Publicado
Actividad biocontroladora in vitro de macrohongos contra diferentes hongos fitopatógenos
In Vitro Biocontrol Activity of Macrofungi Against Different Plant Pathogenic Fungi
ATIVIDADE BIOCONTROLADORA IN VITRO DE MACROHONGOS CONTRA DIFERENTES FUNGOS FITOPATOGENOS
DOI:
https://doi.org/10.15446/abc.v25n2.75303Palabras clave:
Antagonismo, cinética del crecimiento, cultivo dual, parasitismo, metabolitos secundarios (es)Antagonism, growth kinetics, dual culture, parasitism, secondary metabolites (en)
Antagonismo, cinética de crescimento, cultura dual, parasitismo, metabólitos secundários (pt)
Descargas
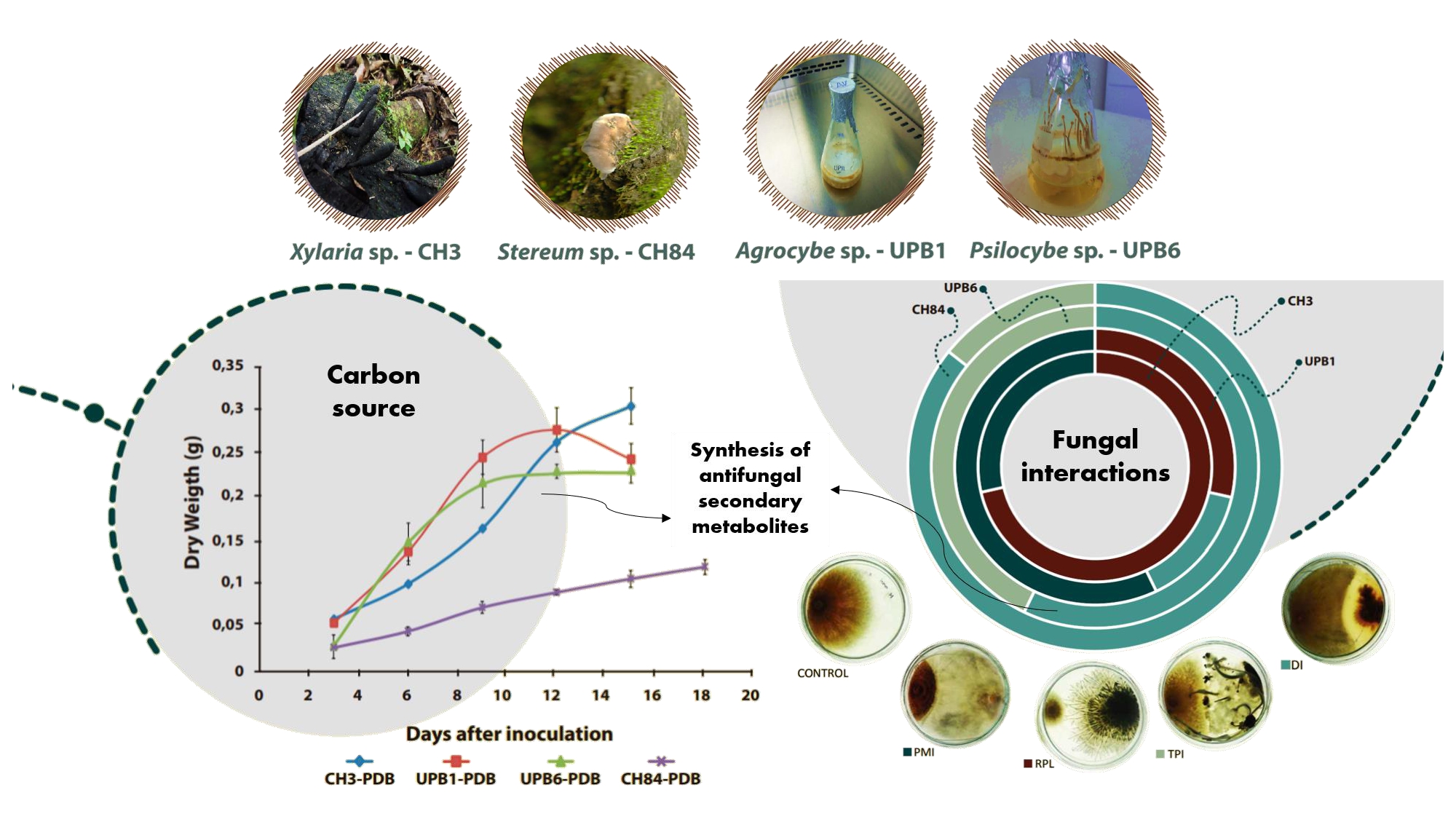
Las enfermedades causadas por hongos en las plantas son una gran preocupación en la producción agrícola. Los macromicetos son una fuente potencial de compuestos antifúngicos que podrían usarse para controlar estas enfermedades. El objetivo de este estudio fue evaluar la actividad biocontroladora in vitro de cuatro macromicetos de los géneros Xylaria, Agrocybe, Psilocybe y Stereum sobre diferentes hongos fitopatógenos. Para ello, se determinó la curva de crecimiento de los macrohongos en dos medios: papa dextrosa (PDA) y salvado de trigo (ST) y se caracterizaron las interacciones y la inhibición in vitro de hongos fitopatógenos. Se realizó la extracción y caracterización de metabolitos secundarios de la biomasa, el medio extracelular y del homogeneizado del micelio y caldo en los hongos con mayor porcentaje de inhibición. Finalmente, se evaluó la actividad antifúngica in vitro de estos extractos. Las curvas de crecimiento cambiaron con la fuente de carbono, tres de cuatro macrohongos mostraron una mayor acumulación de biomasa en PDA que en ST. Las interacciones de Xylaria se clasificaron principalmente como reemplazo, obteniendo el mayor nivel de antagonismo en PDADos de los tres extractos evaluados mostraron actividad antifúngica contra los tres patógenos aislados en concentraciones de 18 μg/ml para extractos metanólicos de biomasa y 2,5 % para el filtrado de homogeneizado con inhibiciones de 10 % a 80 %. La caracterización de los metabolitos de Xylaria mostró como posibles compuestos responsables de la actividad a los ácidos grasos. Este trabajo mostró el potencial de estos hongos para el control de enfermedades fúngicas.
Fungal diseases in plants are a major concern in agricultural production. Macrofungi are a potential source of antifungal compounds that could be used to control these diseases. The aim of this study was to evaluate the in vitro biocontrol activity of four macrofungi of the genus Xylaria, Agrocybe, Psilocybe and Stereum on different plant pathogenic fungi. For this purpose, the growth curve of macrofungi was determined on two carbon sources: potato dextrose and wheat bran, interactions and the in vitro inhibition on phytopathogenic fungi were also characterized. Subsequently, extraction and characterization of secondary metabolites of the intracellular, extracellular and filtrate of homogenized of mycelia and broth culture were performed only on the fungi that showed greater inhibition percentage. Finally, the in vitro antifungal activity of this extracts was evaluated. Growth curves changes with the carbon source, three of four isolates show greater accumulation of biomass in potato dextrose than wheat brand reaching the stationary growth phase longer. Xylaria interactions were classified mostly as replacement, obtaining the highest level of antagonism on potato dextrose. Two of the three extracts assessed showed antifungal activity in all three pathogens isolated at concentrations of 18 µg/ml for biomass methanol extracts and 2.5 % for filtrate of homogenized with inhibitions from 10 % to 80 %. Characterization of the metabolites of Xylariashowed as possible compounds responsible for the activity of the fatty acids. This work showed the potential of these fungi for the control of fungal diseases.
Referencias
Abdel-Motaal FF, Nassar MSM, El-Zayat S.A., El-Sayed M.A., Shin-Ichi I. Antifungal activity of endophytic fungi isolated from Egyptian henbane (Hyoscyamus muticus L.). Pakistan J Bot. 2010;42(4):2883–94.
Aqueveque P, Céspedes CL, Alarcón J, Schmeda-Hirschmann G, Cañumir J.A., Becerra J, et al. Antifungal activities of extracts produced by liquid fermentations of Chilean Stereum species against Botrytis cinerea (grey mould agent). Crop Prot. 2016;89:95–100. Doi:10.1016/j.cropro.2016.07.014
Aqueveque P, Céspedes CL, Becerra J, Aranda M, Sterner O. Antifungal activities of secondary metabolites isolated from liquid fermentations of Stereum hirsutum (Sh134-11) against Botrytis cinerea (grey mould agent). Food Chem Toxicol. 2017;109:1048–54. Doi:10.1016/j.fct.2017.05.036
Artemisia L, Ali M, Haider B. Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of. Ind Crop Prod [Internet]. Elsevier B.V.; 2013;49:400–6. Recuperado a partir de: http://dx.doi.org/10.1016/j.indcrop.2013.05.033
Aziza M, Amrane A. Diauxic growth of Geotrichum candidum AND Penicillium camembertii on amino acids and glucose. brazilian Journal of Chemical Engineering;2012; 29(02):203–10.
Badalyan S, Innocenti G, Grogory N. Interactions between xylotrophic mushrooms and mycoparasitic fungi in dual-culture experiments. Phytopathol. Mediterr. 2004;41:44–8.
Boddy L. Interspecific combative interactions between wood-decaying basidiomycetes; FEMS Microbiology Ecology, 2000;31:185–94.
Castaño, J. Cruz, C. Torres, E. Optimization of the production, purification and characterization of a laccase from the native fungus Xylaria sp. Biocatalysis and Agricultural Biotechnology. 2015;4(4):710-716.
Castiblanco C, Etter A, Ramirez A. Land Use Policy Impacts of oil palm expansion in Colombia : What do socioeconomic indicators show? Land use policy [Internet]. Elsevier Ltd; 2015;44:31–43. Recuperado a partir de: http://dx.doi.org/10.1016/j.landusepol.2014.10.007
Cateni F, Doljak B, Zacchigna M, Anderluh M, Piltaver A, Scialino G, et al. New biologically active epidioxysterols from Stereum hirsutum. Bioorganic Med Chem Lett. 2007;17:6330–4. Doi:10.1016/j.bmcl.2007.08.072
Crespo C, Aime M, Torres E. Exploration for fungi with novel lignocellulolytic enzymes in the Hign Andes Colombian Forest. En: Muñoz F, editor. Memorias del VIII Congreso Latinoam Micol. 4-7 Nov. 2014. Medellin-Colombia; Sayonara Plata Arboleda.
Ghorbanpour M, Omidvari M, Abbaszadeh-Dahaji P, Omidvar R, Kariman K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol Control. 2017(August). Doi:10.1016/j.biocontrol.2017.11.006
He P, He X, Zhang C. Interactions between Psilocybe fasciata and its companion fungus Acremonium strictum. 2006:387–95. Doi:10.1007/s11284-005-0123-0
Hoyos L. 1. Conceptos generales en manejo biológico de fitopatógenos. En: Hoyos L, editor. Enfermedades plantas Control biológico. 1ra ed. Bogotá D.C.: Universidad Nacional de Colombia; 2012; p.1–10.
Hwang K, Uk H, Charusanti P, Palsson BØ, Yup S. Systems biology and biotechnology of Streptomyces species for the production of secondary metabolites. Biotechnol Adv [Internet]. Elsevier Inc.; 2014;32(2):255–68. Recuperado a partir de: http://dx.doi.org/10.1016/j.biotechadv.2013.10.008
Keay SM, Brown AE. Interactions between Psilocybe semilanceata and fungi of its habitat. Mycol Res [Internet]. British Mycological Society; 1989;93(4):554–6. Recuperado a partir de: http://dx.doi.org/10.1016/S0953-7562(89)80054-1
Kim HO, Lim JM, Joo JH, Kim SW, Hwang HJ, Choi JW, et al. Optimization of submerged culture condition for the production of mycelial biomass and exopolysaccharides by Agrocybe cylindracea. Bioresour Technol. 2005;96:1175–82. Doi:10.1016/j.biortech.2004.09.021
Larena I, Torres R, Cal A De, Liñán M, Melgarejo P, Domenichini P, et al. Biological control of postharvest brown rot ( Monilinia spp .) of peaches by Weld applications of Epicoccum nigrum. 2005;32:305–10. Doi:10.1016/j.biocontrol.2004.10.010
Li JF, Qin YK, Tian MQ, Zhang KQ, Li GH. Two new sesquiterpenes from the fungus Stereum sp. NN048997. Phytochem Lett [Internet]. Phytochemical Society of Europe; 2014;10(2):32–4. Recuperado a partir de: http://dx.doi.org/10.1016/j.phytol.2014.07.004
Liu X, Dong M, Zhou XC, Jiang M, Lv X, Jianzhong. Antimicrobial activity of an endophytic Xylaria sp . YX-28 and identification of its antimicrobial compound. 2008:241–7. Doi:10.1007/s00253-007-1305-1
Merck E. Dyeing Reagents for Thin-Layer and Paper Chromatography. Darmstad Germany: Merck; 1980.
Molla AH, Fakhru’l-Razi A, Abd-Aziz S, Hanafi MM, Alam MZ. In-vitro compatibility evaluation of fungal mixed culture for bioconversion of domestic wastewater sludge. World J Microbiol Biotechnol. 2001;17:849–56. Doi:10.1023/A:1013844306960
Morath SU, Hung R, Bennett JW. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol Rev [Internet]. Elsevier Ltd; 2012;26(2–3):73–83. Recuperado a partir de: http://dx.doi.org/10.1016/j.fbr.2012.07.001
Moya A. LA, Torres R. E. Hydrolysis of cellulose and oil palm empty fruit bunches by using consortia of fungi isolated from the soil of Colombian high andean forest. Agron Colomb. 2012;30(3):411–8.
Ngai PHK, Zhao Z, Ng TB. Agrocybin, an antifungal peptide from the edible mushroom Agrocybe cylindracea. Peptides. 2005;26:191–6. Doi:10.1016/j.peptides.2004.09.011
Orlandini N, Leivas M, Sgarioni V, Rangel M, Pereira F, Garcia B, et al. Evaluation of the reproductive toxicity of fungicide propiconazole in male rats. Toxicology [Internet]. Elsevier Ireland Ltd; 2015;335:55–61. Recuperado a partir de: http://dx.doi.org/10.1016/j.tox.2015.06.011
Pal KK, Scholar V, Gardener BM. Biological Control of Plant Pathogens. 2006:1–25. Doi:10.1094/PHI-A-2006-1117-02.Biological
Pandey VK. Anti plant pathogenic properties of higher fungi. Chaudhary Sarwan Kumar Himachal Pradesh Krishi Vishvavidyalaya; 2012.
Paterson RRM. Ganoderma - A therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001. Doi:10.1016/j.phytochem.2006.07.004
Peiris D, Dunn ÆWB, Brown ÆM, Kell ÆDB, Roy I, Hedger ÆJN. Metabolite profiles of interacting mycelial fronts differ for pairings of the wood decay basidiomycete fungus , Stereum hirsutum with its competitors Coprinus micaceus and Coprinus disseminatus. 2008:52–62. Doi:10.1007/s11306-007-0100-4
Pongcharoen W, Rukachaisirikul V, Phongpaichit S, Kühn T, Pelzing M, Sakayaroj J, et al. Metabolites from the endophytic fungus Xylaria sp. PSU-D14. Phytochemistry. 2008;69:1900–2. Doi:10.1016/j.phytochem.2008.04.003
Ramadan U, Grkovic T, Balasubramanian S, Salah M, Quinn RJ, Hentschel U. Elicitation of secondary metabolism in actinomycetes. Biotechnol Adv [Internet]. Elsevier B.V.; 2015;33(6):798–811. Recuperado a partir de: http://dx.doi.org/10.1016/j.biotechadv.2015.06.003
Ramesh V, Karunakaran C, Rajendran A. Optimization of submerged culture conditions for mycelial biomass production with enhanced antibacterial activity of the medicinal macro fungus Xylaria sp . Strain R006 against drug resistant bacterial pathogens. 2014;4(February):88–98. Doi:10.5943/ream/4/1/7
Regueiro J, Olguín N, Simal-gándara J, Suñol C. Toxicity evaluation of new agricultural fungicides in primary cultured cortical neurons. Environ Res [Internet]. Elsevier; 2015;140:37–44. Recuperado a partir de: http://dx.doi.org/10.1016/j.envres.2015.03.013
Richardson SN, Walker AK, Nsiama TK, McFarlane J, Sumarah MW, Ibrahim A, et al. Griseofulvin-producing Xylaria endophytes of Pinus strobus and Vaccinium angustifolium: evidence for a conifer-understory species endophyte ecology. Fungal Ecol [Internet]. Elsevier Ltd; 2014;11(2011):107–13. Recuperado a partir de: http://www.sciencedirect.com/science/article/pii/S175450481400066X
Ruiz B, Chávez A, Forero A, García-Huante Y, Romero A, Sánchez M, et al. Production of microbial secondary metabolites: regulation by the carbon source. Crit Rev Microbiol. 2010;36(2):146–67. Doi:10.3109/10408410903489576
Shaikh AS, Tang YJ, Gin J, Benke PI, Mukhopadhyay A, Keasling JD. Study of Stationary Phase Metabolism Via Isotopomer Analysis of Amino Acids from an Isolated Protein. 2009. Doi:10.1002/btpr.325
Silva GH, De Oliveira CM, Teles HL, Pauletti PM, Castro-Gamboa I, Silva DHS, et al. Sesquiterpenes from Xylaria sp., an endophytic fungus associated with Piper aduncum (Piperaceae). Phytochem Lett. 2010;3:164–7. Doi:10.1016/j.phytol.2010.07.001
Skidmore AM, Dickinson CH. Interactions between germinating spores of Septoria nodorum and phyloplane fungi. Trans Brit Mycol Soci. 1976;66:45–56.
Tarman K, Lindequist U, Wende K, Porzel A, Arnold N, Wessjohann LA. Isolation of a New Natural Product and Cytotoxic and Antimicrobial Activities of Extracts from Fungi of Indonesian. 2011:294–306. Doi:10.3390/md9030294
Valle U, Región DELA, Valle DEL, Cauca DEL, Valle U, R ACB, et al. Macrohongos presentes en el bosque seco tropical de la región del valle del cauca, colombia. 2010:45–54.
Wang X, Huang W, Du J, Li C. Chemical constituents from the fruiting bodies of Xylaria euglossa Fr . and its chemotaxonomic study. Biochem Syst Ecol [Internet]. Elsevier Ltd; 2014;54:157–9. Recuperado a partir de: http://dx.doi.org/10.1016/j.bse.2013.12.018
Wu SH, He J, Li XN, Huang R, Song F, Chen YW, et al. Guaiane sesquiterpenes and isopimarane diterpenes from an endophytic fungus Xylaria sp. Phytochemistry [Internet]. Elsevier Ltd; 2014;105:197–204. Recuperado a partir de: http://dx.doi.org/10.1016/j.phytochem.2014.04.016
Yao J-N, Chen L, Chen H-P, Zhao Z-Z, Zhang S-B, Huang Y, et al. Miscellaneous lanostane triterpenoids with cytotoxicities from fruiting bodies of the basidiomycete Stereum sp. Fitoterapia. 2017(November). Doi:10.1016/j.fitote.2017.11.020
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Mónica de Jesús Narváez-Montaño, Ma. Remedios Mendoza-López, Gabriela Sánchez-Viveros, Juan José Almaraz-Suarez, Rosalba Argumedo-Delira. (2023). Actividad inhibitoria de extractos alcohólicos de hongos comestibles contra Rhizoctonia solani. Revista Mexicana de Ciencias Agrícolas, 14(4), p.615. https://doi.org/10.29312/remexca.v14i4.3200.
2. Jéssica Rembinski, Silvino I. Moreira, Jorge T. De Souza, Alan C.A. Souza, Adriano F. Dorigan, Eduardo Alves, Breno C.M. Juliatti, Fernando C. Julliati. (2022). Trichoderma - Technology and Uses. https://doi.org/10.5772/intechopen.102426.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2020 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).



















