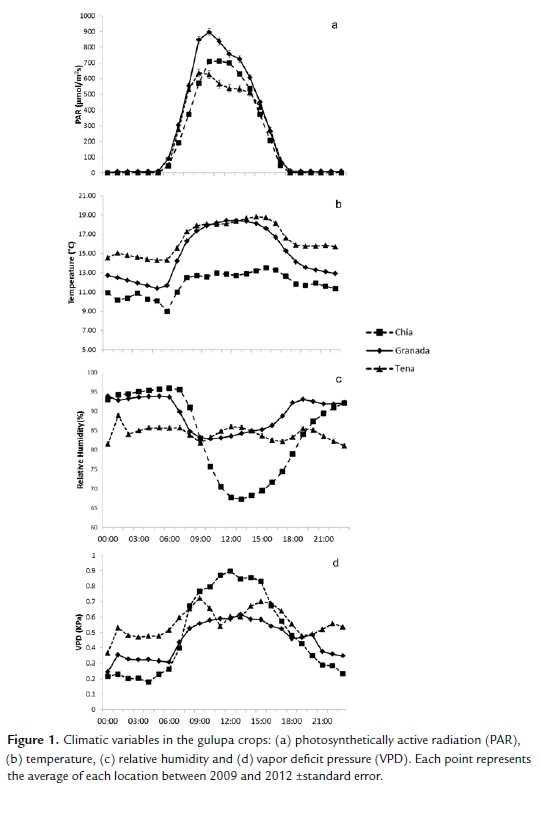
As can be seen in Figure 1, photosynthetically active radiation in Granada and Tena registered a tendency to increase in the morning hours and decrease at midday. Meanwhile, in Chia, the hours of higher radiation were registered at midday. The radiation in Granada was the highest, without exceeding 900 μmol/m2s on average, followed by Chia where the hours of high radiation reached an average of 800 μmol/m2s. In Tena, the radiation in the morning hours reached an average of 700 μmol/m2s and decreased in the afternoon to values close to 500 μmol/m2s which is favored by breaks provided by mist at some hours of the day, which considerably decreased the radiation. As can be seen in Figure 1b, the temperature in the day was similar from morning until afternoon in Granada and Tena (18 °C) but during the night the temperature decreased more in Granada, with a bigger difference between the day and the night (15 °C and 13 °C in Tena and Granada night respectively). By contrast, Chia presented lower temperatures for both the day (13 °C) and the night (11 °C). As can be seen in Figure 1c, the relative humidity presented a higher fluctuation between day and night in Chia, decreasing considerably during the day to values near 65 %, while the night saw values of 95 %. Granada registered values similar to those of Chia in the night but, during the day, these values did not go below 80 %; meanwhile, Tena registered small differences between the day and the night, remaining close to 85 % throughout the 24 hours of the day. Figure 1d shows that in Chia, there was a daily increase in VPD close to 0.9 KPa, related to the decrease in relative humidity but at night the VPD decrease below the other two locations. Granada presented a lower VPD during the day (0.6 KPa) while Tena registered a higher VPD during the night (0.5 KPa) and most part of the day (0.7 KPa).
The soil moisture content did not vary considerably throughout the day in the three locations during the sampling days. In Tena, an edaphic moisture content of between 0.42 and 0.48 m3/m3 was registered. The soil of Chia and Granada registered similar moisture content between 0.19 and 0.28 m3/m3.
Ecophysiological variables
Flowering stage
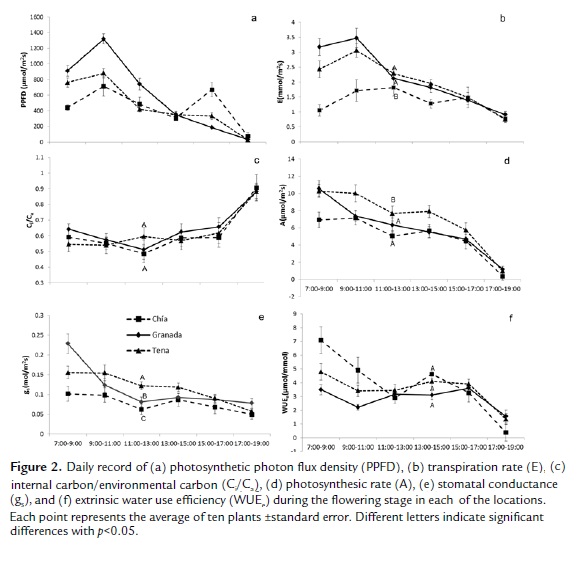
The photosynthetic rate (Fig. 2d) during the day was significantly higher in Tena than in Granada and Chia (χ2=5.33; p<0.01). The trend of the incident radiation (Fig. 2a) was similar to the photosynthesis trend in Tena and Chia while, in Granada, a decrease in the photosynthetic rate was registered in the hours of high radiation (close to 1400 μmol/m2s), which was linked to a decrease in stomatal conductance (Fig.2d) as well as for Ci/Ca (Fig. 2c), and a significant increase in the transpiration rate above of Tena and Chia locations was also registered, which generated a decrease in the extrinsic water use efficiency that was not significant. The total CO2 gain during the sampling days was 37.0 μmol CO2/m2s in Tena, 29.8 μmol CO2/m2s in Granada and 26.0 μmol CO2/m2s in Chia.
The transpiration rate did not show significant differences in Granada and Tena and was significantly lower in Chia (χ2=9.00; p=0.011), which was reflected in the water loss during the two days that was 7.2 mmol H2O/m2s in Chia, 10.42 mmol H2O/m2s in Granada and 10.82 mmol H2O/ m2s in Tena; the radiation behavior was similar to the transpiration trend in the three locations (Fig. 2a and 2b). Although there were no significant differences (χ2=3.00; p=0.22), the water use efficiency was lower in Granada in the first hours of the day, followed by Tena and Chia (Fig. 2f). For its part, the decrease in photosynthesis in the midday hours in Chia, similar to what occurred in Granada, was connected to a significant decrease in stomatal conductance (χ2=21.97; p<0.01) (Fig. 2e) and Ci/Ca (χ2=3.00; p=0.22) (Fig. 2c).
Figure 3b demonstrates a significant decrease of PSII operating efficiency in the plants of Tena (χ2=7.00; p=0.030) in high radiation hours of day, as compared to Granada and Chia, despite the fact that the PAR registered in Tena in the measurement points was similar to that of Granada (Fig. 3a). For its part, the maximum quantum efficiency of photochemistry (Fig. 3c) registered optimal values in Granada, while the plants grown in Chia and Tena had significantly lower values (χ2=71.70; p<0.01).
The leaf water potential during the day can be seen in Figure 3e, which was significantly higher in the plants of Granada (χ2=9.47; p=0.008), where the lowest value was seen in the hours of the highest transpiration, which did not go under -0.8 MPa; followed by the plants sown in Tena and Chia, which did not present significant differences, registering values under -1.0 MPa between 11:00 and 15:00. The decrease in stomatal conductance and the increase in transpiration in the first few hours (Figs. 2e and 2b) were corresponded with a decrease in leaf water potential in Granada. The leaf water potential before morning (Fig. 3d) recovered in Chia, remained high in Granada, and remained low in Tena, with values close to -0.5 MPa (χ2=81.14; p>0.01).
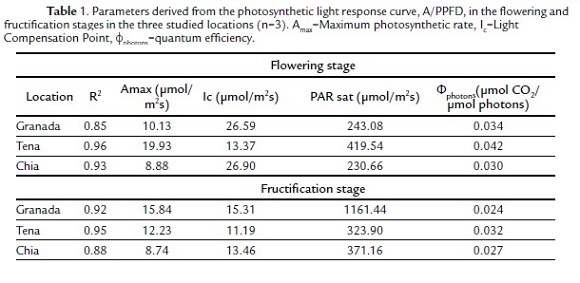
The parameters found using the A/PPFD curve (Table 1) in the flowering stage indicated a higher photosynthetic capacity in Tena at a radiation near 420 μmol/m2s, a lower light compensation point and higher quantum efficiency, followed by Granada and Chia with similar values between them but with a high photosynthetic capacity in Granada.
Fructification stage
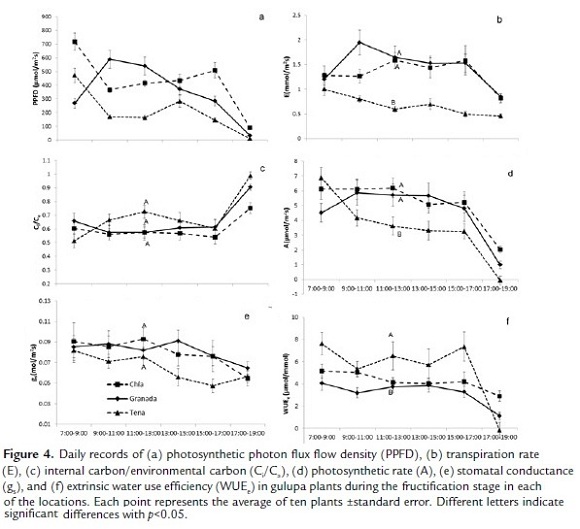
The photosynthetic rate was significantly lower in Tena (χ2=7.60; p<0.01), which was related to the low photosynthetically active radiation registered during the sampling days as seen in Figure 4a; only in the first range of the day where the PPFD was close to 500 μmol/m2s did photosynthesis increased. The photosynthetic rate was similar in Granada and Chia and remained constant with a decrease in the midday hours (Fig. 4d). The total fixed CO2was 26.6 μmol/m2s in Chia, 24.8 μmol/m2s in Granada and 17.7 μmol/m2s in Tena.
Figure 4d demonstrates that the low photosynthesis in Tena was related to the low transpiration (Fig. 4b) along with a very low stomatal conductance (Fig. 4e). This generated a significantly higher water use efficiency in Tena (χ2=6.33; p=0.044) (Fig. 4f). The decrease of gs was not reflected in the decrease in Ci/Ca in Tena, which was similar in Granada and Chia (Fig. 4c). The transpiration rate and stomatal conductance were similar in Granada and Chia.
Figure 5a shows that, despite the fact that there was higher radiation in Granada, the PSII operating efficiency (Fig. 5b) was similar to that of Tena. At the same time, despite the fact that the radiation in Chia in the morning hours was lower than in Granada, the PSII operating efficiency was similar and for the next hours was significantly lower (χ2=6.33; p=0.042). For its part, Fv/Fm registered optimal values in Granada and Tena, however, in Chia, the values had significant differences (χ2=144.76; p<0.001) and indicated possible stress in the plants (Fig. 5c).
Figure 5e evidences a leaf water potential that was significantly lower during the day in plants grown in Chia (χ2=61.50; p=0.0065) than the plants grown in Granada and Tena. The slight changes in stomatal conductance were related to changes in the water potential, which were more evident in Granada. A high water recovery could be seen in Figure 5d for Granada (χ2=81.14; p<0.001), while the plants grown in Chia and Tena registered lower values and, therefore, a lower water recovery by the roots.
The parameters found by the A/PPFD curve for the fructification stage can be seen in Table 1. The maximum photosynthetic rate was higher in Granada, followed by Tena and lower in Chia. Nevertheless, the saturation radiation was considerably higher in Granada and much higher than in the flowering stage, while in Chia and Tena it was low and similar. The light compensation point was higher in Granada, followed by Chia and Tena, while the quantum efficiency was high in Tena but low and similar in Chia and Granada.
DISCUSSION
The higher photosynthetic rate values registered during the day were similar to those shown for passion fruit varieties FB2000 and Gigante Amarelo, but lower than other varieties (Gama et al., 2013). In the three locations, the environmental conditions during the morning favored an increase in the WUEe due to the fact that there are suitable radiation for an increase in photosynthesis while the VPD, temperature, and humidity conditions do not hazard an excessive loss of water by the leaf surface (Bucci et al., 2003; Zweifel et al., 2007; Mengistut et al., 2011). This has also been observed in plants in temperate (Bassow and Bazzas, 1998), Mediterranean (Chaumont et al., 1994; Gatti and Rossi, 2010) and tropical Savanna zones (Eamus et al., 1999).
In the flowering stage in Granada, the instantaneous decrease in photosynthesis was connected to the an increase in radiation to over 1000 μmol/m2s (Fig. 2a), which brought about an increase in leaf temperature and a decrease in stomatal conductance, which have also been observed in Actidinia deliciosa (kiwi), another climbing fruit (Buwalda et al.,1992). Nevertheless, if the average radiation of Granada (Fig. 1a) is compared with the maximum value of the day during the sampling, over 1000 μmol/m2s, it can be observed that it corresponds with values over the average and, therefore, would not be a recurring phenomenon in this location.
Although the water use efficiency was low in the reproductive phase in Granada, connected to a considerable transpiration rate, this did not induce a lower leaf water potential in the plants, indicating an effective water recovery that must be supported by a high water conductance (Hubbard et al., 2001). This agrees with the fact that, in Granada, there are higher radiation, clear skies, and the soil is not water saturated (as was seen by the water soil content), which favor water uptake by the roots and its transport by the xylem due to the fact that it generates a high stomatal sensitivity to environmental changes (Rocha et al., 2004). Cavatte et al., (2012) reported that a low moisture content in the soil and high radiation in the day increase the nocturnal ability for hydration. When comparing the transpiration rate, the stomatal conductance and the water potential, a finer stomatal control was observed for Granada, evidenced by the fact that a slight decrease in stomatal conductance, caused a decrease in the transpiration that was clearly reflected in an increase in the water potential, which although also was observed in Chia, was less evident. The above facts reflected the fact that the best water status was found in the reproductive phase of the plants grown in Granada.
Despite the fact that clear skies are also found in Chia, VPD values that were close to 1.0 KPa (Fig. 1d) did not favor an adequate water recovery because they could generate cavitation in the xylem (Schultz and Matthews, 1997), which is evidenced in the water potential values during the day and the night, despite registering low transpiration rates. The low water potential values before morning that were registered in plants grown in Chia in the fruiting stage, could result from a low temperature during the night that decrease the permeability of the roots and its water conductivity (Matzner and Comstock, 2001; Norisada et al., 2005); these values were close to those reported for grapevines (Vitis vinifera) under water deficit conditions (Tay et al., 2007).
With respect to the chlorophyll fluorescence, the фPSII indicated a more efficient use of the light by the plants grown in Granada because a high incident radiation registered a low decrease of the efficiency factor of photosystem II. In the same manner, Fv/Fm was high in the flowering stage (in Tena only in fruiting stage), as in fructification, and its values indicated an optimal state of photosystem II (Maxwell and Johnson, 2000), which is supported by the climatic conditions of Granada, which include a low VPD both in the day and in the night, an average temperature of 18 °C in the day and 13 °C at night, and a considerable increase in relative humidity that does not decrease considerably in the day. This indicates a high recovery capacity and light use by photosystem II in Granada, which is connected to a high efficiency in the conversion of light intercepted to the biomass, resulting from a high recuperation by photoprotection (Long et al., 2006). Despite the low radiation in Tena, the Fv/Fm was not higher than in Granada and Chia, locations with higher radiation, indicating that other factors, such as the differences between the VPD, temperature and humidity for the day and for the night, which were small in Tena, are important for maintaining the capacity of photosystem II.
The photosynthetic rates in Tena are principally limited by the low average radiation (Fig. 1a) because the environmental conditions that presented during the sampling in the flowering stage and the first range measured in the fructification stage indicate that, with an increase of radiation to over 500 μmol/m2s, photosynthesis increases. At the same time, the mist, besides limiting the available radiation, moistened the surfaces of the leaves, decreasing the diffusion of CO2 to the interior of the leaves (Nobel, 1999; Guy-Letts and Mulligan, 2005). In addition, when radiation decreases considerably, as registered for the fructification stage sampling, the stomatal conductance remains very low, although the Ci/Ca data indicated that there were no stomatal limitations in Tena. Despite the fact that the VPD in Tena was not critical during the day, it increases to 0.7 KPa, which would favor an occasional high decrease in the water potential during the day with respect to Granada. In the flowering stage in Tena the Ψ maintained in the night time hours possibly due to a low difference in humidity, temperature, and VPD between the day and night, which would also be important for water recuperation. Additionally, the changing radiation conditions during the day, connected to the breaks provided by mist, caused instantaneous changes in other variables, such as temperature and humidity, to which the plants could not immediately respond because they need time to recover CO2 uptake when radiation decreases (Long et al., 2006).
In regards to the photosynthetic light curves, in general, with the exception of the PPFDsat in Granada in the fructification stage, the low need for radiation in gulupa was evidenced, which is related to the behavior of subtropical plants in which light is not limiting due to the fact that the stomatal opening is maximum at a relatively low PPFD (Machado et al., 2005; Ribeiro et al., 2005), as verified with the daily tendencies of the three locations where, at low radiation, the photosynthesis increased to up to 7.0 μmol/ m2s in the first hours of the day. Nevertheless, the optimal conditions that are supplied to the leaf when measuring A/ PFFD curves, makes the difference between the results of daily curves (not environmentally controlled) and environmental controlled A/PPFD. For this reason, the Amax is higher than the maximum value of photosynthesis registered in the daily curve at similar or higher radiations.
The A/PPFD curves indicated that Tena has a high photosynthetic capacity, which was more evident in the flowering stage and indicated that the conditions are not stressful for the gulupa found there, but, better yet, are limiting for the increase of photosynthesis in environmental conditions. The results in the flowering stage are related to the fact that a high night temperature increases Amax because it increases the strength of the carbohydrate sinks due to the increase in respiration in darkness that reduces the concentration of carbohydrates in the leaves (Turnbull et al., 2002). In the reproductive phase, in general, the high efficiency in the use of photons and the lower light compensation point registered in Tena are related to the low radiation of the location as seen in undergrowth plants adapted to low quantities of light (Kitao et al., 2000). The photosynthetic capacity was lower in Chia in the two evaluated stages, a product of the low temperatures because in general, tropical species are vulnerable to temperature decreases (Crawford, 1989). Niyogi (1999) and Ribeiro et al., (2005) reported that nighttime cooling decreased the maximum photosynthetic rate, probably due to decrease in the rate of chemical reactions that are part of the photosynthetic system. For its part, although the photosynthetic capacity increased in Granada in the fructification stage, the increase in the saturated radiation was considerable, which verified the plasticity of the plants to changing conditions in the long term because the data measured with the meteorological stations indicated that, during the months previous to the sampling, the radiation increased in Granada (data not shown).The results for the fructification stage were related to high radiation in Granada, which generated a higher saturated radiation as evidenced in Kiwi, A. deliciosa (Laing, 1985; Greer and Halligan, 2001).
Therefore, despite the fact that the photosynthetic capacity is suitable in Tena, as in Granada, in Tena, the specific limitations of the low radiation and the differences between the climatic variables, such as RH, temperature, and VPD, between day and night did not allow for the recovery of the water potential during the night, affecting the good physiological performance of the plants.
CONCLUSIONS
The conditions in Granada were more suitable for the development of gulupa, indicating that a low VPD (close to 0.5 KPa), high relative humidity, and average temperature of 18 °C favored the good ecophysiological performance of gulupa. The differences for humidity, temperature, and VPD between the day and night were important for the recovery of the leaf water status and the photosystems, but must be connected to a low VPD during the day. In Tena, although the low radiation was limiting, the photosynthetic capacity was suitable, indicating that the low water potential during the night has no effect. Nevertheless, the small differences between the day and night for the vapor pressure deficit impeded the recovery of the water status at night. For their part, the low temperatures in Chia and the increase in the VPD in the day did not favor the water status of the plants, generating mechanisms that favor the conservation of water and impede the adequate recovery of photosystem II during the night.
ACKNOWLEDGEMENTS
This study was made possible through the financial support of the Ministerio de Agricultura and Desarrollo Rural of Colombia (Contract N° 239-2008L62227-6867, Agreement N° 054/08 MADR/CIAT/IICA) and Young researchers and innovators grant of COLCIENCIAS. The authors wish to thank the Universidad Nacional de Colombia and OCATI Ltda for their financial and technical support and to especially thank Laura Marcela Flórez Gutiérrez, and Marisol Cruz Aguilar for their active participation in collecting data and Yuli Paola Valencia, Julián Beltrán, Felipe Malagón, Lady Manrique, Juan David Vázquez, and Andrea Rojas for collecting data in the field.
REFERENCES
Abreu PP, Souza MM, Almeida AAF, Santos EA, Freitas JCO, Figueredo AL. Photosynthetic responses of ornamental passion flower hybrids to varying light intensities. Acta Physiol Plant. 2014;36(8):1993-2004. Doi: 10.1007/s11738-014-1574-0.
Allen RG, Pereira LS, Raes D, Smith M. Evapotranspiración del Cultivo. Guías para la determinación de los requerimientos de agua de los cultivos. Estudio para Riego y Drenaje N° 56, Roma: FAO; 2006. p 35-41.
Aro EM, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143(2):113–134. Doi: 10.1016/0005-2728(93)90134-2.
Baker NR. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu Rev Plant Biol. 2008;59:89-113. Doi: 10.1146/annurev.arplant.59.032607.092759.
Bassow SL, Bazzaz, FA. How environmental conditions affect canopy leaf-level photosynthesis in four deciduous tree species. Ecology. 1998;79(8):2660-2675.
Bauerle WL, Wang GG, Bowden JD, Hong CM. An analysis of ecophysiological responses to drought in American Chestnut. Ann For Sci. 2006;63:833-842. Doi: 10.1051/forest:2006066.
Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Stenberg LSL. Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: factors and mechanisms contributing to the refilling of embolized vessels. Plant Cell Environ. 2003;26(10):1633-1645. Doi: 10.1046/j.0140-7791.2003.01082.x.
Buwalda JG, Green TGA, Meekings JS, Coneybear DJ. Measurement of canopy gas exchange of kiwifruit vines using a suite of whole-canopy cuvettes. Environ Exp Bot. 1992;32(4):425-438 Doi: 10.1016/0098-8472(92)90055-7.
Cavatte PC, Oliveira AA, Morais LE, Martins SC, Sanglard LM, DaMatta FM. Could shading reduce the negative impacts of drought on coffee? A morphophysiological analysis. Physiol Plant. 2012;144(2):111:122. Doi: 10.1111/j.1399-3054.2011.01525.x
Chaumont M, Morot-Gaudry JF, Foyer CH. Seasonal and diurnal changes in photosynthesis and carbon partitioning in Vitis vinifera leaves in vines with and without fruit. J Exp Bot. 1994;45(9):1235-1243. Doi: 10.1093/jxb/45.9.1235.
Crawford RMM. Studies in plant survival: Ecological case histories of plant adaptation to adversity. 6th Ed. Oxford: Blackwell Scientific Publications; 1989. 296 p.
Cruz-Aguilar M, Hoyos-Carvajal L, Melgarejo LM. Respuesta fisiológica de la gulupa (Passiflora edulis Sims) frente al ataque por Fusarium spp. In: Melgarejo LM, editor. Ecofisiología del cultivo de la gulupa (Passiflora edulis Sims). Bogotá: Universidad Nacional de Colombia; 2012. p. 91-113.
Eamus D, Myers B, Duff G, Williams D. Seasonal changes in photosynthesis of eight savanna tree species. Tree Physiol. 1999;19(10):665–671. Doi: 10.1093/treephys/19.10.665
Ekstrom, C. MESS: Miscellaneous esoteric statistical scripts, 2013. Available in: http://CRAN.R-project.org/package=MESS.
Foyer CH. The basis for source-sink interactions in leaves. Plant Physiol Biochem. 1987;25(5):649-657.
Gama VN, Cunha JT, de Melo IL, Bacarin MA, Silva DM. Photosynthesic characteristics and quality of five passion fruit varieties under field conditions. Acta Physiol Plant. 2013;35(3):941-948. Doi: 10.1007/s11738-012-1137-1.
Gatti E, Rossi F. Daily and seasonal trends of gas exchange in Pistacia lentiscus L. Acta Physiol Plant. 2010;32(4):809–813. Doi: 10.1007/s11738-009-0448-3.
Greer DH, Halligan EA. Photosynthetic and fluorescence light responses for kiwifruit (Actinidia deliciosa) leaves at different stages of development on vines grown at two different photon flux densities. Aust J Plant Physiol. 2001;28(5):373–382. Doi:10.1071/PP00146.
Guy Letts M, Mulligan M. The impact of light quality and leaf wetness on photosynthesis in north-west Andean tropical montane cloud forest. J Trop Ecol. 2005;21(5):549-557. Doi:10.1017/S0266467405002488.
Higgins SS, Larsen FE, Bendel RB, Radamaker GK, Bassman JH, Bidlake WR, et al. Comparative gas exchange characteristics of potted, glasshouse-grown almond, apple, fig, grape, olive, peach and Asian pear. Sci Hortic. 1992;52(4):313-329. Doi: 10.1016/0304-4238(92)90032-8.
Hubbard RM, Ryan MG, Stiller V, Sperry JS. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ. 2001;24(1):113-121. Doi: 10.1046/j.1365-3040.2001.00660.x.
Jiménez-Neira Y. El cultivo de la Gulupa Passiflora edulis SIMS (Tesis de especialización). Bogotá: Departamento de Agronomía, Facultad de Agronomía, Universidad Nacional de Colombia; 2006. p. 12.
Jiménez Y, Carranza C, Rodríguez M. Manejo integrado del cultivo de gulupa (Passiflora edulis Sims). In: Miranda D, Fischer G, Carranza C, Magnitskiy S, Casierra-Posada F, Piedrahita W, et al., editors. Cultivo, poscosecha y comercialización de las pasifloráceas en Colombia: maracuyá, granadilla, Gulupa y Curuba. Bogotá: Sociedad Colombiana de Ciencias Hortícolas; 2009. p. 159-189.
Kenzo T, Ichie T, Watanabe Y, Yoneda R, Ninomiya I, Koike T. Changes in photosynthesis and leaf characteristics with tree height in five dipterocarp species in a tropical rain forest. Tree Physiol. 2006;26(7):865:873. Doi: 10.1093/treephys/26.7.865.
Kitao M, Lei TT, Koike T, Tobita H, Maruyama Y, Matsumoto Y, et al. Temperature response and photoinhibition investigated by chlorophyll fluorescence measurements for four distinct species of dipterocarp trees. Physiol Plant. 2000;109(3):284–290. Doi:10.1034/j.1399-3054.2000.100309.x.
Laing WA. Temperature and light response curves for photosynthesis in kiwifruit (Actidinia chinensis) cv. Hayward. N Z J Agr Res. 1985;28(1):117-124. Doi: 10.1080/00288233.1985.10427004.
Long SP, Zhu XG, Naidu SL, Ort DR. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006;29(3):315–330. Doi: 10.1111/j.1365-3040.2005.01493.x.
Lüttge U, Scarano FR. Ecophysiology. Revista Bras Bot. 2004;27(1):1-10.
Machado EC, Schmidt PT, Medina CL, Ribeiro RV. Respostas da fotossíntese de três espécies de citros a fatores ambientais. Pesq Agropec Bras. 2005;40(12):1161-1170.
Maxwell K, Johnson GN. Chlorophyll fluorescence —a practical guide. J Exp Bot. 2000;51(345):659-668. Doi: 10.1093/jexbot/51.345.659.
Matzner S, Comstock J. The Temperature dependence of shoot hydraulic resistance: implications for stomatal behaviour and hydraulic limitation. Plant Cell Environ. 2001;24(12):1299-1307. Doi: 10.1046/j.0016-8025.2001.00785.x.
Mengistu T, Sterck FJ, Fetene M, Tadesse W, Bongers, F. Leaf gas exchange in the frankincense tree (Boswellia papyfera) of African dry woodlands. Tree Physiol. 2011;31(7):740-750. Doi: 10.1093/treephys/tpr067.
Mielke MS, Almeida, AAF, Gomes FP. Photosynthetic traits of five neotropical rainforest tree species: interactions between light response curves and leaf-to-air vapour pressure deficit. Braz Arch Biol Technol. 2005;48(5):815-824.
Niyogi KK. Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. Doi: 10.1146/annurev.arplant.50.1.333.
Nobel PS. Physicochemical and Environmental Plant Physiology. 2nd Ed. San Diego, USA: Academic Press; 1999. 474 p.
Norisada M, Hitsuma G, Kuroda K, Yamanoshita T, Masumori M, Tange T, et al. Acacia mangium, a nurse tree candidate for reforestation on degraded sandy soils in the Malay Peninsula. For Sci. 2005;51(5):498–510.
Ocampo J. Diversidad y distribución de las Passifloraceae en el departamento de Huila en Colombia. Acta biol Colomb. 2013;18(3):511-516.
Pandey S, Kumar S, Nagar PK. Photosynthetic performance of Ginkgo biloba L. grown under high and low irradiance. Photosynthetica. 2003;41(4):505-511 Doi: 10.1023/B:PHOT.0000027514.56808.35.
Pinzón IM, Fischer G, Corredor G. Determinación de los estados de madurez del fruto de la gulupa (Passiflora edulis Sims.). Agron Colomb. 2007;25(1):83-95.
Rabbinge R, Jorritsma ITM, Schans J. Damage components of powdery mildew in winter wheat. Neth J Pl Path. 1985;91(5):235-247.
Ribeiro RV, Machado EC, dos Santos MG. Leaf temperature in sweet orange plants under field conditions: influence of meteorological elements. Rev Bras Agrometeorol. 2005;13(2):378-388.
Rocha AV, Su H-B, Vogel CS, Schmid HP, Curtis PS. Photosynthetic and water use efficiency responses to diffuse radiation by an aspen-dominated northern hardwood forest. Forest Sci. 2004;50(6):793-801.
RStudio. RStudio: Integrated development environment for R [Software] Version 0.96.122. Boston (MA); 2012. Available in: http://www.rstudio.org/.
Schaffer B, Andersen PC. Handbook of Environmental Physiology of Fruit Crops, Volume II. Subtropical and Tropical Crops. 1st Ed. Boca Raton, Florida; Editorial CRC Press; 1994. 320 p.
Schultz HR, Matthews MA. High vapour pressure deficit exacerbates xylem cavitation and photoinhibition in shade-grown Piper auritum H.B. & K. during prolonged sunflecks. Oecologia. 1997,110(3):312-319. Doi:10.1007/s004420050164.
Solarte ME, Pérez-Martínez LV, Melgarejo LM. Ecofisiología Vegetal. In: Melgarejo LM editor. Experimentos en Fisiología Vegetal. Bogotá: Universidad Nacional de Colombia; 2010. p. 137-166.
Tay AC, Abdullah AM, Awang M, Furukawa A. Midday depression of photosynthesis in Enkleia malaccensis, a woody climber in a tropical rainforest. Photosynthetica. 2007;45(2):189-193. Doi: 10.1007/s11099-007-0031-3.
Thomas SC, Winner WE. Photosynthetic differences between saplings and adult trees: an integration of field results by meta-analysis. Tree Physiol. 2002;22(2-3):117-127. Doi: 10.1093/treephys/22.2-3.117.
Turnbull MH, Murthy R, Griffin KL. The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant Cell Environ. 2002;25(12):1729–1737. Doi: 10.1046/j.1365-3040.2002.00947.x.
Turner DW, Menzel CM, Simpson DR. Short term drying of half the root systems reduces growth but not water status of photosynthesis in leaves of passionfruit (Passiflora sp.). Sci Hortic. 1996;65(1):25-36. Doi: 10.1016/0304-4238(95)00849-7.
Venables WN, Ripley BD. Modern Applied Statistics with S. 4th Ed. New York: Springer; 2002. 498 p.
Zeiger E, Farquhar GD, Cowan, IR. Stomatal function. 1st Ed. California: Stanford University Press; 1987. 520 p.
Zweifel R, Steppe K, Sterck FJ. Stomatal regulation by microclimate and tree water relations: interpreting ecophysiological field data with a hydraulic plant model.
Referencias
Abreu PP, Souza MM, Almeida AAF, Santos EA, Freitas JCO, Figueredo AL. Photosynthetic responses of ornamental passion flower hybrids to varying light intensities. Acta Physiol Plant. 2014;36(8):1993-2004. Doi: 10.1007/s11738-014-1574-0
Allen RG, Pereira LS, Raes D, Smith M. Evapotranspiración del Cultivo. Guías para la determi-nación de los requerimientos de agua de los cultivos. Estudio para Riego y Drenaje N° 56, Roma: FAO; 2006. p 35-41.
Aro EM, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143(2):113–134. Doi: 10.1016/0005-2728(93)90134-2
Baker NR. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu Rev Plant Biol. 2008;59:89-113. Doi: 10.1146/annurev.arplant.59.032607.092759
Bassow SL, Bazzaz, FA. How environmental conditions affect canopy leaf-level photosynthesis in four deciduous tree species. Ecology. 1998;79(8):2660-2675.
Bauerle, WL, Wang GG, Bowden, JD, Hong, CM. An analysis of ecophysiological responses to drought in American Chestnut. Ann For Sci. 2006;63:833-842. Doi: 10.1051/forest:2006066
Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Stenberg LSL. Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: factors and mechanisms contributing to the refilling of embolized vessels. Plant Cell Environ. 2003;26(10):1633-1645. Doi: 10.1046/j.0140-7791.2003.01082.x
Buwalda JG, Green TGA, Meekings, JS, Coneybear DJ. Measurement of canopy gas exchange of kiwifruit vines using a suite of whole-canopy cuvettes. Environ Exp Bot. 1992;32(4):425-438 Doi: 10.1016/0098-8472(92)90055-7
Cavatte PC, Oliveira AA, Morais LE, Martins SC, Sanglard LM, DaMatta FM. Could shading reduce the negative impacts of drought on coffee? A morphophysiological analysis. Physiol Plant. 2012;144(2):111:122. Doi: 10.1111/j.1399-3054.2011.01525.x
Chaumont M, Morot-Gaudry JF, Foyer CH. Seasonal and diurnal changes in photosynthesis and carbon partitioning in Vitis vinifera leaves in vines with and without fruit. J Exp Bot. 1994;45(9):1235-1243. Doi: 10.1093/jxb/45.9.1235.
Crawford RMM. Studies in plant survival: Ecological case histories of plant adaptation to adver-sity. 6th Ed. Oxford: Blackwell Scientific Publications; 1989. 296 p.
Cruz-Aguilar M, Hoyos-Carvajal L, Melgarejo LM. Respuesta fisiológica de la gulupa (Passiflora edulis Sims) frente al ataque por Fusarium spp. In: Melgarejo LM, editor. Ecofisiología del culti-vo de la gulupa (Passiflora edulis Sims). Bogotá: Universidad Nacional de Colombia; 2012. p. 91-113.
Eamus D, Myers B, Duff G, Williams D. Seasonal changes in photosynthesis of eight savanna tree species. Tree Physiol. 1999;19(10):665–671. Doi: 10.1093/treephys/19.10.665
Ekstrom, C. MESS: Miscellaneous esoteric statistical scripts, 2013. Available in: http://CRAN.R-project.org/package=MESS.
Foyer CH. The basis for source-sink interactions in leaves. Plant Physiol Biochem. 1987;25(5):649-657.
Gama VN, Cunha JT, de Melo IL, Bacarin MA, Silva DM. Photosynthesic characteristics and quality of five passion fruit varieties under field conditions. Acta Physiol Plant. 2013;35(3):941-948. Doi: 10.1007/s11738-012-1137-1
Gatti E, Rossi F. Daily and seasonal trends of gas exchange in Pistacia lentiscus L. Acta Physiol Plant. 2010;32(4):809–813. Doi: 10.1007/s11738-009-0448-3
Greer DH, Halligan EA. Photosynthetic and fluorescence light responses for kiwifruit (Actinidia deliciosa) leaves at different stages of development on vines grown at two different photon flux densities. Aust J Plant Physiol. 2001;28(5):373–382. Doi:10.1071/PP00146
Guy Letts M, Mulligan M. The impact of light quality and leaf wetness on photosynthesis in north-west Andean tropical montane cloud forest. J Trop Ecol. 2005;21(5):549-557. Doi:10.1017/S0266467405002488
Higgins SS, Larsen FE, Bendel RB, Radamaker GK, Bassman JH, Bidlake WR, et al. Compara-tive gas exchange characteristics of potted, glasshouse-grown almond, apple, fig, grape, olive, peach and Asian pear. Sci Hortic. 1992;52(4):313-329. Doi: 10.1016/0304-4238(92)90032-8
Hubbard RM, Ryan MG, Stiller V, Sperry JS. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ. 2001;24(1):113-121.
Jiménez-Neira Y. El cultivo de la Gulupa Passiflora edulis SIMS (Tesis de especialización). Bogo-tá: Departamento de Agronomía, Facultad de Agronomía, Universidad Nacional de Colombia; 2006. p. 12.
Jiménez Y, Carranza C, Rodríguez M. Manejo integrado del cultivo de gulupa (Passiflora edulis Sims). In: Miranda D, Fischer G, Carranza C, Magnitskiy S, Casierra-Posada F, Piedrahita W, et al., editors. Cultivo, poscosecha y comercialización de las pasifloráceas en Colombia: maracuyá, granadilla, Gulupa y Curuba. Bogotá: Sociedad Colombiana de Ciencias Hortícolas; 2009. p. 159-189.
Kenzo T, Ichie T, Watanabe Y, Yoneda R, Ninomiya I, Koike T. Changes in photosynthesis and leaf characteristics with tree height in five dipterocarp species in a tropical rain forest. Tree Phys-iol. 2006;26(7):865:873.
Kitao M, Lei TT, Koike T, Tobita H, Maruyama Y, Matsumoto Y, et al. Temperature response and photoinhibition investigated by chlorophyll fluorescence measurements for four distinct spe-cies of dipterocarp trees. Physiol Plant. 2000;109(3):284–290. Doi:10.1034/j.1399-3054.2000.100309.x.
Laing WA. Temperature and light response curves for photosynthesis in kiwifruit (Actidinia chinensis) cv. Hayward. N Z J Agr Res. 1985;28(1):117-124. Doi: 10.1080/00288233.1985.10427004
Long SP, Zhu XG, Naidu SL, Ort DR. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006;29(3):315–330. Doi: 10.1111/j.1365-3040.2005.01493.x
Lüttge U, Scarano FR. Ecophysiology. Revista Bras Bot. 2004;27(1):1-10.
Machado EC, Schmidt PT, Medina CL, Ribeiro RV. Respostas da fotossíntese de três espécies de citros a fatores ambientais. Pesq Agropec Bras. 2005;40(12):1161-1170.
Maxwell K, Johnson GN. Chlorophyll fluorescence —a practical guide. J Exp Bot. 2000;51(345):659-668. Doi: 10.1093/jexbot/51.345.659
Matzner S, Comstock J. The Temperature dependence of shoot hydraulic resistance: implications for stomatal behaviour and hydraulic limitation. Plant Cell Environ. 2001;24(12):1299-1307. Doi: 10.1046/j.0016-8025.2001.00785.x.
Mengistu T, Sterck FJ, Fetene M, Tadesse W, Bongers, F. Leaf gas exchange in the frankincense tree (Boswellia papyfera) of African dry woodlands. Tree Physiol. 2011;31(7):740-750. Doi: 10.1093/treephys/tpr067
Mielke MS, Almeida, AAF, Gomes FP. Photosynthetic traits of five neotropical rainforest tree species: interactions between light response curves and leaf-to-air vapour pressure deficit. Braz Arch Biol Technol. 2005;48(5):815-824.
Niyogi KK. Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. Doi: 10.1146/annurev.arplant.50.1.333
Nobel PS. Physicochemical and Environmental Plant Physiology. 2nd Ed. San Diego, USA: Ac-ademic Press; 1999. 474 p.
Norisada M, Hitsuma G, Kuroda K, Yamanoshita T, Masumori M, Tange T, et al. Acacia mangi-um, a nurse tree candidate for reforestation on degraded sandy soils in the Malay Peninsula. For Sci. 2005;51(5):498–510.
Ocampo J. Diversidad y distribución de las Passifloraceae en el departamento de Huila en Co-lombia. Acta biol Colomb. 2013;18(3):511-516.
Pandey S, Kumar S, Nagar PK. Photosynthetic performance of Ginkgo biloba L. grown under high and low irradiance. Photosynthetica. 2003;41(4):505-511 Doi: 10.1023/B:PHOT.0000027514.56808.35
Pinzón IM, Fischer G, Corredor G. Determinación de los estados de madurez del fruto de la gu-lupa (Passiflora edulis Sims.). Agron Colomb. 2007;25(1):83-95.
Rabbinge R, Jorritsma ITM, Schans J. Damage components of powdery mildew in winter wheat. Neth J Pl Path. 1985;91(5):235-247.
Ribeiro RV, Machado EC, dos Santos MG. Leaf temperature in sweet orange plants under field conditions: influence of meteorological elements. Rev Bras Agrometeorol. 2005;13(2):378-388.
Rocha AV, Su H-B, Vogel CS, Schmid HP, Curtis PS. Photosynthetic and water use efficiency responses to diffuse radiation by an aspen-dominated northern hardwood forest. Forest Sci. 2004;50(6):793-801.
RStudio. RStudio: Integrated development environment for R [Software] Version 0.96.122. Bos-ton (MA); 2012. Available in: http://www.rstudio.org/
Schaffer B, Andersen PC. Handbook of Environmental Physiology of Fruit Crops, Volume II. Subtropical and Tropical Crops. 1st Ed. Boca Raton, Florida; Editorial CRC Press; 1994. 320 p.
Schultz HR, Matthews MA. High vapour pressure deficit exacerbates xylem cavitation and pho-toinhibition in shade-grown Piper auritum H.B. & K. during prolonged sunflecks. Oecologia. 1997,110(3):312-319. Doi:10.1007/s004420050164
Solarte ME, Pérez-Martínez LV, Melgarejo LM. Ecofisiología Vegetal. In: Melgarejo LM editor. Experimentos en Fisiología Vegetal. Bogotá: Universidad Nacional de Colombia; 2010. p. 137-166.
Tay AC, Abdullah AM, Awang M, Furukawa A. Midday depression of photosynthesis in Enkleia malaccensis, a woody climber in a tropical rainforest. Photosynthetica. 2007;45(2):189-193. Doi: 10.1007/s11099-007-0031-3.
Thomas SC, Winner WE. Photosynthetic differences between saplings and adult trees: an integra-tion of field results by meta-analysis. Tree Physiol. 2002;22(2-3):117-127. Doi: 10.1093/treephys/22.2-3.117
Turnbull MH, Murthy R, Griffin KL. The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant Cell Environ. 2002;25(12):1729–1737. Doi: 10.1046/j.1365-3040.2002.00947.x
Turner DW, Menzel CM, Simpson DR. Short term drying of half the root systems reduces growth but not water status of photosynthesis in leaves of passionfruit (Passiflora sp.). Sci Hortic. 1996;65(1):25-36. Doi: 10.1016/0304-4238(95)00849-7
Venables WN, Ripley BD. Modern Applied Statistics with S. 4th Ed. New York: Springer; 2002. 498 p.
Zeiger E, Farquhar GD, Cowan, IR. Stomatal function. 1st Ed. California: Stanford University Press; 1987. 520 p.
Zweifel R, Steppe K, Sterck FJ. Stomatal regulation by microclimate and tree water relations: interpreting ecophysiological field data with a hydraulic plant model. J Exp Bot. 2007;58(8):2113–2131. Doi:10.1093/jxb/erm050
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Nixon Flórez-Velasco, Gerhard Fischer, Helber Enrique Balaguera-López. (2024). Photosynthesis in fruit crops of the high tropical Andes: A systematic review. Agronomía Colombiana, 42(2), p.e113887. https://doi.org/10.15446/agron.colomb.v42n2.113887.
2. Ruth Amanda Acero Camelo, Manuel Ricardo Esteban Molina, Alfonso Parra Coronado, Gerhard Fischer, Juan Evangelista Carulla Fornaguera. (2021). BASE GROWTH TEMPERATURE AND PHYLLOCHRON FOR KIKUYU GRASS (Cenchrus clandestinus; Poaceae) . Acta Biológica Colombiana, 26(2), p.160. https://doi.org/10.15446/abc.v26n2.83199.
3. JULIANY M. FRANÇA, LUCIMARA R. VENIAL, ELOÁ B. COSTA, EDILSON R. SCHMILDT, OMAR SCHMILDT, PAULA M. BERNARDES, SANDRO D. TATAGIBA, JOSÉ C. LOPES, MARCIA F.S. FERREIRA, RODRIGO S. ALEXANDRE. (2018). Morphophysiology, Phenotypic and Molecular Diversity of Auxin-induced Passiflora mucronata Lam. (Passifloraceae). Anais da Academia Brasileira de Ciências, 90(2), p.1799. https://doi.org/10.1590/0001-3765201820160898.
4. Wendy Tatiana Cárdenas-Pira, Edwin Torres-Moya, Stanislav Magnitskiy, Luz Marina Melgarejo. (2021). Physiological Responses of Purple Passion Fruit (Passiflora Edulis Sims F. Edulis) Plants to Deficiencies of the Macronutrients, Fe, Mn, and Zn during Vegetative Growth. International Journal of Fruit Science, 21(1), p.344. https://doi.org/10.1080/15538362.2021.1890673.
5. D.S. Espinosa, L.M. Melgarejo, M.S. Hernández, S.E. Melo, J.P. Fernández-Trujillo. (2018). Physiological and biochemical characterization of sweet granadilla (Passiflora ligularis JUSS) at different locations. Acta Horticulturae, (1194), p.1459. https://doi.org/10.17660/ActaHortic.2018.1194.204.
6. Gerhard Fischer, Alfonso Parra-Coronado, Helber Enrique Balaguera-López. (2022). Altitude as a determinant of fruit quality with emphasis on the Andean tropics of Colombia. A review.. Agronomía Colombiana, 40(2) https://doi.org/10.15446/agron.colomb.v40n2.101854.
7. Ginna Esperanza Fernández M., Lus Marina Melgarejo, Natalia Alejandra Rodríguez C.. (2015). Algunos aspectos de la fotosíntesis y potenciales hídricos de la granadilla (Passiflora ligularis Juss.) en estado reproductivo en el Huila, Colombia. Revista Colombiana de Ciencias Hortícolas, 8(2), p.206. https://doi.org/10.17584/rcch.2014v8i2.3214.
8. Nakul Rao Rangare, Narayan Lal, Govind Shiurkar, Kunti Banjare, Pragya Singh, Nisha Jangre, Nisha Sahu, Ram Kishor Patel. (2025). Impact of Climate Change on Guava Production: Challenges and Adaptive Strategies. Applied Fruit Science, 67(5) https://doi.org/10.1007/s10341-025-01637-8.
9. Juan Pablo Bernal Moreno, Nohra Rodríguez. (2023). Responses of landraces and commercial cultivars of yellow passion fruit to the prevalence of Fusarium oxysporum. Agronomía Colombiana, 41(1), p.e104450. https://doi.org/10.15446/agron.colomb.v41n1.104450.
10. Hermann Restrepo-Diaz, Alefsi David Sánchez-Reinoso. (2020). Fruit Crops. , p.59. https://doi.org/10.1016/B978-0-12-818732-6.00005-8.
11. Luciene Souza Ferreira, Andressa Leal Generoso, Virginia Silva Carvalho, Fábio Afonso Mazzei Moura de Assis Figueiredo, Rafael Walter, Tiago Massi Ferraz, Jefferson Rangel da Silva, Geraldo de Amaral Gravina, Weverton Pereira Rodrigues, Wagner A. Vendrame, Eliemar Campostrini. (2021). Better light spectral quality and thermal amplitude inside the greenhouse stimulate growth and improve acclimatization of in vitro–grown Cattleya warneri T. Moore. In Vitro Cellular & Developmental Biology - Plant, 57(6), p.883. https://doi.org/10.1007/s11627-021-10162-8.
12. Letícia Cespom Passos, Jefferson Rangel da Silva, Weverton Pereira Rodrigues, Fabrício de Oliveira Reis, Marco Antônio da Silva Vasconcellos, Jose Altino Machado Filho, Eliemar Campostrini. (2018). Leaf photosynthetic responses of passion fruit genotypes to varying sunlight exposure within the canopies. Theoretical and Experimental Plant Physiology, 30(2), p.103. https://doi.org/10.1007/s40626-018-0106-5.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2015 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).