Publicado
In vitro germination and vegetative propagation through bud development of sacha inchi (Plukenetia volubilis L.)
Germinación y propagación vegetativa in vitro a través del desarrollo de yemas de Sacha Inchi (Plukenetia volubilis)
DOI:
https://doi.org/10.15446/abc.v27n1.88727Palabras clave:
2-ip, Axillary buds, Calcium, Kinetin, Micropropagation (en)2-ip, Calcio, Inducción de brotes, Kinetina, Micropropagación (es)
Descargas
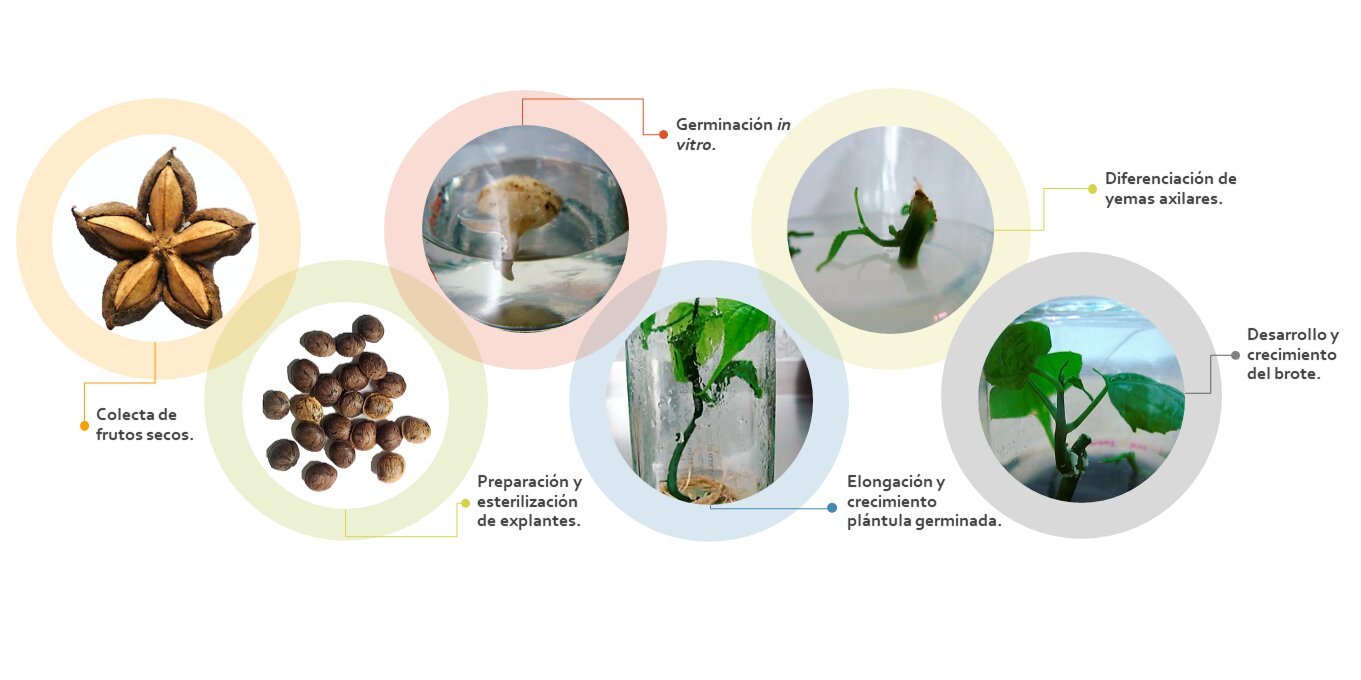
This study describes the in vitro seed germination and micropropagation of Plukenetia volubilis (sacha inchi), an oilseed crop rich in omega-3 fatty acids, with health benefits and several industrial applications. Seed germination was evaluated in different culture media (MS and 1/2 MS), seed coat presence/absence and culture temperature (18 °C and 28 °C). Micropropagation was performed using axillary bud development (ABD) on nodal segments from in vitro seedlings. KIN, BAP and 2-ip were evaluated for ABD, and the effect of modified MS in 453 mg L-1 CaCl2 and 351.62 mg L-1 MgSO4 on ABD and shoot survival was assessed to improve the process. Finally, six treatments were evaluated to optimize ABD and shoot leaf formation. Seed germination of 91.6 % was achieved in MS at 28 °C when the seed coat was removed. ABD was obtained in 45 % and 40 % with 0.4 mg L-1 KIN and 0.6 mg L-1 2-ip, respectively, with the least CAL. The modification in 453 mg L-1 CaCl2 then allowed 76 % ABD and 82 % explant survival. ABD response was optimized to 95 % and 2.45 leaves with MS medium + CaCl2 modification + 10 % coconut water + 0.4 mg L-1 KIN. The same results were obtained by replacing the latter with 0.6 mg L-1 2-ip. Rooting was achieved in MS without PGR, and acclimatization was successful. The results indicate that plant production via germination and vegetative propagation is effective for commercial purposes.
Este estudio describe la germinación in vitro y micropropagación de Plukenetia volubilis, un cultivo oleaginoso rico en omega-3 benéfico para la salud y con múltiples aplicaciones industriales. Se evaluó en la germinación diferentes medios de cultivo (MS y 1/2 MS), presencia-ausencia de testa y temperatura de cultivo (18 ° C y 28 ° C). La micropropagación se realizó vía yemas axilares (ABD) de plántulas in vitro. Se evaluó el efecto de KIN, BAP y 2-ip sobre ABD, seguidamente, para mejorar el proceso se evaluó el efecto de MS modificado en 453 mg L-1 CaCl2 y 351.62 mg L-1 MgSO4 sobre ABD y supervivencia del brote. Finalmente, se evaluaron seis tratamientos para optimizar ABD y la formación de hojas. Se logró una germinación 91,6 % en MS a 28 °C cuando se retiró la testa. Se obtuvo 45 % y 40 % de ABD con 0,4 mg L-1 KIN y 0,6 mg L-1 2-ip respectivamente, ambos con la menor CAL. Posteriormente, la modificación de CaCl2 permitió 76 % ABD y 82 % de supervivencia. Se optimizó ABD al 95 % con 2,45 hojas por brote con el medio: MS + modificación de CaCl2 + 10 % de agua de coco + 0.4 mg L-1 KIN, los mismos resultados se obtuvieron cambiando este último con 0,6 mg L-1 2-ip, se logró enraizamiento en MS sin PGR, y la aclimatización fue exitosa. Los resultados indican que la producción de plantas vía germinación y propagación vegetativa es efectiva con fines comerciales.
Referencias
Agustini ,V., Rahayu, I., Numberi, L., and Mah, Z. (2020). The role of chitosan as a growth driver for orchid culture Dendrobium lasianthera. J.J.Sm. in vitro. Jurnal Biologi Papua, 12(1), 43–49. https://doi.org/10.31957/jbp.1096
Almeida, M., Almeida, C. V., Graner, E., Brondani, G. E., and Abreu-Tarazi, M. F. (2012). Pre-procambial cells are niches for pluripotent and totipotent stem-like cells for organogenesis and somatic embryogenesis in the peach palm: A histological study. Plant Cell Reports, 31(8), 1495–1515. https://doi.org/10.1007/s00299-012-1264-6
Azcón, J., and Talón, M. (2008). Plant Physiology Fundamentals. Editorial McGraw-Hill Interamericana.
Bairu, M. W., Jain, N., Stirk, W. A, Doležal, K., and Van Staden, J. (2009). Solving the problem of shoot-tip necrosis in Harpagophytum procumbens by changing the cytokinin types, calcium and boron concentrations in the medium. South African Journal of Botany, 75(1), 122–127. https://doi.org/10.1016/j.sajb.2008.08.006
Bordignon, S. R., Bovi Ambrosano, M. G., and Viegas Rodrigues, P. H. (2012). In vitro propagation of Sacha inchi. Ciência Rural, 42(7), 1168–1172. https://doi.org/10.1590/S0103-84782012005000049
Burns, D. T., Johnston, E. -L., and Walker, M. J. (2020). Authenticity and the Potability of Coconut Water - a Critical Review. Journal of AOAC International, 103(3), 1-7. https://doi.org/10.1093/jaocint/qsz008
Cachique, D. H., Solsol, H. R., García Sanchez, M. A., Arévalo López, L. A., and Kodahl, N. (2018). Vegetative propagation of the underutilized oilseed crop sacha inchi (Plukenetia volubilis L.). Genetic Resources and Crop Evolution, 65(7), 2027-2036. https://doi.org/10.1007/s10722-018-0659-9
Cardoso, A. A. S., Lopes, M. T. G., Valente, M. S. F., Quisen, R. C., and Chaves, F. C. M. (2018). Seed morphometry, in vitro germination and vegetative propagation in sacha inchi (Plukenetia volubilis L.). Revista Brasileña de Ciencias Agrarias, 13(3), 1–7. https://doi.org/10.5039/agraria.v13i3a5561
Chirinos, R., Necochea, O., Pedreschi, R., and Campos, D. (2016). Sacha inchi (Plukenetia volubilis L.) shell: An alternative source of phenolic compounds and antioxidants. International Journal of Food Science & Technology, 51(4), 986-993. https://doi.org/10.1111/ijfs.13049
Dong, Y., Chen, M., Wang, X., Niu, L., Fu, Q., and Xu, Z. (2016). Establishment of in vitro regeneration system of woody oil crop Plukenetia volubilis. Molecular Plant Breed, 14(2), 462–470.
Gupta, S., Kachhwaha, S., Lal Kothari, S., and Jain, R. (2020). Synergistic effect of cytokinins and auxins enables mass clonal multiplication of drumstick tree (Moringa oleifera Lam.): a wonder. In Vitro Cellular & Developmental Biology - Plant, 56, 458-469. https://doi.org/10.1007/s11627-020-10065-0
Hesami, M., Daneshvar, M. H., and Yoosefzadeh-Najafabadi, M. (2018). Establishment of a protocol for in vitro seed germination and callus formation of Ficus religiosa L., an important medicinal plant. Jundishapur Journal of Natural Pharmaceutical Products, 13(4), 1–8. https://doi.org/10.5812/jjnpp.62682
Kao, K., and Michayluk, M. (1975). Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta, 126(2), 105–110. https://doi.org/10.1007/BF00380613
Kerdiles, O., Layé, S., and Calon, F. (2017). Omega-3 polyunsaturated fatty acids and brain health: Preclinical evidence for the prevention of neurodegenerative diseases. Trends in Food Science & Technology, 69(Part B), 203–213. https://doi.org/10.1016/j.tifs.2017.09.003
Kodahl, N. (2020). Sacha inchi (Plukenetia volubilis L.) from lost crop of the Incas to part of the solution to global challenges. Planta, 251(4), 1-22. https://doi.org/10.1007/s00425-020-03377-3
Kodahl, N., García-Dávila, C. R., Cachique, D., Sørensen, M., and Lütken, H. (2018). An in vitro seed germination protocol for Plukenetia volubilis L. ISHS Acta Horticulturae, 1201, 549–554. https://doi.org/10.17660/ActaHortic.2018.1201.73
Maurer, N. E., Hatta-Sakoda, B., Pascual-Chagman, G., and Rodriguez-Saona, L. E. (2012). Characterization and authentication of a novel vegetable source of omega-3 fatty acids, sacha inchi (Plukenetia volubilis L.) oil. Food Chemistry, 134(2), 1173–1180. https://doi.org/10.1016/j.foodchem.2012.02.143
Millones, C. E., and Vásquez, E. R. (2008). Micropropagación de plantas de sacha inchi (Plukenetia volubilis L.) provenientes de la provincia de Rodríguez de Mendoza, Región Amazonas. Investigaciones Amazonenses, 2, 7–11.
Moreno Martínez, D., and Menchaca García, R. A. (2007). Efecto de los compuestos orgánicos en la propagación in vitro de Stanhopea Tigrina Bateman (Orchidaceae). Foresta Veracruzana, 9(2), 27–32.
Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Patthanajuck, V., and Bunnag, S. (2017). Effect of plant growth regulators on shoot induction of sacha inchi (Plukenetia volubilis L.) in vitro (pp. 494–500). NIGRC KKU-2017.
Rai, I. N., Sudana, I. M., Astawa, I. N. G., Dwiyani, R., and Fitriani, Y. (2019). Direct organogenesis in vitro propagation of local balinese banana with thidiazuron. International Journal of Life Sciences, 3(3), 32–40. https://doi.org/10.29332/ijls.v3n3.360
Restrepo-Osorio, C., Gil-Correal, A., Chamorro-Gutiérrez, L., Ramírez-Ríos, V., Álvarez, J. C., and Villanueva-Mejía, D. (2020). Efficient direct shoot organogenesis and genetic stability in micropropagated sacha inchi (Plukenetia volubilis L.). BMC Research Notes, 13(1), 1–7. https://doi.org/10.1186/s13104-020-05257-1
de la Rosa, R., and Quijada, J. (2013). Germination of Sacha Inchi, Plukenetia Volubilis L. (Mcbride, 1951) (Malpighiales, Euphorbiaceae) Under Four Different Conditions. The Biolgist, 11(1), 9–14.
Sathe, S. K, Kshirsagar, H. H, and Sharma, G. M. (2012). Solubilization, Fractionation, and electrophoretic characterization of inca peanut (Plukenetia volubilis L.) Proteins. Plant Foods for Human Nutrition, 67(3), 247–255. https://doi.org/10.1007/s11130-012-0301-5
Sembiring, R., Hayati, M., and Kesumawati, E. (2020). Formation of potato micro tubers (Solanum tuberosum L.) by using BAP and coconut water in the in vitro culture. IOP Conference Series: Earth and Environmental Science, 425(1), 012072. https://doi.org/10.1088/1755-1315/425/1/012072
Solis, R., Cachique, D., Guerrero-Abab, J., Ruiz Sánchez, M. E, and Tapiay Figueroa, L. (2018). In vitro propagation of sacha inchi through organogenesis. Pesquisa Agropecuária Brasileira, 53(3), 1285–1288. https://doi.org/10.1590/s0100-204x2018001100012
Solis, R., Gonzales, N., Pezo, M., Arévalo, L., and Vallejos-Torres, G. (2019). Rooting of sacha inchi (Plukenetia volubilis) juvenile cuttings in microtunnels. Acta Agronómica, 68(11), 35–40. https://doi.org/10.15446/acag.v68n1.72101
Solis, R., Pezo, M., Diaz, G., Arévalo, L., and Cachique, D. (2016). Vegetative propagation of Plukenetia polyadenia by cuttings: effects of leaf area and indole-3-butyric acid concentration. Brazilian Journal of Biology, 77(1), 580–584. https://doi.org/10.1590/1519-6984.20415
Tenenbaum, A., and Fisman, E. Z. (2018). Omega-3 polyunsaturated fatty acids supplementation in patients with diabetes and cardiovascular disease risk: Does dose really matter? Cardiovascular Diabetology, 17(1), 1–3. https://doi.org/10.1186/s12933-018-0766-0
Thomas, T. D. (2008). The role of activated charcoal in plant tissue culture. Biotechnology Advances, 26(6), 618–631. https://doi.org/10.1016/j.biotechadv.2008.08.003
Thuy, L., and Nhung, N. (2019). Study on the suitable mediums for in vitro culture the stem segment of sacha inchi (Plukenetia volubilis L.) plant. Naresuan University Journal: Science and Technology, 207(14), 121–128.
Tropicos.org. (4 de marzo de 2020). Legacy Names. Missouri Bot. Gard. 2020. http://legacy.tropicos.org/Name/40012207?tab=subordinatetaxa
Tuteja, N., and Mahajan, S. (2007). Calcium Signaling Network in Plants. Plant Signaling & Behavior, 2(2), 79–85. https://doi.org/10.4161/psb.2.2.4176
Viegas Rodrigues, P., Bordignon, S. B., and Bovi Ambrosano, G. (2014). Horticultural performance of in vitro propagated plants of Sacha inchi. Ciência Rural, Santa Maria, 44(6), 1050–1053. https://doi.org/10.1590/S0103-84782014000600016.
Wang, S., Zhu, F., and Kakuda, Y. (2018). Sacha inchi (Plukenetia volubilis L.): Nutritional composition, biological activity, and uses. Food Chemistry, 265, 316–328. https://doi.org/10.1016/j.foodchem.2018.05.055
Watanabe, Y., and Tatsuno, I. (2017). Omega-3 polyunsaturated fatty acids for cardiovascular diseases: present, past and future. Expert Review of Clinical Pharmacology, 10(8), 865–873. https://doi.org/10.1080/17512433.2017.1333902
White, P, R. (1964). The cultivation of animal and plant cells. Soil Science, 97(1), 74. https://doi.org/10.1097/00010694-196401000-00013
Xu, T., Li, T., and Qi, M. (2009). Analysis of calcium content, hormones, and degrading enzymes in tomato pedicel explants during calcium-inhibited abscission. Agricultural Sciences in China, 8(5), 556–563. https://doi.org/10.1016/S1671-2927(08)60246-1
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. MAYRA Bataller Venta, ELIET Veliz Lorenzo, ALEXANDER Perez, GRETHER Barreras, MABEL Villanueva, NIUBIS Ortega, OSCAR Ledea. (2023). Effect of Ozone Treatments on Germination and Disinfection of Sacha Inchi ( Plukenetia volubilis L.) Seeds . Ozone: Science & Engineering, 45(6), p.582. https://doi.org/10.1080/01919512.2023.2192743.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2021 Acta Biológica Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).



















