Publicado
VARIACIÓN EN LA EXPRESIÓN DE GENES DE Apis mellifera L. (Hymenoptera: Apidae) POR EXPOSICIÓN A UNA DOSIS SUBLETAL DE FLUPIRADIFURONA
Variation in Apis mellifera L. (Hymenoptera: Apidae) gene expression by exposure to a sublethal dose of Flupyradifurone
DOI:
https://doi.org/10.15446/abc.v28n3.92972Palabras clave:
agonistas del receptor nicotínico de acetilcolina, efecto subletal, estrés oxidativo, flupiradifurona, disminución de polinizadores (es)nicotinic acetylcholine receptor agonist, sublethal effect, oxidative stress, flupyradifurone, pollinators decline (en)
Descargas
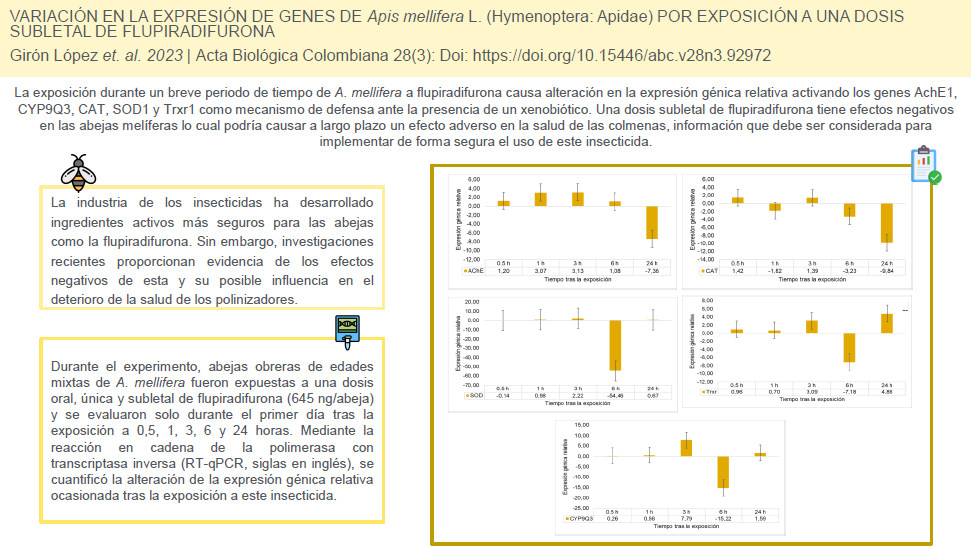
La industria de los insecticidas ha desarrollado ingredientes activos más seguros para las abejas como la flupiradifurona. Sin embargo, investigaciones recientes proporcionan evidencia de los efectos negativos de esta y su posible influencia en el deterioro de la salud de los polinizadores. Durante el experimento, abejas obreras de edades mixtas de A. mellifera fueron expuestas a una dosis oral, única y subletal de flupiradifurona (645 ng/abeja) y se evaluaron solo durante el primer día tras la exposición a 0,5, 1, 3, 6 y 24 horas. Mediante la reacción en cadena de la polimerasa con transcriptasa inversa (RT-qPCR, siglas en inglés), se cuantificó la alteración de la expresión génica relativa ocasionada tras la exposición a este insecticida. Un total de cinco genes fueron cuantificados: tres genes antioxidantes primarios (Catalasa (CAT), Superóxido dismutasa (SOD1) y Tioredoxin dismutasa (Trxr1), un gen de detoxificación (Citocromo P450 9Q3 (CYP9Q3)) y un gen con actividad neuronal (Acetilcolinesterasa, AChE1). El gen endógeno proteina ribosómica S18 (RPS18) permitió estimar la expresión génica relativa como la respuesta a la exposición al insecticida. El gen AChE1 fue sobreexpresado hasta las seis horas tras la exposición; los restantes genes CYP9Q3, CAT, SOD1 y Trxr1 presentaron un comportamiento similar. En una dosis subletal y a intervalos de tiempo corto, la flupiradifurona causa alteración en la expresión relativa de cinco genes en individuos de abejas melíferas tras la exposición oral, lo cual podría causar a largo plazo un efecto adverso en la salud de las colmenas.
The insecticide industry has developed more bee-safe active ingredients such as flupyradifurone. However, recent research provides evidence of the negative effects of this and its possible influence on the deterioration of the health of pollinators. During the experiment, mixed-age worker bees of A. mellifera were exposed to a single, sublethal oral dose of flupyradifurone (645 ng/ bee) and were assessed only for the first day after exposure to 0.5, 1, 3, 6, and 24 hours. Using reverse transcriptase polymerase chain reaction (RT-qPCR), the alteration in gene expression caused y exposure to this insecticide was quantified. A total of five genes were quantified: three primary antioxidant genes (Catalase (CAT), Superoxide dismutase (SOD1) and Thioredoxin dismutase (Trxr1), one detoxification gene (Cytochrome P450 9Q3 (CYP9Q3)) and one gene with neuronal activity (Acetylcholinesterase, AChE1). The endogenous ribosomal protein S18 (RPS18) gene allowed the estimation of relative gene expression as the response to insecticide exposure. The AChE1 gene was overexpressed up to six hours after exposure; the remaining CYP9Q3, CAT, SOD1, and Trxr1 genes presented a similar behavior. At a sublethal dose and at short time intervals, flupyradifurone causes alteration in the relative expression of five genes in individual honeybees after oral exposure, which could cause a long-term adverse effect on hive health.
Referencias
Al Naggar, Y. y Baer, B. (2019). Consequences of a short time exposure to a sublethal dose of Flupyradifurone (Sivanto) pesticide early in life on survival and immunity in the honeybee (Apis mellifera). Scientific Reports, 9(1), 1–11. https://doi.org/10.1038/s41598-019-56224-1
Alburaki, M., Steckel, S. J., Chen, D., McDermott, E., Weiss, M., Skinner, J. A., Kelly, H., Lorenz, G., Tarpy, D. R., Meikle, W. G., Adamczyk, J. y Stewart, S. D. (2017). Landscape and pesticide effects on honey bees: forager survival and expression of acetylcholinesterase and brain oxidative genes. Apidologie, 48(4), 556–571. https://doi.org/10.1007/s13592-017-0497-3
Alburaki, M., Smith, K. D., Adamczyk, J. y Karim, S. (2019). Interplay between Selenium, selenoprotein genes, and oxidative stress in honey bee Apis mellifera L. Journal of Insect Physiology, 117. https://doi.org/10.1016/j.jinsphys.2019.103891
Boily, M., Sarrasin, B., DeBlois, C., Aras, P. y Chagnon, M. (2013). Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: Laboratory and field experiments. Environmental Science and Pollution Research, 20(8), 5603–5614. https://doi.org/10.1007/s11356-013-1568-2
Bryden, J., Gill, R. J., Mitton, R. A. A., Raine, N. E. y Jansen, V. A. A. (2013). Chronic sublethal stress causes bee colony failure. Ecology Letters, 16(12), 1463–1469. https://doi.org/10.1111/ele.12188
Calderone, N. W. (2012). Insect pollinated crops, insect pollinators and US agriculture: Trend analysis of aggregate data for the period 1992-2009. PLoS ONE, 7(5). https://doi.org/10.1371/journal.pone.0037235
Campbell, J. W., Cabrera, A. R., Stanley-Stahr, C. y Ellis, J. D. (2016). An Evaluation of the Honey Bee (Hymenoptera: Apidae) Safety Profile of a New Systemic Insecticide, Flupyradifurone, Under Field Conditions in Florida. Journal of Economic Entomology, 109(5), 1967–1972. https://doi.org/10.1093/jee/tow186
Chakrabarti, P., Carlson, E. A., Lucas, H. M., Melathopoulos, A. P. y Sagili, R. R. (2020). Field rates of SivantoTM (flupyradifurone) and Transform® (sulfoxaflor) increase oxidative stress and induce apoptosis in honey bees (Apis mellifera L.). PLOS ONE, 15(5).https://doi.org/10.1371/journal.pone.0233033
Christen, V., Joho, Y., Vogel, M. y Fent, K. (2019). Transcriptional and physiological effects of the pyrethroid deltamethrin and the organophosphate dimethoate in the brain of honey bees (Apis mellifera). Environmental Pollution, 244, 247–256. https://doi.org/10.1016/j.envpol.2018.10.030
Diaz Meraz, R. A. (2015). Efecto de seis plaguicidas sobre mortalidad en dos especies de abejas: Apis mellifera y Tetragonisca angustula (Hymenoptera: Apidae) [Tesis]. Escuela Agrícola Panamericana. Repositorio Escuela Agrícola Panamericana. https://bdigital.zamorano.edu/server/api/core/bitstreams/3917104b-5b57-404d-90f4-94d100864acf/content
Eban-Rothschild, A. D. y Bloch, G. (2008). Differences in the sleep architecture of forager and young honeybees (Apis mellifera). Journal of Experimental Biology, 211(15), 2408–2416. https://doi.org/10.1242/jeb.016915
Elbert A, Haas M, Springer B, Thielert W. y Nauen R. (2008). Applied aspects of neonicotinoid uses in crop protection. Pest Manag Sci,64(11):1099-105. https://doi.org/10.1002/ps.1616
European and Mediterranean Plant Protection Organization OEPP. (2010). Efficacy evaluation of plant protection products: Side-effects on honeybees. EPPO Bulletin, 40(3), 313–319. https://doi.org/10.1111/j.1365-2338.2010.02418.x
Felton, G. W. y Summers, C. B. (1995). Antioxidant systems in insects. Archives of Insect Biochemistry and Physiology, 29(2), 187–197. https://doi.org/10.1002/arch.940290208
Feyereisen, R. (2012). 8-Insect CYP Genes and P450 Enzymes. In L. I. Gilbert (Ed.), Insect Molecular Biology and Biochemistry (pp. 236–316). Elsevier. https://doi.org/10.1016/B978-0-12-384747-8.10008-X
Glaberman, S., White, K., Steeger, T., Carleton, J. y Winfield, S. (June 25, 2014). Environmental Fate and Ecological Risk Assessment for Foliar, Soil Drench, and Seed Treatment Uses of the New Insecticide Flupyradifurone (BYI 02960). https://www.regulations.gov/document/EPA-HQOPP-2013-0226-0047
Godfray, H. C. J., Blacquière, T., Field, L. M., Hails, R. S., Petrokofsky, G., Potts, S. G., Raine, N. E., Vanbergen, A. J. y McLean, A. R. (2014). A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators.Proceedings of the Royal Society B.281(1786).https://doi.org/10.1098/rspb.2014.0558
González-Varo, J. P., Biesmeijer, J. C., Bommarco, R., Potts, S. G., Schweiger, O., Smith, H. G., Steffan-Dewenter, I., Szentgyörgyi, H., Woyciechowski, M. y Vilà, M. (2013). Combined effects of global change pressures on animal mediated pollination. In Trends in Ecology and Evolution 28(9), (pp. 524–530). Elsevier Current Trends. https://doi.org/10.1016/j.tree.2013.05.008
Gregorc, A., Alburaki, M., Rinderer, N., Sampson, B., Knight, P. R., Karim, S. y Adamczyk, J. (2018). Effects of coumaphos and imidacloprid on honey bee (Hymenoptera: Apidae) lifespan and antioxidant gene regulations in laboratory experiments. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-33348-4
Gyõri, J., Farkas, A., Stolyar, O., Székács, A., Mörtl, M. y Vehovszky, Á. (2017). Inhibitory effects of four neonicotinoid active ingredients on acetylcholine esterase activity. Acta Biológica Hungarica, 68(4), 345–357. https://doi.org/10.1556/018.68.2017.4.1
Hernandez, J., Volland, A., Leyshon, B. J., Juda, M., Ridlon, J. M., Johnson, R. W. y Steelman, A. J. (2018). Effect of imidacloprid ingestion on immune responses to porcine reproductive and respiratory syndrome virus. ScIentIfIc RepoRts, 8(11615). https://doi.org/10.1038/s41598-018-30093-6
Hesselbach, H. y Scheiner, R. (2018). Effects of the novel pesticide flupyradifurone (Sivanto) on honeybee taste and cognition. Scientific Reports, 8(1), 1–8. https://doi.org/10.1038/s41598-018-23200-0
Hesselbach, H. y Scheiner, R. (2019). The novel pesticide flupyradifurone (Sivanto) affects honeybee motor abilities. Ecotoxicology, 28(3), 354–366. https://doi.org/10.1007/s10646-019-02028-y
Li, X., Schuler, M. A. y Berenbaum, M. R. (2007). Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annual Review Entomology, 52, 231–253. https://doi.org/10.1146/annurev.ento.51.110104.151104
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402-8. https://doi.org/10.1006/meth.2001.1262.PMID:11846609.
Macias, J. O., Tapia Gonzalez, J. M., Contreras Escareno, F., Guzman Novoa, E., Medina Flores, C. A. y De la Mora Peña, A. (2018). El efecto de los agroquimicos sobre las abejas meliferas (Apis mellifera) y su relación con el síndrome del colapso de las colonias. Universidad Michoacana de San Nicolás Hidalgo. Universidad de Zacatecas http://ricaxcan.uaz.edu.mx/jspui/handle/20.500.11845/974
Manjon, C., Troczka, B. J., Zaworra, M., Beadle, K., Randall, E., Hertlein, G., Singh, K. S., Zimmer, C. T., Homem, R. A., Lueke, B., Reid, R., Kor, L., Kohler, M., Benting, J., Williamson, M. S., Davies, T. G. E., Field, L. M., Bass, C. y Nauen, R. (2018). Unravelling the Molecular Determinants of Bee Sensitivity to Neonicotinoid Insecticides. Current Biology, 28(7), 1137-1143. https://doi.org/10.1016/j.cub.2018.02.045
Mao, W., Schuler, M. A. y Berenbaum, M. R. (2011). CYP9Qmediated detoxification of acaricides in the honey bee (Apis mellifera). Proceedings of the National Academy of Sciences of the United States of America, 108(31), 12657–12662. https://doi.org/10.1073/pnas.1109535108
Martínez-López, A., Lesher, J. y Jiménez-García, M. (2013). Comparación de tres métodos para la extracción de ARN total a partir de hojas de cacao. Biotecnología Vegetal, 13(2). Recuperado de https://revista.ibp.co.cu/index.php/BV/article/view/100
Moon, K., Lee, S. H. y Kim, Y. H. (2018). Evaluation of reference genes for quantitative real-time PCR to investigate seasonal and labor-specific expression profiles of the honey bee abdomen. Journal of Asia-Pacific Entomology, 21(4), 1350–1358. https://doi.org/10.1016/j.aspen.2018.10.014
Nauen, R., Jeschke, P., Velten, R., Beck, M. E., Ebbinghaus-Kintscher, U., Thielert, W., Wölfel, K., Haas, M., Kunz, K., & Raupach, G. (2015). Flupyradifurone: a brief profile of a new butenolide insecticide. Pest Management Science, 71(6), 850–862. https://doi.org/10.1002/ps.3932
Palmer, M. J., Moffat, C., Saranzewa, N., Harvey, J., Wright, G. A. y Connolly, C. N. (2013). Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nature Communications, 4(1), 1–8. https://doi.org/10.1038/ncomms2648
Parkinson, R. H., Zhang, S. y Gray, J. R. (2020). Neonicotinoid and sulfoximine pesticides differentially impair insect escape behavior and motion detection. Proceedings of the National Academy of Sciences of the United States of America, 117(10), 5510–5515. https://doi.org/10.1073/pnas.1916432117
Pham-Huy, L. A., He, H. y Pham-Huy, C. (2008). Free radicals, antioxidants in disease and health. In International Journal of Biomedical Science 4(2), pp. 89–96. Master Publishing Group. www.ijbs.org DOI: https://doi.org/10.59566/IJBS.2008.4089
Qi, S., Wang, D., Zhu, L., Teng, M., Wang, C., Xue, X. y Wu, L. (2018). Effects of a novel neonicotinoid insecticide cycloxaprid on earthworm, Eisenia fetida. Environmental Science and Pollution Research, 25(14), 14138–14147. https://doi.org/10.1007/s11356-018-1624-z
Quiroga-Murcia, D. E., Zotti, M. J., Zenner de Polanía, I. y Pech-Pech, E. E. (2017). Toxicity evaluation of two insecticides on Tetragonisca angustula and Scaptotrigona xanthotricha (Hymenoptera: Apidae). Agronomia Colombiana, 35(3), 340–349. https://doi.org/10.15446/agron.colomb.v35n3.65447
Rand, E. E. D., Smit, S., Beukes, M., Apostolides, Z., Pirk, C. W. W.y Nicolson, S. W. (2015). Detoxification mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Scientific Reports, 5(11779). https://doi.org/10.1038/srep11779
Scharlaken, B., de Graaf, D. C., Goossens, K., Brunain, M., Peelman, L. J. y Jacobs, F. J. (2008). Reference Gene Selection for Insect Expression Studies Using Quantitative Real-Time PCR: The Head of the Honeybee, Apis mellifera, After a Bacterial Challenge. Journal of Insect Science, 8(1), 1–10. https://doi.org/10.1673/031.008.3301
Shugart, L. y Theodorakis, C. (1994). Environmental genotoxicity: probing the underlying mechanisms. Environmental Health Perspectives, 102(Suppl. 12), 13–17. https://doi.org/10.1289/ehp.94102s1213
Stahl, B. A., Slocumb, M. E., Chaitin, H., DiAngelo, J. R. y Keene, A. C. (2017). Sleep-Dependent Modulation of Metabolic Rate in Drosophila. Sleep, 40(8). https://doi.org/10.1093/sleep/zsx084
Tan, K., Wang, C., Dong, S., Li, X. y Nieh, J. C. (2017). The pesticide flupyradifurone impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Scientific Reports, 7(1). https://doi.org/10.1038/s41598-017-18060-z
Tosi, S. y Nieh, J. C. (2019). Lethal and sublethal synergistic effects of a new systemic pesticide, flupyradifurone (Sivanto ®), on honeybees. Proceedings of the Royal Society B: Biological Sciences, 286(1900). https://doi.org/10.1098/rspb.2019.0433
Toutant, J. P. (1989). Insect acetylcholinesterase: Catalytic properties, tissue distribution and molecular forms. Progress in Neurobiology, 32(5), 423–446. https://doi.org/10.1016/0301-0082(89)90031-2
Verma, R. S., Mehta, A. y Srivastava, N. (2007). In vivo chlorpyrifos induced oxidative stress: Attenuation by antioxidant vitamins. Pesticide Biochemistry and Physiology, 88(2), 191–196. https://doi.org/10.1016/j.pestbp.2006.11.002
Williamson, S. M.y Wright, G. A. (2013). Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. Journal of Experimental Biology, 216(10), 1799–1807. https://doi.org/10.1242/jeb.083931
Zhou, X., Ma, C., Li, M., Sheng, C., Liu, H. y Qiu, X. (2010). CYP9A12 and CYP9A17 in the cotton bollworm, helicoverpa armigera: Sequence similarity, expression profile and xenobiotic response. Pest Management Science, 66(1), 65–73. https://doi.org/10.1002/ps.1832
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
1. La aceptación de manuscritos por parte de la revista implicará, además de su edición electrónica de acceso abierto bajo licencia Attribution-NonCommercial-ShareAlike 4.0 (CC BY NC SA), la inclusión y difusión del texto completo a través del repositorio institucional de la Universidad Nacional de Colombia y en todas aquellas bases de datos especializadas que el editor considere adecuadas para su indización con miras a incrementar la visibilidad de la revista.
2. Acta Biológica Colombiana permite a los autores archivar, descargar y compartir, la versión final publicada, así como las versiones pre-print y post-print incluyendo un encabezado con la referencia bibliográfica del articulo publicado.
3. Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
4. Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos institucionales, en su página web o en redes sociales cientificas como Academia, Researchgate; Mendelay) lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).



















