Bacillus effect on the germination and growth of tomato seedlings (Solanum lycopersicum L).
Efecto de Bacillus sobre la germinación y crecimiento de plántulas de tomate (Solanum lycopersicum L)
DOI:
https://doi.org/10.15446/abc.v22n1.57375Palabras clave:
biotechnology, indole, PGPB, seedlings, tomato. (en)biotecnología, indoles, PGPB, plántulas, tomate. (es)
Descargas
The capacity to solubilize phosphate and to produce indole compounds Indole Acetic Acid type, was evaluated in 15 strains isolated from castor bean lignocellulosic residues (Ricinus communis). To determine the solubilizing activity of phosphates a qualitative test by using Pikovskaya culture medium was employed and for the evaluation of the production of indole compounds (IAA) a Salkowsky colorimetric analysis technique was applied. Among the microorganisms tested, the Bacillus pumilus GIBI 206 demonstrated capacity to solubilize phosphates and Bacillus subtilis GIBI 200 showed of capacity to solubilize phosphates and to produce Indoleacetic Acid (IAA). To determine the effect of the Bacillus subtilis strain on germination and growth promotion, tomato seeds (Solanum lycopersicum ‘Santa Clara’) were inoculated; the inoculation of the seeds along with the microorganism revealed statistically significant differences, during the germination stage compared to the control treatment. Nevertheless, it revealed a positive influence on the development of tomato plants, originating a significant increase on the mass and length of its stem and root. The results of this research offer the possibility of using the Bacillus subtilis as a growth promoter in tomato seedlings and in the formulation of bio-products.
DOI: https://doi.org/10.15446/abc.v22n1.57375
Bacillus EFFECT ON THE GERMINATION AND GROWTH OF TOMATO SEEDLINGS (Solanum lycopersicum L)
Efecto de Bacillus sobre la germinación y crecimiento de plántulas de tomate (Solanum lycopersicum L)
Teresa CABRA CENDALES1, Cristian Alonso RODRÍGUEZ GONZÁLEZ1, Claudia Patricia VILLOTA CUÁSQUER1, Omar Alberto TAPASCO ALZATE2, Annia HERNÁNDEZ RODRÍGUEZ3.
1 Grupo de investigaciones Biológicas GIBI, Instituto de Microbiología y Biotecnología Agroindustrial, Facultad de Salud, Universidad Católica de Manizales UCM. Carrera 23 n.º 60-63. Manizales, Colombia.
2 Grupo de investigación en Estadística y Matemáticas, Departamento de Matemáticas, Facultad de Ciencias Exactas y Naturales, Universidad de Caldas. Cll 65 n.º 26-10, bloque B, oficina B 408. Manizales, Colombia.
3 Directora de Ciencia y Técnica, Universidad de la Habana. Calle M n.º 255/ 21 y 19, Vedado, Plaza de la Revolución. La Habana, Cuba.
For correspondence. tcabra@ucm.edu.co
Received: 11th May 2016, Returned for revision: 1st August 2016, Accepted: 2nd November 2016.
Associate Editor: Howard Junca.
Citation/Citar este artículo como: Cabra Cendales T, Rodríguez González CA, Villota Cuásquer CP, Tapasco Alzate OA, Hernández Rodríguez A. Bacillus effect on the germination and growth of tomato seedlings (Solanum lycopersicum L). Acta biol. Colomb. 2017;22(1):37-44. DOI: https://doi.org/10.15446/abc.v22n1.57375
ABSTRACT
The capacity to solubilize phosphate and to produce indole compounds Indole Acetic Acid type, was evaluated in 15 strains isolated from castor bean lignocellulosic residues (Ricinus communis). To determine the solubilizing activity of phosphates a qualitative test by using Pikovskaya culture medium was employed and for the evaluation of the production of indole compounds (IAA) a Salkowsky colorimetric analysis technique was applied. Among the microorganisms tested, the Bacillus pumilus GIBI 206 demonstrated capacity to solubilize phosphates and Bacillus subtilis GIBI 200 showed of capacity to solubilize phosphates and to produce Indoleacetic Acid (IAA). To determine the effect of the Bacillus subtilis strain on germination and growth promotion, tomato seeds (Solanum lycopersicum 'Santa Clara') were inoculated; the inoculation of the seeds along with the microorganism revealed statistically significant differences, during the germination stage compared to the control treatment. Nevertheless, it revealed a positive influence on the development of tomato plants, originating a significant increase on the mass and length of its stem and root. The results of this research offer the possibility of using the Bacillus subtilis as a growth promoter in tomato seedlings and in the formulation of bio-products.
Keywords: biotechnology, indole, PGPB, seedlings, tomato.
RESUMEN
La capacidad para solubilizar fosfatos y producir compuestos indólicos del tipo Ácido Indol Acético, se evaluó en 15 cepas aisladas de residuos lignocelulósicos de higuerilla (Ricinus communis). Para determinar la actividad solubilizadora de fosfatos se realizó una prueba cualitativa utilizando medio de cultivo Pikovskaya y para evaluar la producción de compuestos indólicos (AIA) se empleó la técnica colorimétrica de Salkowsky. Entre los microorganismos evaluados, Bacillus pumilus GIBI 206 mostró tener la capacidad para solubilizar fosfatos y Bacillus subtilis GIBI 200 evidenció capacidad para solubilizar fosfatos y producir Ácido Indolacético (AIA). Para determinar el efecto de la cepa Bacillus subtilis sobre la germinación y promoción de crecimiento, se inoculó en semillas de tomate (Solanum lycopersicum cultivar Santa Clara); la inoculación de las semillas con el microorganismo no mostró diferencias estadísticamente significativas en el tiempo de germinación en comparación con el tratamiento control, sin embargo mostró influir positivamente en el desarrollo de las plantas de tomate generando un aumento significativo sobre la masa y longitud del tallo y de la raíz. Los resultados de esta investigación ofrecen la posibilidad de utilizar a Bacillus subtilis como promotora de crecimiento en plántulas de tomate y en la formulación de bioinsumos.
Palabras clave: biotecnología, indoles, PGPB, plántulas, tomate.
INTRODUCTION
The indiscriminate use of chemicals in agriculture has a negative impact on the environment, originating the accumulation of toxic substances, which pollute the soil, the water, and water tables, causing a biodiversity loss (Camelo et al., 2011). One of the strategies currently used for the agricultural clean production is the use of plant growth promoting bacteria (PGPR, plant growth promoting rhizobacteria (Cubillos et al., 2009).
The term PGPR was proposed to describe the bacteria that inhabit the plants rhizosphere and which have a positive effect on the crops (Kloepper and Schroth, 1993).
Subsequently, Bashan and Holguín (1998) proposed a new classification for the PGPR, which includes all the beneficial bacteria considering their particular role, to the extent that the term is divided into PGPR (plant growth promoting bacteria) and biocontrol-PGPB (biocontrol-plant growth promoting bacteria).
The PGPB can promote the plant growing through direct or indirect channels. The direct effects can be demonstrated in the absence of other microorganisms (that is, the plant only interacts with the microorganism under analysis), whereas the indirect mechanisms can be observed in the interaction between the microorganism of interest with a phytopathogenic, whereby the harmful effects on a plant are reduced (Acebo-Guerrero et al., 2015). The PGPB direct effects are: the phytohormone synthesis, the siderophores production, the mineral solubilization and the atmospheric nitrogen fixation (Estrada et al., 2013; Kaur and Reddy, 2014; Reis et al., 2015).
The influence on the absorption of mineral elements due to the raise of ionic flow on the radicular surface associated with PGPB has been also observed (Whipps, 2001). The biocontrol mechanisms widely recognized, mediated by PGPB, are the competition for nutrients or space, the inhibitory chemical compounds synthesis as the siderophores, the antibiotics, lythic enzymes, and biosurfactants (Acebo-Guerrero et al., 2015), as well as the induction of systemic acquired resistance in plants (Hernández-Rodríguez et al., 2014; Chandler et al., 2015).
A wide array of bacterial genre is considered within this classification: Pseudomonas, Burkholderia, Bacillus, Azospirillum, Herbaspirillum, Enterobacter y Azotobacter, among others (Kennedy et al., 2004). Several researches have informed the genres Pseudomonas, Azospirillum, Azotobacter, Bacillus y Streptomyces, as part of the tomato rhizosphere microbial community, being the genre of Azospirillum, the most predominant. Additionally, the effect of its inoculation on growth, its nutritional condition and performance is highlighted (Solanum lycopersicum L) (Terry-Alfonso et al., 2005; Widnyana Ketut and Cokorda Javandira, 2016), similarly, various species of the genre Bacillus have been used to confirm its capacity to promote plant growing in tomato plants. In this regard, Gül et al. (2008), used two commercial strains of Bacillus amyloliquefaciens (FZB24 and FZB42) to determine their effect on tomato production in closed and open systems, regarding the presence of different quantity of nutrients. Myresiotis (2014) demonstrated that tomato plants inoculated with a combination of the B. subtilis GB03 and B. pumilus SE34 strains enhanced the absorption of nutrients and the supression of pathogens.
In Colombia, 15 % of the horticultural area and 9.6 % of the fresh vegetables exported belong to tomato. In the year 2010, the area planted was of 16,227 ha, with a production of 546,322 t, and for the year 2013, the harvested area was of 16,704 ha, with a production of 683538 t, for a total average per year of 40.9 t/ha.(Arie et al., 2007; Morales et al., 2009; Ceballos et al., 2012). Considering Colombian diversity and its capacities of agricultural use, with emphasis in tomato crops, this study has the aim of determine the effect of native strains de Bacillus on the germination and the development of the diverse biometric parameters of tomato crops.
MATERIALS AND METHODS
Microbial cultures
Fifteen native bacterial strains were used, these strains were previously isolated from the castor bean lignocellulose residues (Ricinus communis) and characterized through traditional and molecular techniques by Cabra-Cendales et al. (2015). These strains are deposited in the microrganisms bank at The Catholic University of Manizales (UCM) with the codes GIBI 187, GIBI 188, GIBI 189, GIBI 192, GIBI 193, GIBI 194, GIBI 195, GIBI 197, GIBI 199, GIBI 200, GIBI 201, GIBI 203, GIBI 204, GIBI 206, GIBI 208. The strain with code GIBI 200 was inoculated in tomato seeds Santa Clara in order to confirm its effect on it.
Production of Indole compounds of Indole Acetic Acid (IAA) type
For a quantitative determination of the Indole compounds production, the bacteria to be evaluated were inoculated in Trypticase Soy Agar (TSA) supplemented with DL-Tryptophan 0.1/L as inductor of auxins production (Tien et al., 1979). They were incubated at 30 oC and kept under constant agitation at 120 rpm during 96 h. Successively, they were centrifuged for 10 minutes at 5000 rpm and the agar cultures free from cells were added with the Salkowski reactive (Glickmann et al., 1995) in a 1:2. relation. The incubation process was done at an above room-ambient condition temperature and darkness for 30 minutes; afterwards the absorbance was read at 540 nm with a spectrophotometer type Pharo300 by Merck. In parallel, a pattern curve was done with synthetic (IAA) in concentrations of μg.mL-1 to 30 μg.mL-1. The experiment was repeated three times with five replicas per strain.
Phosphate solubilizing
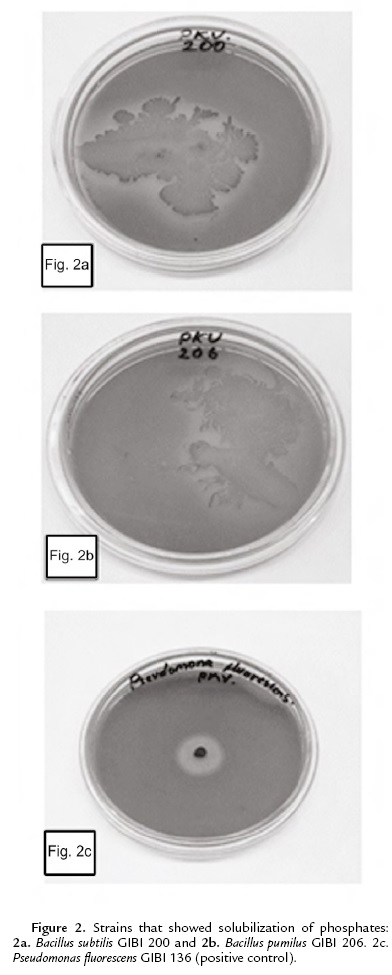
The capacity of phosphate solubilizing was determined in the Pikovskaya culture medium supplemented with Ca3 (PO4)2 (MercK). The plates were incubated at 30 ºC for seven days (Mehta and Nautiyal, 2001). As a positive control the bacteria Pseudomonas fluorescens GIBI 136 was used.
Bioassay of plant-microorganism interaction, peat and seeds inoculation
To prepare the bacterial inoculum a plates of nutritive agar was taken from the growing bacterium colony and suspended in 5ml of Trypticase Soy broth at pH 7.0. It was kept in agitation at 120 rpm and at 30 °C for 48 to 72 h. Afterwards, the 5 ml of Trypticase Soy broth were moved with the growing microorganism at 45 ml of the same culture medium. Subsequently, it was taken at a volume of 500 ml, incubated at 30 °C at constant agitation until achieving a concentration of 108 cells/ml. The concentration was verified through spectrophotometry at a wavelength (λ) of 600 nm seeking for an absorbance between 0.9 and 1 equivalent to a concentration of 1x108 c/mL and by counting the colony-forming units UFC/mL through the total viable count. The microorganism feasibility and purity tests were made in triplicate in Trypticase Soy Agar plates.
Commercial peat was used as substrate for planting seeds (Sphagnum), it sterilized was three times for one hour at 121 °C, afterwards, each kilogram was impregnated with 300 ml of the inoculum and incubated for 96 hours at 30 oC. Posterior to this, a count on the microorganism plate was done and a 7.9 x 108 UFC/g count was obtained.
Experimental Conditions
One hundred twenty (120) Solanum lycopersicum L. Santa Clara commercial seeds were disinfected in a sodium hypochlorite solution at 5 % for a minute, in ethanol at 70 % for a minute and after that, successive washes with sterile distilled water. Sixty seeds were inoculated with the microorganism solution in Trypticase Soy broth, with a concentration of 108 cells/ml. They were agitated for an hour at 120 rpm. As negative control sixty treated seeds with sterile distilled water were used (Reyes et al., 2008).
The seeds were planted at random in germination trays containing commercial peat coming from Canadian peatlands (Sphagnum), assigning a well to each of them randomly. Twelve grooves with ten seeds each distributed in six grooves with seeds and microorganism-inoculated peat and six grooves of negative control (peat seeds treated with Trypticase Soy (TS) only were established. Germination trays were watered with 2 ml sterile distilled water for nine days, after which the maximum of germination in treatments was obtained.
The seedlings obtained were left planted up to the age of 37 days where two real leaves had developed. Posterior to this, fifteen plants for treatment were selected at random and the following growing parameters were determined: longest root length, stem length, leaves and root moist and dry mass of matter, according to the Camargo and Ávila (2014) method.
Biometric Analysis
The design was completely randomized using as response variable the production of IAA and as a controlled factor the type of microorganism and the Duncan multiple range test was applied for multiple comparisons between treatments using SPSS version 19.0. The germination results were compared with respect to the control group (uninoculated seeds) using the SPSS 18.0 program. Subsequently, the method of Principal Component Analysis (PCA) was used, using the R 2.15.1 program in order to track the effect of microorganisms in various biometric indicators of the plant.
RESULTS
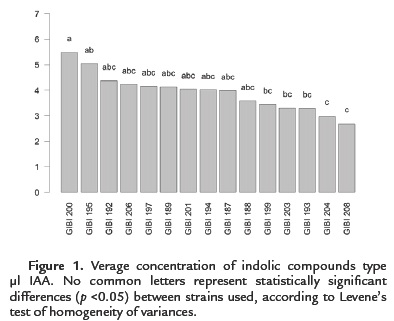
100 % of the strains (15) have the ability to produce indole compounds IAA type. However, only 13.3 % (2) solubilized phosphates. All strains produce low levels of IAA in the range of 2.49 to 5.47 µg / ml (Figure. 1). Bacillus subtilis strain GIBI 200 obtained the highest production average of this metabolite (5.47 µg / ml), whereas the Bacillus sp. strain GIBI 208 showed the lowest production value of IAA (2.49 µg / ml).
As for the solubilization of phosphates, only Bacillus subtilis GIBI 200 and Bacillus pumilus GIBI 206 showed halos around the colony, which shows that these strains have the potential to solubilize inorganic phosphate (Fig. 2). Bacillus subtilis strain GIBI 200 was selected to continue this study for their ability to produce IAA and solubilize phosphates, mechanisms that may be involved in the plant growth-promoting activity.
Effect of Bacillus subtilis strain GIBI 200 on tomato crop
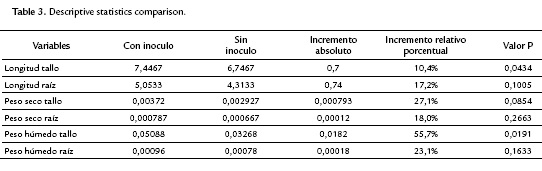
The maximum germination of tomato seeds was obtained on day nine, for both inoculated and uninoculated seeds. The percentage of germination of inoculated seeds was 86.7 % (52 seeds) and of uninoculated was 80 % (48 seeds). No statistical significance between the inoculated tomato seeds and the control sample (p=0.401) was demonstrated. However, high mortality was observed in the control seedlings (51.6 %, 31), while in inoculated plants it was 6.6 % (four seedlings), indicating that the inoculation with the strain Bacillus subtilis GIBI 200 reduced substantially seedling mortality.
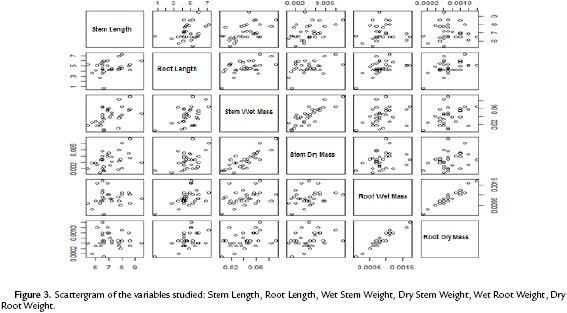
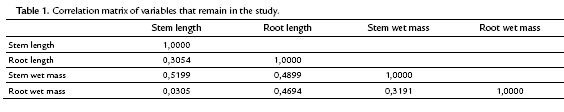
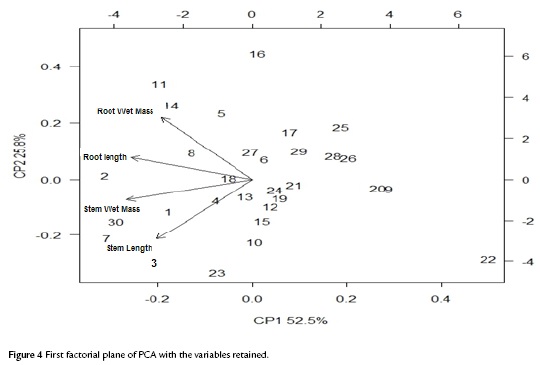
The scattergram (Fig. 3) evidences a positive correlation between wet and dry root mass with a correlation coefficient of 0.9474. Similarly, it evidences a strong correlation between the wet and dry mass of the stem with a correlation coefficient of 0.8227. These high correlations indicate the presence of redundant information in the variables involved. Therefore, to improve the quality of the information reflected in the plane, this information (values of the wet mass of root and shoot) was deleted. Table. 1 shows the correlation matrix of the variables that remain in the study.
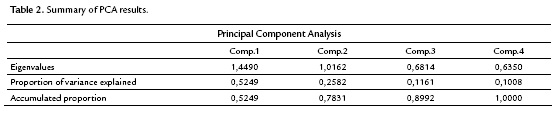
The correlation among the variables involved indicates that a principal component analysis is appropriate to group quantitative variables that demonstrate interdependence. Table 2 shows that the first component retains 52.5 % of the variability, while the second component retains only 25.8 %, so the first factorial plane explains about 78.3 % of the total variability of the data. Furthermore, it is detected that the first two components are the only ones having eigenvalues that exceed 1.0, indicating that the first factorial plane is the illustrative enough to explain the interdependence present in the data studied, without a significant loss of information.
The factorial plane (Fig. 4) shows that in general, vectors that identify the variables have high magnitude, indicating that all the variables studied are well represented and confirms that the analysis of other factorial planes is not needed. The first principal component accounts for the size of the plants whose growth is registered to the left, that is, larger plants are located in the second and third quadrant, while smaller are located to the right of plane. The second component is an indicator of the shape of plants, and contrasts mainly the variables wet mass of root and stem length. This first factorial plane shows a positive impact associated with inoculation of tomato plants with Bacillus subtilis GIBI 200 related to the increase in stem and root lengths, as well as fresh mass of root and stem; if you consider that plants labeled with numbers 1 to 15 correspond to plants inoculated with the microorganism, whereas plants registered with the numbers 16 to 30 served as a control group. This first factorial plane shows that plants with high biophysical measurements are located in the quadrants of the left of the plane, which are mostly inoculated plants with the bacteria. It also shows a 0.7 and 0.74 mm increase for inoculated plants with respect to uninoculated plants, on their average stem and root lengths respectively. Similarly, increases in the other variables studied were recorded, although the only statistically significant differences (p<0.05) are the length and stem wet weight (Table 3)
DISCUSSION
Bacillus is one of the most studied genus due to the plant growth promoting capacity in crops of economic importance (Rojas et al., 2011; Hernández-Rodríguez et al, 2014; Myresiotis et al., 2014; Widnyana Ketut and Cokorda Javandira, 2016). These bacteria are not usually important producers of auxin, however, some strains with the capacity to produce between 16 and 55 µg/ml of IAA have been reported (Tsavkelova, 2006; Felici et al., 2008; Rojas et al., 2011). The genus Bacillus includes B.megaterium species and B. subtilis that can produce organic acids as primary mechanism of solubilization of phosphate, but may also act as phytases enzymes excreted into the culture medium (Fernandez et al., 2005).
In this research, the studied strains produce between 2.49 and 5.47 µg/ml IAA. These values are similar to those previously reported by Luna Martínez et al. (2013), who typified four Bacillus strains with a range of concentration from 2.3 a 6.8 µg/ml, they achieved an increase in the germination and fresh mass of the tomato seedlings.
Bacillus subtilis GIBI 200 strain showed an auxin production and phosphate solubilization capacity although the strain did not show a significant effect (p<0.05) on the tomato seeds germination. The results obtained do no correspond to those reported by Lagunas et al. (2001); Izzeddin and Medina (2011); Luna-Martínez et al. (2013), who showed that the inoculation of tomato seeds with the Bacillus strains increase the germination percentages in 5 or 6 %.
The seed germination depends on the viability of the embryo and the breaking of dormancy generated by environmental conditions (Luna-Martínez et al., 2013). The PGPB can influence the latter case since the reduction in ethylene levels due to the ACC deaminase activity of bacteria in the seed increases germination, along with the production of IAA that stimulates cell division in order to promote the growth of the embryo (Glick et al., 2007; Jalili et al., 2009).
With regard to growth, it was found that the strain Bacillus subtilis GIBI 200 has plant growth promoting effect on 37-day-old tomato seedlings, with significant increases (p≤ 0.05) in length and fresh weight of stem and root. The results coincide with those reported by other authors who demonstrated the positive effect of inoculation of tomato plants with strains of Bacillus (Lagunas et al., 2001; Luna Martinez et al, 2013.) And other bacterial genera such as Azospirillum (Terry-Alfonso et al., 2005), Pseudomonas (Gravel et al., 2007) and Rhizobium (Santillana et al., 2005). This behavior could be related to the production of metabolites of IAA type by the inoculated strain. The IAA induces plant growth by increasing cell division and differentiation of tissue, effects that are reflected in a higher content of biomass (Lagunas et al., 2001; Santillana et al., 2005). It has also been argued that the IAA absorbed by the seeds and roots of plants could stimulate the activity of the ACC synthase enzyme, which is involved in the synthesis of ethylene. Low ethylene concentrations promote the growth of root hairs of inoculated plants and the surface area of the root (Luna Martinez et al., 2013), which brings increase in water and nutrient intake by the plant, thereby increase in plant growth.
CONCLUSIONS
Bacillus subtilis GIBI 200 promotes the growth of tomato. The major effects of inoculation of the strain were observed in the increase of length and fresh weight of stem and root in young plants. This could be related to the production of indole compounds of IAA type and solubilization of phosphate by the strain; however, other mechanisms could be influencing the beneficial effects obtained in vivo, although other experiments should be developed to demonstrate this hypothesis. The results indicate the agro-biological capabilities of Bacillus subtilis GIBI 200 and its potential use as a microbial inoculant on tomato crop.
ACKNOWLEDGMENT
The authors express their sincere gratitude to the Directorate of Research and Graduate Studies at the Catholic University of Manizales for the funding provided for the development of this research, according to Agreement No 9 January 27, 2011, issued by the Academic Council.
CONFLICT OF INTEREST
The authors declare that they have not conflict of interest.
REFERENCES
Acebo-Guerrero Y, Hernández-Rodríguez A, Vandeputte O, Miguélez-Sierra Y, Heydrich-Pérez M, Ye L, El Jaziri, M. Characterization of Pseudomonas chlororaphis from Theobroma cacao L. rhizosphere with antagonistic activity against Phytophthora palmivora (Butler). J Appl Microbiol. 2015;119(4):1112-1126. Doi: 10.1111/jam.12910.
Arie T, Takahashi H, Kodama M, Teraoka T. Tomato as a model plant for plant-pathogen interactions. Plant Biotechnol. 2007;24(1):135-147. Doi: 10.1007/978-3-642-60234-4_2
Bashan Y, Holguin G. Proposal for the division of Plant Growth- Promoting Rhizobacteria into two classification: biocontrol-PGPB (Plant Growth-Promoting Bacteria) and PGPB. Soil Biol Biochem. 1998;30(8-9):1225-1228.
Cabra-Cendales T, Meneses-Cabezas DC, Galeano-Vanegas NF. Identificación de microorganismos asociados a residuos de higuerilla (Ricinus communis). Rev Colomb Quim. 2015;44(2):10-15. Doi: 10.15446/rev.colomb.quim.
Camargo-Cepeda DF, Ávila ER. Efectos del Trichoderma sp. sobre el crecimiento y desarrollo de la arveja (Pisum sativum L.). Ciencia y Agricultura. 2014;11(1):91-100.
Camelo M, Vera SP, Bonilla RR. Mecanismos de acción de las rizobacterias promotoras del crecimiento vegetal. Corpoica cienc tecnol. Agropecu.2011. Available in: http://agris.fao.org/agris-search/search/display.do?f=2012/CO/CO1201.xml;CO2012003937 Accessed.
Ceballos-Aguirre N, Vallejo-Cabrera FA, Arango-Arango N. Evaluación del contenido de antioxidantes en introducciones de tomate tipo cereza (Solanum spp). Acta Agron. 2012;61(3):230-238.
Chandler S, Van Hese N, Coutte F, Jacques P, Höfte M, De Vleesschauwer D. Role of cyclic lipopeptides produced by Bacillus subtilis in mounting induced immunity in rice (Oryza sativa L.). Physiol Mol Plant P. 2015; 91:20-30. Doi: 10.1016/j.pmpp.2015.05.010.
Cubillos JR, Castellanos DE, Argüello H. Selección de Microorganismos promotores de crecimiento vegetal (ácido indol acético) a partir de muestras de suelo rizosferico, como primera etapa en el desarrollo de un biofertilizante. Rev Bras Agroecol. 2009;4(2):páginas.
Estrada GA, Divan Baldani VL, de Oliveira DM, Urquiaga S, Baldani JI. Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil. 2013;369:115-129. Doi: 10.1007/s11104-012-1550-7.
Felici C, Vettori L, Giraldi E, Forino LMC, Toffanin A, Tagliasacchi AM, Nuti M. Single and co-inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicon esculentum: effects on plant growth and rhizosphere microbial community. Appl Soil Ecol. 2008;40(2):260-270. Doi: http://doi.org/10.1016/j.apsoil.2008.05.002.
Fernández LA, Zalba P, Gómez MA, Sagardoy MA. Bacterias solubilizadoras de fosfato inorgánico aisladas de suelos de la región sojera. Rev Cienc Suelo Nutr Veg. 2005;23(1):31-37.
Glick B R, Cheng Z, Czarny J, Duan J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol. 2007;119(3):329-339.
Glickmann E, Dessaux Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61(2):793-796.
Gravel V, Antoun H, Tweddell RJ. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem. 2007;39(8):1968-1977.
Gül A, Kidoglu F, Tüzel Y. Effects of nutrition and Bacillus amyloliquefaciens on tomato (Solanum lycopersicum, L.) growing in perlite. Span J Agric Res. 2008;6(3):422-429.
Hernández-Rodríguez A, Ruíz-Beltrán Y, Acebo-Guerrero Y, Miguélez-Sierra Y, Heydrich-Pérez M. Microbial antagonists to manage black pod rot in Theobroma cacao L. Their present status and perspective use in Cuba. Rev Protección Veg. 2014;29(1):11-19. Doi: 101159/000207196. 11.
Izzeddin N, Medina L. Efecto del control biológico por antagonistas sobre fitopatógenos en vegetales de consumo humano. Salus. 2011;15(3):8-18.
Jalili F, Khavazi K, Pazira E, Nejati A, Rahmani H A, Sadaghiani H R, Miransari M. Isolation and characterization of ACC deaminase-producing fluorescent pseudomonads, to alleviate salinity stress on canola (Brassica napus L.) growth. J Plant Physiol. 2009;166(6):667-674. Doi: 10.1007/s11103-008-9435-0.
Kaur G, Reddy MS. Influence of P-solubilizing bacteria on crop yield and soil fertility at multilocational sites. Eur J Soil Biol. 2014;61:35-40. Doi: 10.1046/j.1351-0754.2003.0567.x.
Kennedy IR, Choudhury AT, Kecskes ML. Non-symbiotic bacterial diazotrophs in crop-farming systems: can their potential for plant growth promotion bebetter exploited?. Soil Biol Biochem. 2004;36(8):1229-1244.
Kloepper JW, Metting Jr FB. Plant growth-promoting rhizobacteria as biological control agents. Soil microbial ecology: applications in agricultural and environmental management.1992:255-274.
Lagunas-Lagunas J, Zavaleta-Mejía E, Osada-Kawasoe S, Aranda-Ocampo S, Luna-Romero I, Vaquera-Huerta H. Bacillus firmus como agente de control biológico de Phytophthora capsici Leo. en jitomate (Lycopersicon esculentum Mill.). Rev Mex Fitop. 2001;19(1):57-65.
Luna Martínez L, Martínez Peniche RA, Hernández Iturriaga M, Arvizu Medrano SM, Pacheco Aguilar JR. Caracterización de rizobacterias aisladas de tomate y su efecto en el crecimiento de tomate y pimiento. Rev Fitotec Mex. 2013;36(1):63 - 69.
Mehta S, Nautiyal CS. An Efficient Method for Qualitative screening of Phosphate-Solubilizing Bacteria. Curr Microbiol. 2001;43:51-56.
Morales FJ, Tamayo PJ, Castaño M, Olaya C, Martínez AK, Velasco AC. Enfermedades virales del tomate (Solanum Lycopersicum L.) en Colombia. Fitopatología Colombiana. 2009;33(1):23-27.
Myresiotis CK, Vryzas Z, Papadopoulou-Mourkidou E. Enhanced root uptake of acibenzolar-S-methyl (ASM) by tomato plants inoculated with selected Bacillus plant growth-promoting rhizobacteria (PGPR). App Soil Ecol. 2014;77:26-33. Doi: http://doi.org/10.1016/j.apsoil.2014.01.005.
Reis VM, Baldani VLD, Baldani J I. Isolation, identification and biochemical characterization of Azospirillum spp. and other nitrogen-fixing bacteria. In Handbook for Azospirillum. Switzerland: Springer International Publishing. 2015. p. 3-26.
Reyes I, Alvarez L, El-Ayoubi H, Valery A. Selección y evaluación de rizobacterias promotoras del crecimiento en pimentón y maíz. Bioagro. 2008;20(1):37-48.
Rojas MM, Tejeda B, Larrea JA, Mahillon J, Heydrich M. Aislamiento y caracterización de cepas de Bacillus asociadas al cultivo del arroz (Oryza sativa L.). Rev Bras Agroecol. 2011;6(1):90-99.
Santillana N, Arellano C, Zúñiga D. Capacidad del Rhizobium de promover el crecimiento en plantas de tomate (Lycopersicon esculentum Miller). Ecol Appl. 2005;4(4):1-2. Doi: 10.1111/1365-2664.12845.
Terry-Alfonso E, Leyva-Galán A, Hernández-Rodríguez A. Microorganismos benéficos como biofertilizantes eficientes para el cultivo del tomate (Lycopersicon esculentum, Mill). Rev Colomb Biotecnol. 2005;7(2):47.
Tien TM, Gasking MH, Hubbell DH. Plant growth substance produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennistem americanun L.) Appl. Environ Microbiol. 1979;37(4):219-226.
Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI. Microbial producers of plant growth stimulators and their practical use: a review. Appl Biochem Microbiol. 2006;42(2): 117-126. Doi: 10.1134/S0003683806020013.
Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 2001;52: 487-511.
Widnyana Ketut I, Cokorda Javandira. Activities Pseudomonas spp. and Bacillus sp. to stimulate germination and seedling growth of tomato plants. Agric Agric Sci Procedia. 2016;9:419-423. Doi: 10.1016/j.aaspro.2016.02.158
Referencias
Acebo‐Guerrero Y, Hernández‐Rodríguez A, Vandeputte O, Miguélez‐Sierra Y, Heydrich‐Pérez M, Ye L, El Jaziri, M. Characterization of Pseudomonas chlororaphis from Theobroma cacao L. rhizosphere with antagonistic activity against Phytophthora palmivora (Butler). J Appl Microbiol. 2015;119(4):1112-1126. Doi:10.1111/jam.12910
Arie T, Takahashi H, Kodama M, Teraoka T. Tomato as a model plant for plant-pathogen interactions. Plant Biotechnol. 2007;24(1):135-147. Doi:10.1007/978-3-642-60234-4_2
Bashan Y, Holguin G. Proposal for the division of Plant Growth- Promoting Rhizobacteria into two classification: biocontrol-PGPB (Plant Growth-Promoting Bacteria) and PGPB. Soil Biol Biochem. 1998;30(8-9):1225-1228.
Cabra-Cendales T, Meneses-Cabezas DC, Galeano-Vanegas NF. Identificación de microorganismos asociados a residuos de higuerilla (Ricinus communis). Rev Colomb Quim. 2015;44(2):10-15. Doi:10.15446/rev.colomb.quim
Camargo-Cepeda DF, Ávila ER. Efectos del Trichoderma sp. sobre el crecimiento y desarrollo de la arveja (Pisum sativum L.). Ciencia y Agricultura. 2014;11(1):91-100.
Camelo M, Vera SP, Bonilla RR. Mecanismos de acción de las rizobacterias promotoras del crecimiento vegetal. Corpoica cienc tecnol. Agropecu.2011. Available in:http://agris.fao.org/agris- search/search/display.do?f=2012/CO/CO1201.xml;CO2012003937 Accessed
Ceballos-Aguirre N, Vallejo-Cabrera FA, Arango-Arango N. Evaluación del contenido de antioxidantes en introducciones de tomate tipo cereza (Solanum spp). Acta Agron. 2012;61(3):230-238.
Chandler S, Van Hese N, Coutte F, Jacques P, Höfte M, De Vleesschauwer D. Role of cyclic lipopeptides produced by Bacillus subtilis in mounting induced immunity in rice (Oryza sativa L.). Physiol Mol Plant P. 2015;91:20–30. Doi:10.1016/j.pmpp.2015.05.010
Cubillos JR, Castellanos DE, Argüello H. Selección de Microorganismos promotores de crecimiento vegetal (ácido indol acético) a partir de muestras de suelo rizosferico, como primera etapa en el desarrollo de un biofertilizante. Rev Bras Agroecol. 2009;4(2):páginas.
Estrada GA, Divan Baldani VL, de Oliveira DM, Urquiaga S, Baldani JI. Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil. 2013;369:115–129. Doi:10.1007/s11104-012-1550-7
Felici C, Vettori L, Giraldi E, Forino LMC, Toffanin A, Tagliasacchi AM, Nuti M. Single and co-inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicon esculentum: effects on plant growth and rhizosphere microbial community. Appl Soil Ecol. 2008;40(2):260-270. Doi:http://doi.org/10.1016/j.apsoil.2008.05.002
Fernández LA, Zalba P, Gómez MA, Sagardoy MA. Bacterias solubilizadoras de fosfato inorgánico aisladas de suelos de la región sojera. Rev Cienc Suelo Nutr Veg. 2005;23(1):31-37.
Glick B R, Cheng Z, Czarny J, Duan J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol. 2007;119(3):329-339.
Glickmann E, Dessaux Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61(2):793-796.
Gravel V, Antoun H, Tweddell RJ. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem. 2007;39(8):1968-1977.
Gül A, Kidoglu F, Tüzel Y. Effects of nutrition and Bacillus amyloliquefaciens on tomato (Solanum lycopersicum, L.) growing in perlite. Span J Agric Res. 2008;6(3):422-429.
Hernández-Rodríguez A, Ruíz-Beltrán Y, Acebo-Guerrero Y, Miguélez-Sierra Y, Heydrich-Pérez M. Microbial antagonists to manage black pod rot in Theobroma cacao L. Their present status and perspective use in Cuba. Rev Protección Veg. 2014;29(1):11-19. Doi:101159/000207196. 11.
Izzeddin N, Medina L. Efecto del control biológico por antagonistas sobre fitopatógenos en vegetales de consumo humano. Salus. 2011;15(3):8-18.
Jalili F, Khavazi K, Pazira E, Nejati A, Rahmani H A, Sadaghiani H R, Miransari M. Isolation and characterization of ACC deaminase-producing fluorescent pseudomonads, to alleviate salinity stress on canola (Brassica napus L.) growth. J Plant Physiol. 2009;166(6):667-674. Doi:10.1007/s11103-008-9435-0
Kaur G, Reddy MS. Influence of P-solubilizing bacteria on crop yield and soil fertility at multilocational sites. Eur J Soil Biol. 2014;61:35-40. Doi:10.1046/j.1351-0754.2003.0567.x
Kennedy IR, Choudhury AT, Kecskes ML. Non-symbiotic bacterial diazotrophs in crop-farming systems: can their potential for plant growth promotion bebetter exploited?. Soil Biol Biochem. 2004;36(8):1229-1244.
Kloepper JW, Metting Jr FB. Plant growth-promoting rhizobacteria as biological control agents. Soil microbial ecology: applications in agricultural and environmental management.1992:255-274.
Lagunas-Lagunas J, Zavaleta-Mejía E, Osada-Kawasoe S, Aranda-Ocampo S, Luna-Romero I, Vaquera-Huerta H. Bacillus firmus como agente de control biológico de Phytophthora capsici Leo. en jitomate (Lycopersicon esculentum Mill.). Rev Mex Fitop. 2001;19(1):57-65.
Luna Martínez L, Martínez Peniche RA, Hernández Iturriaga M, Arvizu Medrano SM, Pacheco Aguilar JR. Caracterización de rizobacterias aisladas de tomate y su efecto en el crecimiento de tomate y pimiento. Rev Fitotec Mex. 2013;36(1):63 – 69.
Mehta S, Nautiyal CS. An Efficient Method for Qualitative screening of Phosphate-Solubilizing Bacteria. Curr Microbiol. 2001;43:51-56.
Morales FJ, Tamayo PJ, Castaño M, Olaya C, Martínez AK, Velasco AC. Enfermedades virales del tomate (Solanum Lycopersicum L.) en Colombia. Fitopatología Colombiana. 2009;33(1):23-27.
Myresiotis CK, Vryzas Z, Papadopoulou-Mourkidou E. Enhanced root uptake of acibenzolar-S-methyl (ASM) by tomato plants inoculated with selected Bacillus plant growth-promoting rhizobacteria (PGPR). App Soil Ecol. 2014;77:26-33. Doi:http://doi.org/10.1016/j.apsoil.2014.01.005
Reis VM, Baldani VLD, Baldani J I. Isolation, identification and biochemical characterization of Azospirillum spp. and other nitrogen-fixing bacteria. In Handbook for Azospirillum. Switzerland: Springer International Publishing. 2015. p. 3-26.
Reyes I, Alvarez L, El-Ayoubi H, Valery A. Selección y evaluación de rizobacterias promotoras del crecimiento en pimentón y maíz. Bioagro. 2008;20(1):37-48.
Rojas MM, Tejeda B, Larrea JA, Mahillon J, Heydrich M. Aislamiento y caracterización de cepas de Bacillus asociadas al cultivo del arroz (Oryza sativa L.). Rev Bras Agroecol. 2011;6(1):90-99.
Santillana N, Arellano C, Zúñiga D. Capacidad del Rhizobium de promover el crecimiento en plantas de tomate (Lycopersicon esculentum Miller). Ecol Appl. 2005;4(4):1-2. Doi: 10.1111/1365-2664.12845
Terry-Alfonso E, Leyva-Galán A, Hernández-Rodríguez A. Microorganismos benéficos como biofertilizantes eficientes para el cultivo del tomate (Lycopersicon esculentum, Mill). Rev Colomb Biotecnol. 2005;7(2):47.
Tien TM, Gasking MH, Hubbell DH. Plant growth substance produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennistem americanun L.) Appl. Environ Microbiol. 1979;37(4):219-226.
Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI. Microbial producers of plant growth stimulators and their practical use: a review. Appl Biochem Microbiol. 2006;42(2): 117-126. Doi:10.1134/S0003683806020013
Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 2001;52: 487-511.
Widnyana Ketut I, Cokorda Javandira. Activities Pseudomonas spp. and Bacillus sp. to stimulate germination and seedling growth of tomato plants. Agric Agric Sci Procedia. 2016;9:419-423. Doi:10.1016/j.aaspro.2016.02.158
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Amirhossein Bahramisharif, Laura E. Rose. (2019). Efficacy of biological agents and compost on growth and resistance of tomatoes to late blight. Planta, 249(3), p.799. https://doi.org/10.1007/s00425-018-3035-2.
2. Henrique Fonseca Elias de Oliveira, Thiago Dias Silva, Jhon Lennon Bezerra da Silva, Priscila Jane Romano Gonçalves Selaria, Marcos Vinícius da Silva, Marcio Mesquita, Josef Augusto Oberdan Souza Silva, Rhuanito Soranz Ferrarezi. (2025). Enhancing Cherry Tomato Performance Under Water Deficit Through Microbial Inoculation with Bacillus subtilis and Burkholderia seminalis. Horticulturae, 11(10), p.1157. https://doi.org/10.3390/horticulturae11101157.
3. Livia Pappalettere, Susanna Bartolini, Annita Toffanin. (2024). Enhancement of Tomato Seed Germination and Growth Parameters through Seed Priming with Auxin-Producing Plant Growth Promoting Bacteria Strains. Seeds, 3(3), p.479. https://doi.org/10.3390/seeds3030032.
4. Farjana Sultana, M. Motaher Hossain, Ying Ma. (2022). Assessing the potentials of bacterial antagonists for plant growth promotion, nutrient acquisition, and biological control of Southern blight disease in tomato. PLOS ONE, 17(6), p.e0267253. https://doi.org/10.1371/journal.pone.0267253.
5. Aroldo F. L. Machado, Ernandes S. Barbosa, Vera L. D. Baldani, Fernando R. de Souza, Lucas C. Martins, Junior Borella. (2023). Herbicide does not impact diazotrophic bacteria-promoting growth of red rice plants. Weed Control Journal, 22 https://doi.org/10.7824/wcj.2023;22:00800.
6. Gabriel Ruiz-Aymá, Ricardo Romero-Arguelles, Esther E. Rios-Del Toro, Alexa Juarez-Gaspar, Alina Olalla-Kerstupp, Marco Loredo-Tovias, José I. González-Rojas, Licet Villarreal-Treviño, Antonio Guzmán-Velasco, Mayra A. Gomez-Govea. (2026). Postbiotic metabolites present in the supernatants of Lysinibacillus xylanilyticus and Bacillus cereus promote the germination and growth of Hibiscus sabdariffa and Prosopis juliflora. Frontiers in Microbiology, 16 https://doi.org/10.3389/fmicb.2025.1741549.
7. Slimane Mokrani, El-hafid Nabti. (2021). Soil Microbiomes for Sustainable Agriculture. Sustainable Development and Biodiversity. 27, p.31. https://doi.org/10.1007/978-3-030-73507-4_2.
8. Thiago Dias Silva, Rhuanito Soranz Ferrarezi, Priscila Jane Romano Gonçalves Selari, Jhon Lennon Bezerra da Silva, Marcos Vinícius da Silva, Marcio Mesquita, Henrique Fonseca Elias de Oliveira. (2025). Microbial Inoculation Strategies for Optimal Cherry Tomato Production. Physiologia Plantarum, 177(6) https://doi.org/10.1111/ppl.70655.
9. Farheen Nazli, Najm-ul-Seher, Muhammad Yahya Khan, Moazzam Jamil, Sajid Mahmood Nadeem, Maqshoof Ahmad. (2020). Plant Disease Management Strategies for Sustainable Agriculture through Traditional and Modern Approaches. Sustainability in Plant and Crop Protection. 13, p.111. https://doi.org/10.1007/978-3-030-35955-3_6.
10. Leobardo Serrano-Carreón, Sergio Aranda-Ocampo, Karina A. Balderas-Ruíz, Antonio M. Juárez, Edibel Leyva, Mauricio A. Trujillo-Roldán, Norma A. Valdez-Cruz, Enrique Galindo. (2022). A case study of a profitable mid-tech greenhouse for the sustainable production of tomato, using a biofertilizer and a biofungicide. Electronic Journal of Biotechnology, 59, p.13. https://doi.org/10.1016/j.ejbt.2022.06.003.
11. Ivana Danilov, Vanja Vlajkov, Zdravko Šumić, Anita Milić, Aleksandra Tepić Horecki, Tatjana Dujković, Nemanja Živanović, Nataša Simin, Marija Lesjak, Jovana Grahovac. (2024). Valorization of Strawberry Juice Production Wastewater: Possibilities for Polyphenols Recovery and Plant Biostimulant Production. Foods, 13(20), p.3224. https://doi.org/10.3390/foods13203224.
12. U. V. Ogugua, I. P. Ogbuewu, C. A. Mbajiorgu, P. Adriaanse. (2025). Meta-analysis of biofertilizer effects of Bacillus species on tomato yield. Scientific Reports, 15(1) https://doi.org/10.1038/s41598-025-12711-2.
13. Amanul Islam, Shahinur Kabir, Abul Khair. (2019). Characterization and Evaluation of Bacillus siamensis Isolate for its Growth Promoting Potential in Tomato. Agriculture (Pol'nohospodárstvo), 65(2), p.42. https://doi.org/10.2478/agri-2019-0005.
14. Francisco Castillo Reyes, David Castillo Quiroz, Jesús Eduardo Sáenz Ceja, Agustín Rueda Sánchez, J. Trinidad Sáenz Reyes. (2022). Efectos del pretratamiento con Trichoderma y Bacillus en la germinación de semillas de Agave victoriae-reginae T. Moore. Revista Mexicana de Ciencias Forestales, 13(69), p.56. https://doi.org/10.29298/rmcf.v13i69.844.
15. Sarahi Rubio-Tinajero, Eduardo Osorio-Hernández, Benigno Estrada-Drouaillet, José Hugo Tomás Silva-Espinosa, Rafael Delgado-Martínez, César Alejandro Espinoza-Ahumada, Claudio Ríos-Velasco, Jorge Ariel Torres-Castillo. (2022). In vitro biocontrol of Fusarium oxysporum with antagonistic microorganisms and In vivo effect on Solanum lycopersicum L.. Journal of Environmental Science and Health, Part B, 57(8), p.625. https://doi.org/10.1080/03601234.2022.2093590.
16. Sajjad Hyder, Amjad Shahzad Gondal, Anam Sehar, Aimen Razzaq Khan, Nadia Riaz, Zarrin Fatima Rizvi, Rashid Iqbal, Mohamed S. Elshikh, Khaloud M. Alarjani, Muhammed Habib ur Rahman, Muhammad Rizwan. (2024). Use of ginger extract and bacterial inoculants for the suppression of Alternaria solani causing early blight disease in Tomato. BMC Plant Biology, 24(1) https://doi.org/10.1186/s12870-024-04789-z.
17. Pallavi Bhardwaj, Abhishek Chauhan, Anuj Ranjan, Saglara S. Mandzhieva, Tatiana Minkina, Usha Mina, Vishnu D. Rajput, Ashutosh Tripathi. (2023). Assessing Growth-Promoting Activity of Bacteria Isolated from Municipal Waste Compost on Solanum lycopersicum L.. Horticulturae, 9(2), p.214. https://doi.org/10.3390/horticulturae9020214.
18. Helen Kalorizou, Dimitra Stefanopoulou, Paschalis Giannoulis, Stefanos Leontopoulos. (2025). Effect of Selective Substrates on Germination of Pomegranate (Punica granatum) and Trifoliate Orange (Poncirus trifoliata) Seeds with and Without the Presence of Plant-Beneficial Microorganisms. Seeds, 4(1), p.12. https://doi.org/10.3390/seeds4010012.
19. Bastien Cochard, Basile Giroud, Julien Crovadore, Romain Chablais, Lucas Arminjon, François Lefort. (2022). Endophytic PGPR from Tomato Roots: Isolation, In Vitro Characterization and In Vivo Evaluation of Treated Tomatoes (Solanum lycopersicum L.). Microorganisms, 10(4), p.765. https://doi.org/10.3390/microorganisms10040765.
20. René Díaz‐Herrera, Victor Navarro‐Macias, Olga B. Alvarez‐Pérez, Janeth Ventura‐Sobrevilla, Anna Ilina, J. A. Ascacio‐Valdés, Cristóbal N. Aguilar, Mónica L. Chávez‐González, Miguel A. Medina‐Morales, Yadira Karina. Reyes Acosta, Roberto Arredondo‐Valdés. (2025). Harnessing Microorganisms as Sustainable Green Technology for Enhanced Seed Germination and Crop Growth. Environmental Quality Management, 34(4) https://doi.org/10.1002/tqem.70103.
21. Marino Costa-Santos, Nuno Mariz-Ponte, Maria Dias, Luísa Moura, Guilhermina Marques, Conceição Santos. (2021). Effect of Bacillus spp. and Brevibacillus sp. on the Photosynthesis and Redox Status of Solanum lycopersicum. Horticulturae, 7(2), p.24. https://doi.org/10.3390/horticulturae7020024.
22. Lavinia - Diana – Nicoleta Buturugă - Barbu, Maria-Cristina Lumînare , Daniel Nicolae Cojanu , Sorina Dinu , Oana - Alina Boiu - Sicuia , Narcisa Băbeanu . (2023). THE INFLUENCE OF SOME BACTERIAL STRAINS ON THE GERMINATION OF GREEN SORREL SEEDS (RUMEX ACETOSA L.). Romanian Journal for Plant Protection, 16, p.113. https://doi.org/10.54574/RJPP.16.14.
23. Diana A. Al-Quwaie, Aminah Allohibi. (2025). Isolation, identification, and molecular characterization of plant growth-promoting rhizobacteria as an environmentally friendly fungicide against leaf blight disease in Alternaria terricola-infected tomato plants. Journal of Plant Pathology, 107(3), p.1497. https://doi.org/10.1007/s42161-025-01933-y.
24. Vanja Vlajkov, Ivana Pajčin, Snežana Vučetić, Stefan Anđelić, Marta Loc, Mila Grahovac, Jovana Grahovac. (2023). Bacillus-Loaded Biochar as Soil Amendment for Improved Germination of Maize Seeds. Plants, 12(5), p.1024. https://doi.org/10.3390/plants12051024.
25. Soufiane Alami, Kaoutar Kaddouri, Mouad Lamrabet, Zohra Chaddad, Omar Bouhnik, Meryeme Bennis, Hanaa Abdelmoumen, Mustapha Missbah El Idrissi. (2024). Soil Bacteria. , p.451. https://doi.org/10.1007/978-981-97-3473-3_16.
26. Dimitra Douka, Tasos-Nektarios Spantidos, Polina C. Tsalgatidou, Panagiotis Katinakis, Anastasia Venieraki. (2024). Whole-Genome Profiling of Endophytic Strain B.L.Ns.14 from Nigella sativa Reveals Potential for Agricultural Bioenhancement. Microorganisms, 12(12), p.2604. https://doi.org/10.3390/microorganisms12122604.
27. Anna Krzepiłko, Katarzyna Matyszczuk, Małgorzata Ostrowska, Agata Święciło. (2025). Influence of simultaneous treatment of seeds with ZnONPs and Bacillus subtilis on the biological quality parameters of red cabbage seedlings. Acta Scientiarum Polonorum Hortorum Cultus, 24(1), p.33. https://doi.org/10.24326/asphc.2025.5388.
28. V. S. Maslennikova, V. P. Tsvetkova, A. F. Petrov, A. V. Pastukhova. (2021). Influence of the bacillus genus bacteria on the growth and productivity of tomatoes of spok variety. Bulletin of NSAU (Novosibirsk State Agrarian University), (1), p.56. https://doi.org/10.31677/2072-6724-2021-58-1-56-63.
29. Thanawan Gateta, Wasan Seemakram, Thanapat Suebrasri, Saranya Chantawong, Chaiya Klinsukon, Jindarat Ekprasert, Sophon Boonlue. (2025). The Dual Role of Bacillus sp. KKU-RE-018 Isolated from Medicinal Plants in Controlling Anthracnose Disease and Enhancing the Growth of Chili Plants. Plants, 14(19), p.3010. https://doi.org/10.3390/plants14193010.
30. Gordana Tamindžić, Dragana Miljaković, Maja Ignjatov, Jegor Miladinović, Vuk Đorđević, Dragana Milošević, Dušica Jovičić, Slobodan Vlajić, Dragana Budakov, Mila Grahovac. (2024). Impact of Simultaneous Nutrient Priming and Biopriming on Soybean Seed Quality and Health. Plants, 13(18), p.2557. https://doi.org/10.3390/plants13182557.
31. Ebtesam A. Gashash, Nahid A. Osman, Abdulaziz A. Alsahli, Heba M. Hewait, Ashmawi E. Ashmawi, Khalid S. Alshallash, Ahmed M. El-Taher, Enas S. Azab, Hany S. Abd El-Raouf, Mohamed F. M. Ibrahim. (2022). Effects of Plant-Growth-Promoting Rhizobacteria (PGPR) and Cyanobacteria on Botanical Characteristics of Tomato (Solanum lycopersicon L.) Plants. Plants, 11(20), p.2732. https://doi.org/10.3390/plants11202732.
32. Tasos-Nektarios Spantidos, Dimitra Douka, Panagiotis Katinakis, Anastasia Venieraki. (2025). Genomic Insights into Plant Growth Promotion and Biocontrol of Bacillus velezensis Amfr20, an Olive Tree Endophyte. Horticulturae, 11(4), p.384. https://doi.org/10.3390/horticulturae11040384.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).