Publicado
APORTES Y DIFICULTADES DE LA METAGENÓMICA DE SUELOS Y SU IMPACTO EN LA AGRICULTURA.
Contributions and difficulties of soil metagenomics and its impact on agriculture
DOI:
https://doi.org/10.15446/abc.v26n3.85760Palabras clave:
Metagenómica, Suelo, Agricultura, Inoculantes, Bacterias Promotoras del crecimiento PGPR´s. (es)Metagenomics, Soil, Agriculture, Inoculants, PGPR´s growth promoting bacteria. (en)
Metagenômica, Solo, Agricultura, Inoculantes, Bactérias Promotoras de Crescimento PGPR´s. (pt)
Descargas
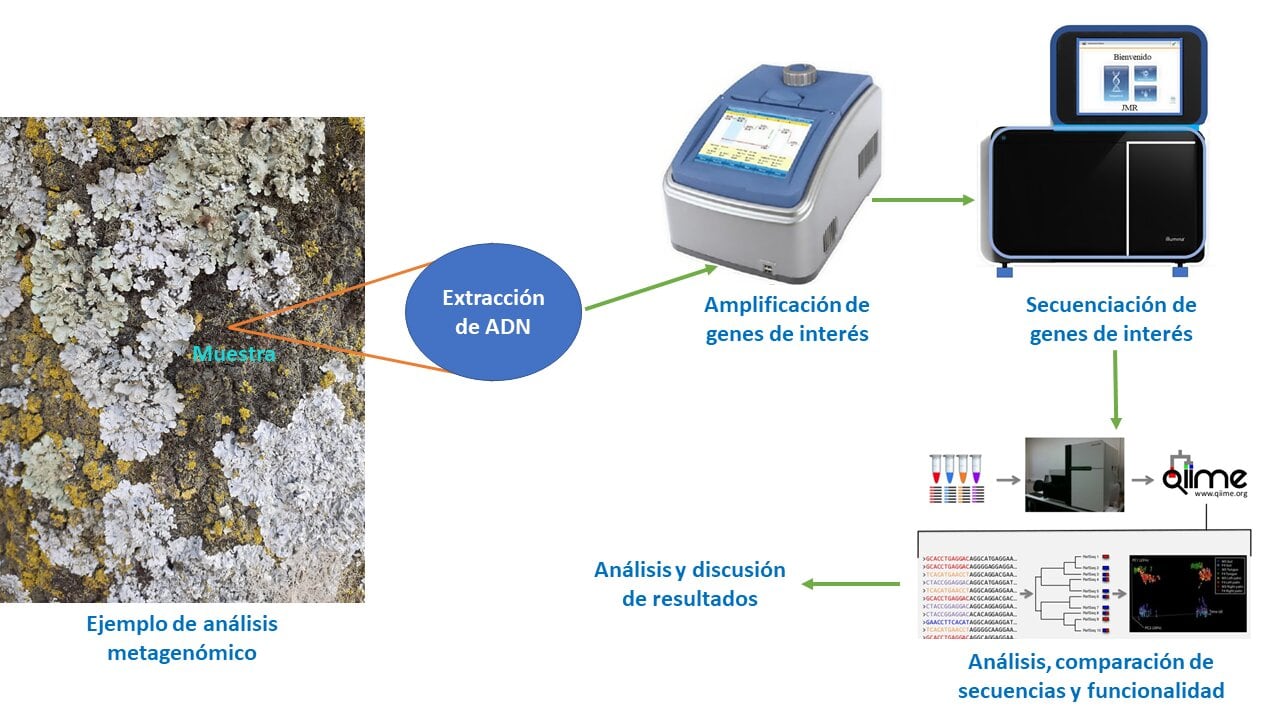
Los microorganismos son de gran interés porque colonizan todo tipo de ambiente, sin embargo, uno de los problemas al que nos enfrentamos para conocer su diversidad biológica es que no todos los microorganismos son cultivables. El desarrollo de nuevas tecnologías como la generación de vectores de clonación aunado al desarrollo de técnicas de secuenciación de alto rendimiento ha favorecido el surgimiento de una nueva herramienta llamada metagenómica, la cual nos permite estudiar genomas de comunidades enteras de microorganismos. Debido a que ningún ambiente es idéntico a otro, es importante mencionar que dependiendo del tipo de muestra a analizar será el tipo de reto al cual nos enfrentaremos al trabajar con metagenómica, en el caso específico del suelo existen diversas variantes como la contaminación del suelo con metales pesados o diversos compuestos químicos que podrían limitar los estudios. Sin embargo, pese a las limitaciones que el mismo ambiente presenta, la metagenómica ha permitido tanto el descubrimiento de nuevos genes como la caracterización de las comunidades microbianas que influyen positivamente en el desarrollo de plantas, lo cual en un futuro podría generar un gran impacto en la agricultura. En este artículo se realizó una revisión de diversas investigaciones que han empleado metagenómica, reportadas en las bases de datos de PudMed y Google Schoolar, con el objetivo de examinar los beneficios y limitaciones de las diversas metodologías empleadas en el tratamiento del ADN metagenómico de suelo y el impacto de la metagenómica en la agricultura.
Microorganisms are of great interest because they colonize all types of environment, however, one of the problems we face in knowing biological diversity is that not all microorganisms are cultivable. The development of new technologies such as the generation of cloning vectors coupled with the development of high performance sequencing techniques, have favored the emergence of a new tool in science called metagenomics, which allows us to study genomes of entire communities. Since all environments are different, the type of challenge that we will face when working with metagenomics is going to change depending of the type of sample, in the specific case of soils, there are several variables, such as soil contamination with heavy metals or chemical compounds that could limit metagenomic studies. However, despite the limitations that the environment presents, with the help of metagenomics, both gene discovery and the characterization of microbial communities that positively influence plant development have been achieved, which could generate a greater impact on agriculture in the future. In this article a review of several investigations that have used metagenomics, reported in the PudMed and Google Schoolar databases was carried out, with the aim of examining the benefits and limitations of the various methodologies used in the treatment of metagenomic DNA from soil and the impact of metagenomics in agriculture.
Referencias
Abreu, N.A. and Taga, M.E. (2016). Decoding molecular interactions in microbial communities. FEMS Microbiol Rev, 40(5), 648-663. https://doi.org/10.1093/femsre/fuw019
Al-Amoudi, S., Razali, R., Essack, M., Amini, MS., Bougouffa, S., Archer J.A.C, Lafi F. F. and Bajic, (2016). Metagenomics as a preliminary screen for antimicrobial bioprospecting. Gene, 594(2), 248-258. https://doi.org/10.1016/j.gene.2016.09.021
Ambardar, S., Singh, H.R., Gowda, M. and Vakhlu, J. (2016). Comparative Metagenomics Reveal Phylum Level Temporal and Spatial Changes in Mycobiome of Belowground Parts of Crocus sativus. PloS One, 11(9):e0163300. https://doi.org/10.1371/journal.pone.0163300
Apolinar-Hernández, M.M., Peña-Ramírez, Y.J., Pérez-Rueda, E., Canto-Canché, B.B., De Los Santos-Briones, C. and O’Connor-Sánchez, A. (2016). Identification and in silico characterization of two novel genes encoding peptidases S8 found by functional screening in a metagenomic library of Yucatán underground water. Gene, 593(1), 154-161. https://doi.org/10.1016/j.gene.2016.08.009
Baez-Rogelio, A., Morales-García, Y.E., Quintero-Hernández, V., Muñoz-Rojas, J. (2017). Next generation of microbial inoculants for agriculture and bioremediation. Microb Biotechnol, 10(1), 19-21. https://doi.org/10.1111/1751-7915.12448
Berbegal, C., Borruso, L., Fragasso, M., Tufariello, M., Russo, P., Brusetti, L., Spano, G. and Capozzi, V. (2019). A Metagenomic-Based Approach for the Characterization of Bacterial Diversity Associated with Spontaneous Malolactic Fermentations in Wine. Int J Mol Sci, 20(16), 3980. https://doi.org/10.3390/ijms20163980
Bouhajja, E., Agathos, S.N. and George, I.F. (2016). Metagenomics: Probing pollutant fate in natural and engineered ecosystems. Biotechnol Adv, 34(8), 1413-1426. https://doi.org/10.1016/j.biotechadv.2016.10.006
Bruner, E.A., Okubara, P.A., Abi-Ghanem, R., Brown, D.J. and Reardon, C.L. (2015). Use of pressure cycling technology for cell lysis and recovery of bacterial and fungal communities from soil. BioTechniques, 58(4), 171-180. https://doi.org/10.2144/000114273
Bryan, N.S. and Ivy, J.L. (2015). Inorganic nitrite and nitrate: evidence to support consideration as dietary nutrients. Nutr Res, 35(8), 643-654. https://doi.org/10.1016/j.nutres.2015.06.001
Buckley, K.M. and Ettensohn, C.A. (2019). Techniques for analyzing gene expression using BAC-based reporter constructs. Methods Cell Biol, 151, 197-218. https://doi.org/10.1016/bs.mcb.2019.01.004
de Castro, A.P., Sartori da Silva, M.R.S., Quirino, B.F., da Cunha Bustamante, M.M. and Krüger, R.H. (2016). Microbial Diversity in Cerrado Biome (Neotropical Savanna) Soils. PloS One, 11(2):e0148785. https://doi.org/10.1371/journal.pone.0148785
Chekanov, K., Kublanovskaya, A. and Lobakova, E. (2019). Eukaryotic Sequences in the 16SrRNA Metagenomic Dataset of Algal-bacterial Consortia of the White Sea Coastal Zone. J Eukaryot Microbiol, 66(5), 853-856. https://doi.org/10.1111/jeu.12722
Dabdoub, S.M., Ganesan, S.M. and Kumar, P.S. (2016). Comparative metagenomics reveals taxonomically idiosyncratic yet functionally congruent communities in periodontitis. Sci Rep, 6, 38993. https://doi.org/10.1038/srep38993
Dai, L., Zhang, G., Yu, Z., Ding, H., Xu, Y. and Zhang, Z. (2019). Effect of Drought Stress and Developmental Stages on Microbial Community Structure and Diversity in Peanut Rhizosphere Soil. Int J Mol Sci, 20(9), 2265. https://doi.org/10.3390/ijms20092265
Davis, K.F., Chhatre, A., Rao, N.D., Singh, D., Ghosh-Jerath, S., Mridul, A. Poblete-Cazenave, M., Pradhan, N. and DeFries, R. (2019). Assessing the sustainability of post-Green Revolution cereals in India. Proc Natl Acad Sci U S A, 116(50), 25034-25041. https://doi.org/10.1073/pnas.1910935116
Diaz-Torres, M.L., McNab, R., Spratt, D.A., Villedieu, A., Hunt, N., Wilson, M. and Mullany, P. (2003). Novel tetracycline resistance determinant from the oral metagenome. Antimicrob Agents Chemother, 47(4), 1430-1432. https://doi.org/10.1128/AAC.47.4.1430-1432.2003
Duan, C.-J., Xian, L., Zhao, G.-C., Feng, Y., Pang, H., Bai, X.-L., Tang, J.-L., Ma, Q.-S. and Feng, J.-X. (2009). Isolation and partial characterization of novel genes encoding acidic cellulases from metagenomes of buffalo rumens. J Appl Microbiol, 107(1), 245-256. https://doi.org/10.1111/j.1365-2672.2009.04202.x
Elend, C., Schmeisser, C., Leggewie, C., Babiak, P., Carballeira, J.D., Steele, H.L., Reymond, J.-L., Jaeger, K.-E. and Streit, W.R. (2006). Isolation and biochemical characterization of two novel metagenome-derived esterases. Appl Environ Microbiol, 72(5), 3637-3645. https://doi.org/10.1128/AEM.72.5.3637-3645.2006
Ettenauer, J.D., Piñar, G., Lopandic, K., Spangl, B., Ellersdorfer, G., Voitl, C. and Sterflinger, k. (2012). Microbes on building materials--evaluation of DNA extraction protocols as common basis for molecular analysis. Sci Total Environ, 439, 44-53. https://doi.org/10.1016/j.scitotenv.2012.09.005
Eyice, Ö., Namura, M., Chen, Y., Mead, A., Samavedam, S. and Schäfer, H.(2015). SIP metagenomics identifies uncultivated Methylophilaceae as dimethylsulphide degrading bacteria in soil and lake sediment. ISME J, 9(11),2 336-2348. https://doi.org/10.1038/ismej.2015.37
Faner, R., Sibila, O., Agustí, A., Bernasconi, E., Chalmers, J.D., Huffnagle, G.B., Manichanh, C., Molyneaux, P.L., Paredes, R., Brocal, V.C., Ponomarenco, J., Sethi, S., Dorca, J. and Monsó, E. (2017). The microbiome in respiratory medicine: current challenges and future perspectives. Eur Respir J, 49(4),1602086. https://doi.org/10.1183/13993003.02086-2016
Fonseca, J.P., Hoffmann, L., Cabral, B.C.A., Dias, V.H.G., Miranda, M.R., de Azevedo Martins, A.C., Boschiero, C., Rodrigues Bastos, W. and Silva, R. (2018). Contrasting the microbiomes from forest rhizosphere and deeper bulk soil from an Amazon rainforest reserve. Gene, 642, 389-397. https://doi.org/10.1016/j.gene.2017.11.039
Gillespie, D.E., Brady, S.F., Bettermann, A.D., Cianciotto, N.P., Liles, M.R., Rondon, M.R., Clardy, J., Goodman, R.M. and Handel, J. (2002) Isolation of antibiotics turbomycin a and B from a metagenomic library of soil microbial DNA. Appl Environ Microbiol, 68(9), 4301-4306. https://doi.org/10.1128/AEM.68.9.4301-4306.2002
Gobbi, A., Santini, R.G., Filippi, E., Ellegaard-Jensen, L., Jacobsen, C.S. and Hansen, L.H. (2019). Quantitative and qualitative evaluation of the impact of the G2 enhancer, bead sizes and lysing tubes on the bacterial community composition during DNA extraction from recalcitrant soil core samples based on community sequencing and qPCR. PloS One, 14(4):e0200979. https://doi.org/10.1371/journal.pone.0200979
Gudeta, D.D., Bortolaia, V., Pollini, S., Docquier, J.-D., Rossolini, G.M., Amos, G.C.A., Wellington, E.M.H. and Guardabassi, L. (2016). Expanding the Repertoire of Carbapenem-Hydrolyzing Metallo-ß-Lactamases by Functional Metagenomic Analysis of Soil Microbiota. Front Microbiol, 7:1985. https://doi.org/10.3389/fmicb.2016.01985
He, B., Wang, X., Yang, C., Zhu, J., Jin, Y. and Fu, Z. (2020). The regulation of autophagy in the pesticide-induced toxicity: Angel or demon?. Chemosphere, 242:125138. https://doi.org/10.1016/j.chemosphere.2019.125138
Hemmat-Jou, M.H., Safari-Sinegani, A.A., Mirzaie-Asl, A. and Tahmourespour, A.(2018). Analysis of microbial communities in heavy metals-contaminated soils using the metagenomic approach. Ecotoxicol Lond Engl, 27(9), 1281-1291. https://doi.org/10.1007/s10646-018-1981-x
Hjelmsø, M.H., Hellmér, M., Fernandez-Cassi, X., Timoneda, N., Lukjancenko, O., Seidel, M., Elsässer, D., Aarestrup, F.M, Löfstrom, C., Bofill-Mas, S., Abril, J.F., Girones, R. and Schultz, A.C. (2017). Evaluation of Methods for the Concentration and Extraction of Viruses from Sewage in the Context of Metagenomic Sequencing. PloS One, 12(1):e0170199. https://doi.org/10.1371/journal.pone.0170199
Högfors-Rönnholm, E., Christel, S., Engblom, S. and Dopson, M. (2018). Indirect DNA extraction method suitable for acidic soil with high clay content. MethodsX, 5,136-140. https://doi.org/10.1016/j.mex.2018.02.005
Holland, J.E., Bennett, A.E., Newton, A.C., White, P.J., McKenzie, B.M., George, T.S., Pakeman, R.J., Bailey, J.S., Fornara, D.A. and Hayes, R.C. (2018). Liming impacts on soils, crops and biodiversity in the UK: A review. Sci Total Environ. 610-611, 316-332. https://doi.org/10.1016/j.scitotenv.2017.08.020
Hong, C.E., Kim, J.U., Lee, J.W., Bang, K.H. and Jo, I.H. (2019). Metagenomic analysis of bacterial endophyte community structure and functions in Panax ginseng at different ages. 3 Biotech, 9(8):300. https://doi.org/10.1007/s13205-019-1838-x
Jiang, L., You, W., Zhang, X., Xu, J., Jiang, Y., Wang, K., Zhao, Z., Chen, B., Zhao, Y., Mahboob, S., Al-Ghanim, K.A., Ke, C. and Xu, P. (2016). Construction of the BAC Library of Small Abalone (Haliotis diversicolor) for Gene Screening and Genome Characterization. Mar Biotechnol (N Y), 18(1), 49-56. https://doi.org/10.1007/s10126-015-9666-4
Jung, H.-M. and Kim, Y.-H.(2018). Simultaneous Overexpression of Integrated Genes by Copy Number Amplification of a Mini-Yeast Artificial Chromosome. J Microbiol Biotechnol, 28(5), 821-825. https://doi.org/10.4014/jmb.1711.11061
Karunarathne, A., Gunnell, D., Konradsen, F. and Eddleston, M. (2020). How many premature deaths from pesticide suicide have occurred since the agricultural Green Revolution? Clin Toxicol Phila, 58(4), 227-232. https://doi.org/10.1080/15563650.2019.1662433
Kashi, F.J. (2016). An Improved Procedure of the Metagenomic DNA Extraction from Saline Soil, Sediment and Salt. Int. Lett. Nat. Sci, 60, 38-45. https://doi.org/10.18052/www.scipress.com/ILNS.60.38
Katz, M., Hover, B.M. and Brady, S.F. (2016). Culture-independent discovery of natural products from soil metagenomes. J Ind Microbiol Biotechnol, 43(2-3), 129-141. https://doi.org/10.1007/s10295-015-1706-6
Katzke, N., Knapp, A., Loeschcke, A., Drepper, T. and Jaeger, K.-E. (2017). Novel Tools for the Functional Expression of Metagenomic DNA. Methods Mol Biol Clifton NJ, 1539, 159-196. https://doi.org/10.1007/978-1-4939-6691-2_10
King, P., Pham, L.K., Waltz, S., Sphar, D., Yamamoto, R.T., Conrad, D., Taplitz, R., Torriani, F. and Forsyth, R.A. (2016). Correction: Longitudinal Metagenomic Analysis of Hospital Air Identifies Clinically Relevant Microbes. PloS One,11(12):e0169376. https://doi.org/10.1371/journal.pone.0169376
Kuhn, R., Böllmann, J., Krahl, K., Bryant, I.M. and Martienssen, M. (2017). Comparison of ten different DNA extraction procedures with respect to their suitability for environmental samples. J Microbiol Methods, 143, 78-86. https://doi.org/10.1016/j.mimet.2017.10.007
Kumar, V., AlMomin, S., Al-Aqeel, H., Al-Salameen, F., Nair, S. and Shajan, A. (2018). Metagenomic analysis of rhizosphere microflora of oil-contaminated soil planted with barley and alfalfa. PloS One, 13(8):e0202127. https://doi.org/10.1371/journal.pone.0202127
Lacerda-Júnior, G.V., Noronha, M.F., Cabral, L., Delforno, T.P., de Sousa, S.T.P., Fernandes-Júnior, P.I., Melo, I.S. and Oliveira, V.M. (2019). Land Use and Seasonal Effects on the Soil Microbiome of a Brazilian Dry Forest. Front Microbiol, 10, 648. https://doi.org/10.3389/fmicb.2019.00648
Liu Y, Yang D, Zhang N, Chen L, Cui Z, Shen Q. and Zhang, R. (2016). Characterization of Uncultured Genome Fragment from Soil Metagenomic Library Exposed Rare Mismatch of Internal Tetranucleotide Frequency. Front Microbiol,7,2081. https://doi.org/10.3389/fmicb.2016.02081
Llacsa, L.X., Solis-Castro, R.L., Mialhe, E. and García-Seminario, R. (2019). Metagenomic Analysis of the Bacterial and Fungal Community Associated to the Rhizosphere of Tabebuia chrysantha and T. billbergii. Curr Microbiol, 76(9), 1073-1080. https://doi.org/10.1007/s00284-019-01725-5
Mahenthiralingam, E., Baldwin, A., Drevinek, P., Vanlaere, E., Vandamme, P., LiPuma, J.J. and Dowson, C.G. (2006). Multilocus sequence typing breathes life into a microbial metagenome. PloS One, 1(1):e17. https://doi.org/10.1371/journal.pone.0000017
Makhalanyane, T.P., Van Goethem, M.W. and Cowan, D.A. (2016). Microbial diversity and functional capacity in polar soils. Curr Opin Biotechnol, 38, 159-166. https://doi.org/10.1016/j.copbio.2016.01.011
Manoharan, L., Kozlowski, J.A., Murdoch, R.W., Löffler, F.E., Sousa, F.L. and Schleper, C. (2019). Metagenomes from Coastal Marine Sediments Give Insights into the Ecological Role and Cellular Features of Loki- and Thorarchaeota. MBio, 10(5)e02039. https://doi.org/10.1128/mBio.02039-19
Mazziotti, M., Henry, S., Laval-Gilly, P., Bonnefoy, A. and Falla, J. (2018). Comparison of two bacterial DNA extraction methods from non-polluted and polluted soils. Folia Microbiol (Praha), 63(1), 85-92. https://doi.org/10.1007/s12223-017-0530-y
Mendes, L.W. and Tsai, S.M. (2018). Distinct taxonomic and functional composition of soil microbiomes along the gradient forest-restinga-mangrove in southeastern Brazil. Antonie Van Leeuwenhoek. 2018;111(1):101-114. https://doi.org/10.1007/s10482-017-0931-6
Mirete, S., Morgante, V. and González-Pastor, J.E. (2016). Functional metagenomics of extreme environments. Curr Opin Biotechnol, 38, 143-149. https://doi.org/10.1016/j.copbio.2016.01.017
Morales-García, Y.E., Baez, A., Quintero-Hernández, V., Molina-Romero, D., Rivera-Urbalejo, A.P., Pazos-Rojas, L.A. and Muñoz-Rojas, J. (2019). Bacterial Mixtures, the Future Generation of Inoculants for Sustainable Crop Production. En: Maheshwari DK, Dheeman S, editores. Field Crops Sustain Manag PGPR. Cham: Springer International Publishing, 11-44. https://doi.org/10.1007/978-3-030-30926-8_2
Mori, T., Mizuta, S., Suenaga, H. and Miyazaki, K. (2008) Metagenomic screening for bleomycin resistance genes. Appl Environ Microbiol, 74(21), 6803-6805. https://doi.org/10.1128/AEM.00873-08
Mtimka, S., Pillay, P., Rashamuse, K., Gildenhuys, S. and Tsekoa, T.L. (2020). Functional screening of a soil metagenome for DNA endonucleases by acquired resistance to bacteriophage infection. Mol Biol Rep, 47(1), 353-361. https://doi.org/10.1007/s11033-019-05137-3
Mueller-Spitz, S.R., Vonderheide, A.P., Shann, J.R., Caruso, J.A. and Kinkle, B.K. (2006). Use of SEC-ICP-MS with a collision cell for determining the interaction of chromium with DNA extracted from metal-contaminated soils. Anal Bioanal Chem, 386(1), 142-151. https://doi.org/10.1007/s00216-006-0575-2
Munar, M.P., Takahashi, H. and Okamura, Y. (2019). Discovery of a Novel Gene Conferring Tellurite Tolerance Through Tellurite Reduction to Escherichia coli Transformant in Marine Sediment Metagenomic Library. Mar Biotechnol (N Y), 21(6), 762-772. https://doi.org/10.1007/s10126-019-09922-w
Narayan, A., Jain, K., Shah, A.R. and Madamwar, D. (2016). An efficient and cost-effective method for DNA extraction from athalassohaline soil using a newly formulated cell extraction buffer. 3 Biotech, 6(1):62. https://doi.org/10.1007/s13205-016-0383-0
National Research Council (US) Committee on Metagenomics: Challenges and Functional Applications. The New Science of Metagenomics: Revealing the Secrets of Our Microbial Planet. Washington (DC): National Academies Press (US); 2007. https://doi.org/10.17226/11902
Nesme ,J., Achouak, W., Agathos, S.N., Bailey, M., Baldrian, P., Brunel, D., Frostegar, A., Heulin, T., Jansson, J., Jurkevitch, E., Kruus, K., Kowalchuk G.A., Lagares, A., Lappin-Scott, H.M., Lemanceau, P., Le Paslier, T., Mandic-Mulec, I., Murrell, J.C., Myrold, D.D… Simonet, P. (2016). Back to the Future of Soil Metagenomics. Front Microbiol, 7, 73. https://doi.org/10.3389/fmicb.2016.00073
Pellacani, C. and Costa, L.G. (2018). Role of autophagy in environmental neurotoxicity. Environ Pollut, 235, 791-805. https://doi.org/10.1016/j.envpol.2017.12.102
Piel, L., Pescher, P. and Späth, G.F. (2018). Reverse Epidemiology: An Experimental Framework to Drive Leishmania Biomarker Discovery in situ by Functional Genetic Screening Using Relevant Animal Models. Front Cell Infect Microbiol, 8, 325. https://doi.org/10.3389/fcimb.2018.00325
Pitta, D.W., Indugu, N., Kumar, S., Vecchiarelli, B., Sinha, R., Baker, L.D., Bhukya, B. and Ferguson, J.D. (2016). Metagenomic assessment of the functional potential of the rumen microbiome in Holstein dairy cows. Anaerobe, 38, 50-60. https://doi.org/10.1016/j.anaerobe.2015.12.003
Prabha, R., Singh, D.P., Verma, M.K., Sahu, P. and Kumar, P. (2018). Bacterial diversity in rhizosphere of Paspalum scrobiculatum L. (kodo millet) is revealed with shotgun metagenome sequencing and data analysis. Data Brief, 20, 1653-1657. https://doi.org/10.1016/j.dib.2018.09.006
Puri, R.R., Adachi, F., Omichi, M., Saeki, Y., Yamamoto, A., Hayashi, S., Ali, M.A. and Itoh, K. (2019). Metagenomic study of endophytic bacterial community of sweet potato (Ipomoea batatas) cultivated in different soil and climatic conditions. World J Microbiol Biotechnol, 35(11), 176. https://doi.org/10.1007/s11274-019-2754-2
Pyzik, A., Ciezkowska, M., Krawczyk, P.S., Sobczak, A., Drewniak, L., Dziembowski, A. and Lipinski, L. (2018). Comparative analysis of deep sequenced methanogenic communities: identification of microorganisms responsible for methane production. Microb Cell Fact, 17(1), 197. https://doi.org/10.1186/s12934-018-1043-3
Ravi, R.K., Walton, K. and Khosroheidari, M. (2018). MiSeq: A Next Generation Sequencing Platform for Genomic Analysis. Methods Mol Biol Clifton NJ, 1706, 223-232. https://doi.org/10.1007/978-1-4939-7471-9_12
Ravin, N.V., Mardanova, A.V. and Skryabin, K.G. (2015). [Metagenomics as a Tool for the Investigation of Uncultured Microorganisms. Genetika, 51(5), 519-528. https://doi.org/10.1134/S1022795415050063
Rawat, N. and Joshi, G.K. (2019). Bacterial community structure analysis of a hot spring soil by next generation sequencing of ribosomal RNA. Genomics, 111(5), 1053-1058. https://doi.org/10.1016/j.ygeno.2018.06.008
Rehman, Z.U., Ali, M., Iftikhar, H. and Leiknes, To. (2019). Genome-resolved metagenomic analysis reveals roles of microbial community members in full-scale seawater reverse osmosis plant. Water Res, 149, 263-271. https://doi.org/10.1016/j.watres.2018.11.012
Roeh, S., Weber, P., Rex-Haffner, M., Deussing, J.M., Binder, E.B. and Jakovcevski, M. (2017). Sequencing on the SOLiD 5500xl System - in-depth characterization of the GC bias. Nucleus, 8(4), 370-380. https://doi.org/10.1080/19491034.2017.1320461
Sáenz, J.S., Roldan, F. and Junca, H. (2019). Arbeli Z. Effect of the extraction and purification of soil DNA and pooling of PCR amplification products on the description of bacterial and archaeal communities. J Appl Microbiol, 126(5), 1454-1467. https://doi.org/10.1111/jam.14231
Salam, L.B., Obayori, S.O., Nwaokorie, F.O., Suleiman, A. and Mustapha, R. (2017). Metagenomic insights into effects of spent engine oil perturbation on the microbial community composition and function in a tropical agricultural soil. Environ Sci Pollut Res Int, 24(8), 7139-7159. https://doi.org/10.1007/s11356-017-8364-3
Salazar, J.K., Carstens, C.K., Ramachandran, P., Shazer, A.G., Narula, S.S., Reed, E., Ottesen, A. and Schill, K.M. (2018). Metagenomics of pasteurized and unpasteurized gouda cheese using targeted 16S rDNA sequencing. BMC Microbiol, 18(1), 189. https://doi.org/10.1186/s12866-018-1323-4
Sánchez-Sanhueza, G., Bello-Toledo, H., González-Rocha, G., Gonçalves, A.T., Valenzuela, V. and Gallardo-Escárate, C. (2018). Metagenomic study of bacterial microbiota in persistent endodontic infections using Next-generation sequencing. Int Endod J, 51(12), 1336-1348. https://doi.org/10.1111/iej.12953
Schimann, H., Bach, C., Lengelle, J., Louisanna, E., Barantal, S., Murat, C. and Buée, M. (2017). Diversity and Structure of Fungal Communities in Neotropical Rainforest Soils: The Effect of Host Recurrence. Microb Ecol, 73(2), 310-320. https://doi.org/10.1007/s00248-016-0839-0
Shao, K. and Gao, G. (2018). Soil microbial communities of three grassland ecosystems in the Bayinbuluke, China. Can J Microbiol, 64(3), 209-213. https://doi.org/10.1139/cjm-2017-0585
Shen, F., Li, Y., Zhang, M., Awasthi, M.K., Ali, A., Li, R., Wang, Q. and Zhang, Z. (2016). Atmospheric Deposition-Carried Zn and Cd from a Zinc Smelter and Their Effects on Soil Microflora as Revealed by 16S rDNA. Sci Rep, 6, 39148. https://doi.org/10.1038/srep39148
Smułek, W., Sydow, M., Zabielska-Matejuk, J. and Kaczorek, E. (2020). Bacteria involved in biodegradation of creosote PAH - A case study of long-term contaminated industrial area. Ecotoxicol Environ Saf, 187, 109843. https://doi.org/10.1016/j.ecoenv.2019.109843
Solden, L., Lloyd, K. and Wrighton, K. (2016). The bright side of microbial dark matter: lessons learned from the uncultivated majority. Curr Opin Microbiol, 31, 217-226. https://doi.org/10.1016/j.mib.2016.04.020
Stach, J.E.M., Bathe, S., Clapp, J.P. and Burns, R.G. (2001). PCR-SSCP comparison of 16S rDNA sequence diversity in soil DNA obtained using different isolation and purification methods. FEMS Microbiol Ecol, 36(2-3), 139-151. https://doi.org/10.1111/j.1574-6941.2001.tb00834.x
Steffan, J.J., Brevik, E.C.and Burgess, L.C. (2018). Cerdà A. The effect of soil on human health: an overview. Eur J Soil Sci, 69(1), 159-171. https://doi.org/10.1111/ejss.12451
Stoops, J., Ruyters, S., Busschaert, P., Spaepen, R., Verreth, C., Claes, J., Lieves, B. and Van Campenhout, L. (2015). Bacterial community dynamics during cold storage of minced meat packaged under modified atmosphere and supplemented with different preservatives. Food Microbiol, 48, 192-199. https://doi.org/10.1016/j.fm.2014.12.012
Tavares, T.C.L, Normando, L.R.O, de Vasconcelos, A.T.R., Gerber, A.L., Agnez-Lima, L.F. and Melo, V.M.M. (2016). Metagenomic analysis of sediments under seaports influence in the Equatorial Atlantic Ocean. Sci Total Environ, 557-558, 888-900. https://doi.org/10.1016/j.scitotenv.2016.03.141
Técher, D., Martinez-Chois, C., D’Innocenzo, M., Laval-Gilly, P., Bennasroune, A., Foucaud, L. and Falla, J. (2010). Novel perspectives to purify genomic DNA from high humic acid content and contaminated soils. Sep Purif Technol, 75(1), 81-86. https://doi.org/10.1016/j.seppur.2010.07.014
Terrón-González, L., Martín-Cabello, G., Ferrer, M. and Santero, E. (2016). Functional Metagenomics of a Biostimulated Petroleum-Contaminated Soil Reveals an Extraordinary Diversity of Extradiol Dioxygenases. Appl Environ Microbiol, 82(8), 2467-2478. https://doi.org/10.1128/AEM.03811-15
Tocchetti, A., Donadio, S. and Sosio, M. (2018). Large inserts for big data: artificial chromosomes in the genomic era. FEMS Microbiol Lett, 365(9). https://doi.org/10.1093/femsle/fny064
Tsurumaru, H., Okubo, T., Okazaki, K., Hashimoto, M., Kakizaki, K., Hanzawa, E., Takahashi, H., Asanome, N., Tanaka, F., Sekiyama, V., Ikeda, S. and Minamisawa, K. (2015). Metagenomic analysis of the bacterial community associated with the taproot of sugar beet. Microbes Environ, 30(1), 63-69. https://doi.org/10.1264/jsme2.ME14109
Vassilev, N., Vassileva, M., Lopez, A., Martos, V., Reyes, A., Maksimovic, I., Eichler-Löbermann, B. and Malusá, E. 82015). Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl Microbiol Biotechnol, 99(12), 4983-4996. https://doi.org/10.1007/s00253-015-6656-4
Vejan, P., Abdullah, R., Khadiran, T., Ismail, S. and Nasrulhaq Boyce. A. (2016). Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability-A Review. Molecules, 21(5), 573. https://doi.org/10.3390/molecules21050573
Vigneron, A., Alsop, E.B., Cruaud, P., Philibert, G., King, B., Baksmaty, L., Lavallée, D., Lomans, B.P., Kyrpides, N.C., Head, I.M. and Tsesmetzis, N. (2017). Comparative metagenomics of hydrocarbon and methane seeps of the Gulf of Mexico. Sci Rep, 7(1), 16015. https://doi.org/10.1038/s41598-017-16375-5
Wagner, A.O., Praeg, N., Reitschuler, C. and Illmer, P. Effect of DNA extraction procedure, repeated extraction and ethidium monoazide (EMA)/propidium monoazide (PMA) treatment on overall DNA yield and impact on microbial fingerprints for bacteria, fungi and archaea in a reference soil. Appl Soil Ecol. 2015;93:56-64. https://doi.org/10.1016/j.apsoil.2015.04.005
Watanabe, N., Kryukov, K., Nakagawa, S., Takeuchi, J.S., Takeshita, M., Kirimura, Y., Mitsuhashi, S., Ishihara, T., Aoki, H., Inokuchi, S., Imanishi, T. and Inoue, S. (2018). Detection of pathogenic bacteria in the blood from sepsis patients using 16S rRNA gene amplicon sequencing analysis. PloS One, 13(8):e0202049. https://doi.org/10.1371/journal.pone.0202049
Xu, J., Zhang, Y., Zhang, P., Trivedi, P., Riera, N., Wang, Y., Liu, X., Fan, G., Tang, J., Coletta-Filho, H.D., Cubero, J., Deng, X., Ancona, V., Lu, Z., Zhong, B., Caroline Roper, M., Capote, N., Catara, V., Pietersen, G… Wang. N. (2018). The structure and function of the global citrus rhizosphere microbiome. Nat Commun, 9(1), 4894. https://doi.org/10.1038/s41467-018-07343-2
Yan, K., Dong, Z., Wijayawardena, M.A.A., Liu, Y., Naidu, R. and Semple, K. (2017). Measurement of soil lead bioavailability and influence of soil types and properties: A review. Chemosphere, 184, 27-42. https://doi.org/10.1016/j.chemosphere.2017.05.143
Zhang, J.J., Tang. X., Zhang, M., Nguyen, D. and Moore, B.S. (2017). Broad-Host-Range Expression Reveals Native and Host Regulatory Elements That Influence Heterologous Antibiotic Production in Gram-Negative Bacteria. MBio, 8(5). https://doi.org/10.1128/mBio.01291-17
Zhou, J., Bruns, M.A. and Tiedje, J.M. (1996). DNA recovery from soils of diverse composition. Appl Environ Microbiol, 62(2), 316-322. https://doi.org/10.1128/AEM.62.2.316-322.1996
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Judith A. Sánchez-Ledesma, Bernardo Águila, Roberto Garibay-Orijel, Cristina García-De la Peña, Erika Nava-Reyna. (2025). Soil mycobiome structure across central and peripherial zones in a pecan nut agroecosystem [Carya illinoinensis (Wangenh.) K. Koch]. Folia Oecologica, 52(2), p.149. https://doi.org/10.2478/foecol-2025-0015.
2. Priscila Heredia Reto, Rosita Castillo Rogel, Gabriela Palomino Lucano, Jean Louis Falen, Ricardo David Avellan Laguno, Karina Zapata Vidaurre, Marisol Saavedra Febre, Gabriel Reyes Calle, Juan Zingg Rosell, Jimmy Lopez Perez, José Morán Rosillo, Eric Mialhe, Benoit Diringer, Ying Ma. (2025). Assessing microbial diversity in open-pit mining: Metabarcoding analysis of soil and pit microbiota across operational and restoration stages. PLOS ONE, 20(4), p.e0320923. https://doi.org/10.1371/journal.pone.0320923.
3. Melissa Álvarez Peña, Ramiro Álvarez Valenzuela. (2025). Gestión del Conocimiento. Perspectiva Multidisciplinaria (Libro 81). , p.21. https://doi.org/10.59899/Ges-cono-81-C1.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2021 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).



















