Tomato spotted wilt virus (TSWV), weeds and thrip vectors in the tomato (Solanum lycopersicum L.) in the Andean region of Cundinamarca (Colombia)
Keywords:
Frankliniella occidentalis, Thrips palmi, Thrips tabaci, weeds, Tospovirus. (es)Downloads
ABSTRACT
The presence and distribution of the TSWV, weeds and thrip vectors in major tomato producing areas in the Andean de- partment of Cundinamarca (Oriente, Sumapaz and Ubate provinces) were assessed with the DAS ELISA technique, evalu- ating the presence of the TSWV in tomato tissue, associated thrips and weeds. High incidences were observed in different provinces of the Andean department of Cundinamarca. The average viral incidence reached 23.3% in Sumapaz, 19.4% in Oriente and 4% in Ubate. The symptoms observed were: brown spots and concentric rings in the leaf area, stems and fruits; browning and spotting in the flower; and wilting in the leaves, stems and flowers. The thrip species with the highest presence were Frankliniella occidentalis, followed by Thrips palmi and Thrips tabaci. We determined the important role of weeds as inoculum sources and vector reservoirs for the species Emilia sonchifolia and Amaranthus dubius.
CROP PROTECTION
Tomato spotted wilt virus (TS WV), weeds and thrip vectors in the tomato (Solanum lycopersicum L.) in the Andean region of Cundinamarca (Colombia)
Tomato spotted wilt virus (TSWV), malezas y vectores de trips en el tomate (Solanum lycopersicum L.) en la región andina de Cundinamarca (Colombia)
Everth E. Ebratt R.1, Rocio Acosta A.2, Olga Y. Martínez B.3,Omar Guerrero G.4 and Walther Turizo A.5
1Tibaitatá Research Center, Instituto Colombiano Agropecuario (ICA). Mosquera (Colombia). everth.ebratt@ica.gov.co2Faculty of Sciences, Universidad de los Andes. Bogota (Colombia).
3Master of Science, Instituto Politécnico Nacional (CEPR OBI). Yautepec (Mexico).
4Agroecological Engineering Program, Corporación Universitaria Minuto de Dios. Bogota (Colombia).
5Department of Agronomy, Faculty of Agronomy, Universidad Nacional de Colombia. Bogota (Colombia).
Received for publication: 10 March, 2012. Accepted for publication: 29 March, 2013.
ABSTRACT
The presence and distribution of the TSWV, weeds and thrip vectors in major tomato producing areas in the Andean department of Cundinamarca (Oriente, Sumapaz and Ubate provinces) were assessed with the DAS ELISA technique, evaluating the presence of the TSWV in tomato tissue, associated thrips and weeds. High incidences were observed in different provinces of the Andean department of Cundinamarca. The average viral incidence reached 23.3% in Sumapaz, 19.4% in Oriente and 4% in Ubate. The symptoms observed were: brown spots and concentric rings in the leaf area, stems and fruits; browning and spotting in the flower; and wilting in the leaves, stems and flowers. The thrip species with the highest presence were Frankliniella occidentalis, followed by Thrips palmi and Thrips tabaci. We determined the important role of weeds as inoculum sources and vector reservoirs for the species Emilia sonchifolia and Amaranthus dubius.
Key words: Frankliniella occidentalis, Thrips palmi, Thrips tabaci, weeds, Tospovirus.
RESUMEN
La presencia y distribución de TSWV, arvenses y los trips vectores en las principales zonas productoras de tomate en la región andina del departamento de Cundinamarca (provincias de Oriente, Sumapaz y Ubaté), se confirmó mediante la técnica DAS ELISA , se evaluó la presencia del virus TSWV en tejido de tomate, arvenses y trips asociados. Se observaron incidencias altas en diferentes provincias de la región andina del departamento de Cundinamarca. La incidencia viral alcanzó promedios de 23,3% en el Sumapaz, el 19,4% en Oriente y el 4% en Ubaté. Los síntomas característicos observados correspondieron a manchas de color café y anillos concéntricos en el área foliar, tallos y frutos, bronceado, manchas en flor y marchitez en hojas, tallos y flores. Las especies de trips con mayor presencia fueron Frankliniella occidentalis, seguida de Thrips palmi y Thrips tabaci. Se determinó el importante papel de las arvenses como fuente de inóculo y reservorio de vectores en las especies Emilia sonchifolia y Amaranthus dubius.
Palabras clave: Frankliniella occidentalis, Thrips palmi, Thrips tabaci, arvenses, Tospovirus.
Introduction
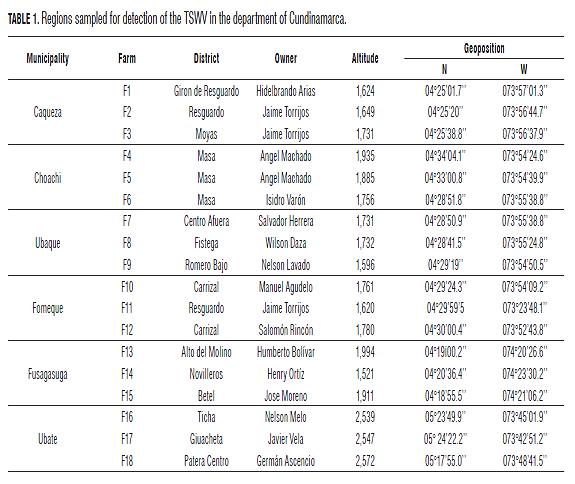
In Colombia, the tomato (Solanum lycopersicum L.) covers 11,304 ha with the principal participation in the departments of Norte de Santander, Boyacá, Antioquia, Santander and Cundinamarca, the latter of which has 600 ha planted, both in open fields and under cover (Encuesta Nacional Agropecuaria 2011; Agrocadenas, 2010).
Phytosanitary problems caused by viral agents are of interest because of the difficulty in diagnosis and the high handling costs. Among these is the Tomato spotted wilt virus (TSWV), which is a pathogen that, once it infects plants, cannot be controlled (Persley et al., 2007; Tamayo and Jaramillo, 2006). The TSWV belongs to the Bunyanviridae family and the Tospovirus genus and is widely distributed throughout the world in more than 500 species of ornamental, vegetable and fruit plants (Agrios, 2005), with losses estimated at 25 to 50% (Cho et al., 1998; Cho et al., 1998). In Colombia, the losses in ornamental crops due to the TSWV are above 70% and in the tomato, the incidence is reported at 20 to 30% (Corredor, 1999; Tamayo and Jaramillo, 2006; Rodríguez et al., 2008).
The dispersion of the TSWV is attributed to the complex plant, virus and insect vector interaction (Mound and Teulon, 1995) with an important role of thrips as vectors of the virus (Wijkamp et al., 1993; Corredor, 1999; Kritzman et al., 2002; Hogenhout et al., 2008); thrips transmit four virus groups corresponding to the genera Ilarvirus, Sobemovirus, Carmovirus and Tospovirus (Morse and Hoodle, 2006). Currently, 5,500 species of thrips are known and only 0.2% are reported as viral transmitters. According to Mound (1996, 2005) and Ohnishi et al. (2006), among the species involved, efficient vectors of Tospovirus include Frankliniella occidentalis, F. schultzei, F. intonsa, F. fusca, F. bispinosa, F. zucchini, F. cephalica, Thrips tabaci, Thrips palmi, T. setosus and Scirtothrips dorsalis (Nagata et al., 2004; Funderburk et al., 2007). At this time, of the 235 genera of the Thripinae subfamily and 1,700 valid species, only eleven species in three genera are reported as efficient Tospovirus vectors in the world (Mound, 1996; Mound and Teulon, 1996; Ohnishi et al., 2006).
In Colombia, the TSWV was detected in 1987 on the Sabana de Bogota in pompom plants (Chrysanthemum sp.), however, it was mistaken for other diseases and not until 1990 was the true causal agent determined by serological techniques (Angarita, 1995). Currently, the method used to diagnose infections with the TSWV is serological tests by the Enzyme-Linked Immuno-Sorbent Assay ELISA method, because of their convenience, speed, ease of use and efficiency, allowing the examination of a large number of samples in a short time (Gonsalves and Trujillo, 1986; Huguenot et al., 1990; Wang and Gonsalves, 1990; De Avila et al., 1993, De Avila et al., 1990).
Although TSWV detection is done with the DAS ELISA technique on plant material, Gonsalves and Trujillo (1986), Cho et al. (1988) and Nagata et al. (2002) modified this test to detect the TSWV in thrip individuals. In Colombia, this technique has been applied to ornamental plants, thrips, plants, weeds, and tomato crops (Vasquez, 1996; Corredor, 1999; Tamayo and Jaramillo, 2006, Rodríguez et al., 2008). The complex nature of the interaction between thrips, Tospoviruses and host plants was first recognized when it was discovered that the TSWV multiplies in vector insects with a persistent circulative-propagative type virus-thrip ratio (Ullman et al., 1993; Wijkamp et al., 1993; Hogenhout et al., 2008); however, Ullman (1996) put forth that infective, adult thrips are only produce when the acquisition of the virus occurs in the larval stages. Corredor (1999), showed that the minimum time required by F. occidentalis nymphs to ingest and obtain the TSWV was 5 min, increasing the likelihood of ingesting more virus particles and the efficient acquisition of the virus, extending the feeding time in infected leaves; and determined that the minimum latency time required by the TSWV in F. occidentalis nymphs was 8 d.
This study was undertaken to determine the distribution of the TSWV (Tomato spotted wilt virus) and associated thrips as vectors in the tomato (S. lycopersicum) in the Andean department of Cundinamarca; specifically to detect the presence and distribution of the virus in tomato-producing provinces, establishing viral prevalence, diagnosis and characterization of symptoms in respect to genetic material of field tomatoes and the presence of the virus in plants, weeds and thrips associated with the crop.
Materials and methods
This research was conducted at the C. I. Tibaitata of Instituto Colombiano Agropecuario (ICA-Corpoica), National Phytosanitary Laboratory Diagnosis, entomology area (04°41"46.7"" N, 74°12"12.8"" W and 2569 m a.s.l.). The field monitoring was conducted in three regions of the Cundinamarca department: Oriente province in the municipalities of Fomeque, Choachi, Ubaque and Caqueza; Sumapaz province in the municipality of Fusagasuga; and Ubate province in the municipality of Ubate (Tab.1). Three tomato producing farms were randomly selected in each municipality, for a total of 18. Sampling was conducted in each crop, with random sites in three rows, two lateral and one central to the planted area. In each row, five tomato plants were taken randomly, for a total of 15 plants per crop, observing the presence of viral symptoms, presence and capture of thrips and collecting associated weeds. The prevalence of the TSWV was determined by the number of plants with symptoms over the total number of sampled plants in each parcel, as according to Farooq and Akanda (2007).
Plant sample
The tomato sample consisted of two leaves from the upperthird of the plant, inflorescences and fruit, the latter of which was used only in the characterization of viral symptoms. Each sample was wrapped in paper towels and then in foil with the basic data of location and type of plant material planted. In the laboratory, the presence of the TSWV was determined with the DAS -ELISA technique with Agdia® (Elkhart, IN ) commercial kits and the recommended protocol: the plate was sensitized with the capture antibody (1:200), which was diluted in the buffer media (1X), 100 mL of the mixture were placed in each well and incubated overnight in a moist chamber at 4°C. Then the plate was washed with a cleaning buffer (1X PBST) three times. The samples were macerated in a general extraction buffer (1X GEB), one gram of the sample in 10 mL of extraction buffer 1X, 100 mL of the sample were added to each well, together with the respective reaction controls (positive, negative and blank ). Then, the plate was incubated overnight in a moist chamber at 4°C. After incubation, the plate was washed with a cleaning buffer 1X (1X PBST) seven times. The conjugated antibody with alkaline phosphatase (1:200) was diluted in the ECI-1X buffer, 100 mL of the enzyme conjugate-ECI 1X buffer mixture were added to each well, and the plate was incubated for two hours at room temperature. After incubation, the plate was washed with a cleaning buffer 1 X (1X PBST) eight times and 100 mL of the PNP solution were added to each well. The plate was incubated in a moist chamber in the dark for one hour and the measurements or readings were taken at an absorbance of 405 nm (A405), in a Opsys Mr-Dymex® (Richfield, MN) spectrophotometer, every 15 min.
Insect material
Thrip material was collected on each of the 18 farms with taps on the upper-third, leaves and inflorescences of the tomato plants, preferably symptomatic, over a flat surface with white cotton fabric and the help of a 20X magnifying glass and 000 brush, capturing and introducing the thrips into vials with 150 µL of extraction buffer or 75% ethyl alcohol. Identification of the thrips was carried out with the microassembly methodology proposed by Mound and Geoffrey (1998); genus and species were determined using the keys and descriptions proposed by Mound and Marullo (1996), Mound and Gillespie (1997), and EPP O (2002). The species identified were compared with literature reports on TSWV vectors according to Mound (2001), Murray (2001) and Kirk (2001); also the focal thrip species was selected according to the highest number of capture events, presence and efficiency as a TSWV vector, as according to Ullman (1993, 1996). For the detection of the TSWV in thrips, the DAS ELISA technique was implemented, modified for insect materials from the methodology described by Matsuura et al. (2002), Corredor (1999) and Sakurai (personal communication).
Thrip samples, like the plants, were visually analyzed spectrophotometrically at an absorbance of 405 nm (A405) in an OPSYS MR-DYMEX reader, using DAS -ELISA Agdia® protocol. For detection of TSWV in thrips, adult insects were used per sample. With this methodology, TSWV values greater than twice the average of the negative controls were considered positive, as according to Bautista et al. (1995), Sakurai et al. (2004) and Farooq and Akanda (2007).
Weed plant material
The identification of the weeds present in the crop was carried out by direct comparison with photographs of the proposed field manual by Cayon and Mendoza (1989). Of the weeds observed, the predominant species were selected as according to Lucena (1992) and Persley et al. (2007) and viral presence was determined by a DAS -ELISA test (Agdia®).
Results and discussion
TS WV in producing regions
The results indicated the presence of the TSWV in the Oriente province, in the municipalities of Caqueza, Choachi, Ubaque and Fomeque, with symptoms characterized by reduced plant growth, chlorosis and brown spots on leaves and stems; and brown, concentric rings on the surfaces of the fruits. In the province of Sumapaz in Fusagasuga, symptoms similar to those mentioned were observed, along with brown spots dispersed on the leaflets and ribs; brown, concentric rings across the entire surface of fruits and cracks in ripe tomatoes. In the province of Ubate, plants were observed with scattered brown spots on the leaf surface and concentric rings on the underside. According to Antignus et al. (1997), low plant development is characterized by little foliage; along with new shoots that are symptomatic and slow growing in flowers and fruits. Initially, the mid-leaves had dispersed, chlorotic spots before the appearance of dark, brown spots, as affirmed by Cho et al. (1989) and Farooq and Akanda (2007), characterizing these symptoms as initial chlorosis in leaves and shoots which can lead to browning and necrosis.
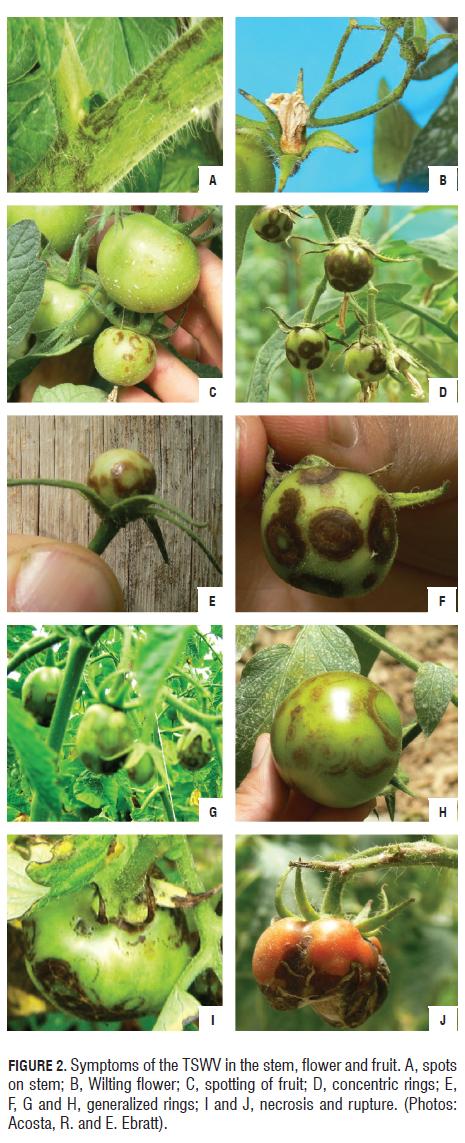
The most representative symptom corresponded to spots in the form of concentric rings as described by Adkins (2005), Sang-Hoon et al. (2005), Persley et al. (2007), and Farooq and Akanda (2007). These symptoms began at the base of the leaflets and spread towards the apex, or clustered in isolated patches (Fig.1A). The rings were clearly observed on the underside of the affected leaves (Fig.1B), the spots may appear scattered throughout the leaves (Fig.1C) or concentrated towards the ribs (Fig.1D) and wilted and crinkled leaves (Fig.1 E and F ).
The young fruits showed irregular spots which may be poorly marked (Fig.2); no ripe fruit was found with characteristics of irregular, yellow or orange mottling, as opposed to Cho et al. (1989), Morris (2004) and Persley et al. (2007); however, it was seen that infected, ripe fruit showed necrosis (Fig.2J), which even led to tissue breakdown. In some cases, according to Agrios (2005) and Persley et al. (2007), brown, concentric rings that start near the stem or particularly scattered throughout the fruit are seen (Fig.2C). The stems presented dark brown spots, some dispersed in the form of rings and others grouped together to form a general spot (Fig.2). The DAS ELISA technique (Agdia®) was adequate for detecting the TSWV in tomato plants and weeds because of its sensitivity and speed of viral localization according to Gonsalves and Trujillo (1986), Okazaki et al. (2007), Sherwood et al. (1989), Huguenot et al. (1990), and Wang and Gonsalves (1990). The analysis of the samples, using this technique, allowed for qualitative results which indicated the presence or absence of the TSWV in the plant material analyzed (Tab.2).
Prevalence of the TS WV in the tomato
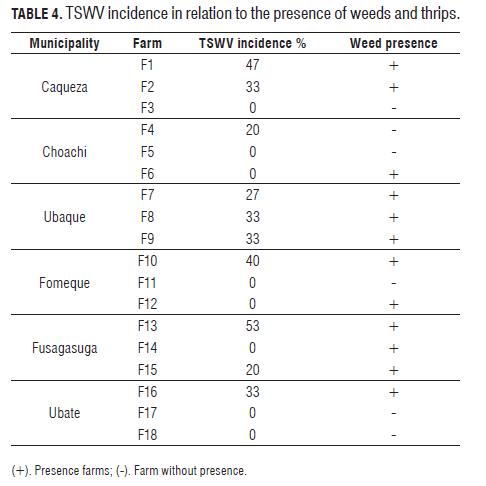
The 18 farms evaluated presented ranges of from 20 to 53% for incidence of the TSWV; at least one property in each municipality presented viral incidence (Fig.3). Thrip populations in each culture were found to average 1.3 individuals per plant, representing a low level of infestation for insect pests, however, there were relatively high incidences of the TSWV, possibly due to mechanical propagation according to Ullman (1996, 1993); however, low levels of thrips (0.1thrips/plant) have the ability to propagate the TSWV to the host when the virus pressure is high, according to Bennison et al. (2002). It was also determined that 44.4% of the crops sampled had a TSWV incidence of zero, possibly due to the control measures aimed at thrips and weed control, which could directly influence the reduction of the spread of the TSWV, as indicated by Momol et al. (2002).
Presence of the TS WV in cultivated tomato materials
Tomato materials grown in the Andean region of Cundinamarca were represented, by percentage, by the varieties and hybrids: "Calima" (27.7%), "Daniela" (16.6%), "Sheila" (11.1 %), "Granitio" (11.1%), "Alboran" (5.5%), "Kyndio" (5.5%), "Rocio" (5.5%), "Santa Clara" (5, 5%), "Yola" (5.5%) and "Rolin" (5.5%) (Tab.3). According to the technical specifications of the materials planted, they have no resistance to the TSWV, allowing high incidences and possibly facilitating viral dispersion in the presence of thrip vectors and weeds in crop areas.
The 70% of the material analyzed presented a TSWV infection, and even higher in cultures with a high thrip infestation and presence of weeds (Tab.4), this confirms the findings of Persley et al. (2007), regarding the use of resistant varieties as an effective control method for managing the TSWV. In Colombia, three hybrids are currently marketed as TSWV resistant, but they are little used in the producing regions, possibly due to their high costs and adaptability to growing conditions, moreover, they do not have adequate commercial dissemination. Mitidieri et al. (1996) stated that the use of commercial hybrids with resistance to the TSWV is meant as a phytosanitary measure to reduce the incidence of this disease, in combination with other cultural, biological and chemical management practices that target vectors and weeds.
TS WV weed plants
The results showed that farms without the presence of weeds (F3, F4, F17 and F18) (Tab.4) showed low to no viral impact except farm F4, which could have presented an early infection from nursery seedlings; while farms with plenty of weeds (F1, F6, F7, F9, F10, F11, F12, F14 and F15) reached incidence of viral levels of 20 and 53%. According to Duffus (1971), the epidemiology of viral illness may be a phenomenon that involves the interaction of cultures, insect vectors and host weeds as sources of inoculum and vector hosts.
Prevalence of the TS WV in relation to weeds and thrips
In the 14 farms with weeds and thrips (Tab.4), the species recognized were: red spinach (Amaranthus dubius), guasca (Galinsoga parviflora) and lilac tasselflower (Emilia sonchifolia), all reported as hosts of the TSWV by Lucena (1992), and Amaranthus sp. a thrip host plant according to Persley et al. (2007). Of these weeds, 21.4% of the samples analyzed demonstrated a positive response to the TSWV with the DAS ELISA test (Agdia®). The presence of weeds and thrips in 77.7% of the sampled tomato crops and the presentation in 71.4% of symptoms characteristic of the TSWV indicate that the poor management of these plants allows for the spread of the disease by acting as viral reservoirs (Duffus, 1971; Tamayo and Jaramillo, 2006), this is consistent with that proposed by Okazaki et al. (2007), who argued that thrips and weeds play an important role in the cycles of TSWV infection in cultivated fields.
This study found that the presence of the TSWV is positively correlated with the presence of weeds (r = 0.6740, P = 0.002, n = 54). There is also a positive correlation between the presence of thrips with the presence of weeds (r = 0.4725, P = 0.048, N = 54), but there was no correlation between the presence of the TSWV and thrip vectors (r = 0.3636, P = 0,138, n = 54), making them occasional transmitters of the TSWV according to Nagata et al. (2004), who found a low probability of finding viruliferous thrips and, due to the host conditions, that they prefer weeds associated with the crop. This suggests that for TSWV management, weed eradication could be the first step and possibly the most effective way to break the cycle of TSWV infection, as framed within an integrated Tospovirus and thrip management program in tomato crops.
Identification of thrip species
Of the individuals collected in the tomato plants and weeds, three predominant species were identified: Frankliniella occidentalis, dark and pale forms, Thrips palmi and Thrips tabaci (Fig.4). It was determined that F. occidentalis was found in 100% of the capture events, followed by T. tabaci at 88% and T. palmi at 55% (Tab.5).
Detection of the TS WV in F. occidentalis
In the detection of the TSWV with the DAS ELISA test, the adult stage of F. occidentalis was chosen because of their continued presence in crops and role as a more efficient vector of the TSWV, as reported by Ullman (1996) and Okazaki et al. (2007). In this work the validity of the methodology used for the detection of viral particles in the insect vector was determined. This coincides with that proposed by Cho et al. (1988), Wijkamp et al. (1993), Bautista et al. (1995), Matsuura et al. (2002) and Sakurai (2004), who endorsed the use of the test in different species of thrips.
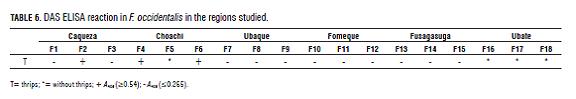
In this study, samples of adult F. occidentalis were positive for the TSWV at 16.6%, in contrast to the average viral prevalence in tomato plants of from 18.83 to 55.55% on the sampled farms (Tab.2; Tab.6).
These results agree with those of Maris et al. (2004), reporting a proportional ratio of thrip populations with regard to the presence of the TSWV; however, in the present study, there was no correlation between the TSWV infected plants and the presence of thrips, possibly due to the low probability of the presence of infective insect vectors of TSWV (21.4%) in the sample, according to Nagata et al. (2004), who identified a TSWV transmission efficiency by F. occidentalis of 31.6%. Medeiros et al. (2004) and van de Wetering et al. (1996) observed that insects collected in symptomatic tomato plants tested negative for the presence of the TSWV as an autoimmune response of the adult stage of the insect to the viral infection prevents detection. Currently, it is known that adult thrips carrying viral particles only acquire and infect the TSWV during the development of immature stages in affected plants (EPP O, 2002; Ullman, 1996), making them economically important insects that are difficult to manage with implementation of unilateral chemical control tactics in complete ignorance of the insectvirus- weed relationship.
Although this study did not consider or evaluate the role of colored forms, especially the dark form of F. occidentalis, as possibly more efficient transmitters of the TSWV, since there is evidences from Sakimura (1969) that the dark forms of several species of thrips are infective vectors responsible for the high prevalence of the TSWV in several crop species.
Conclusions
The disease known as "tomato spotted wilt" or "Black Death" is related to the presence of the TSWV in weeds and the thrip vectors F. occidentalis, T. palmi and T. tabaci associated with tomato crops in the Andean region of the Cundinamarca department.
The presence of asymptomatic weeds that are positive for the TSWV and which are associated with the crop confirms their role as viral reservoirs and hosts for thrip vectors. This raises the imminent need for managing weed plants associated with crops, as they are a key element of high phytosanitary risk in the production of the tomato S. lycopersicum L.
Acknowledgements
The authors wish to thank the Instituto Colombiano Agropecuario (ICA), Seccional Cundinamarca; Dr. Jorge Evelio Ángel Díaz; Corporación Universitaria Minuto de Dios (Uniminuto); Dr. Luis F. Salazar; to tomato producers of the municipalities of Caqueza, Choachi, Ubaque, Fomeque, Fusagasuga y Ubate; to Jaime Torrijos official of the Instituto Colombiano Agropecuario and officials the municipals Secretarias de Agricultura.
Literature cited
Agrios, G. 2005. Plant pathology. 5th ed. Elsevier Academic Press, Burlington, MA.
Agrocadenas. 2010. Acuerdo de competitividad de la cadena de hortalizas. In: http://www.google.com.co/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0CDAQFjAA&url=http%3A%2F%2Fwww.sich.unal.edu.co%2Fsich%2Fdatos_publicos%2Facuerdo_competitividad_hortalizas.pdf&ei=9Kl2UY fEJq7x0wGo8IGIBQ&usg=AFQjCNGSxlWnOm2aiJODxR1v0Q8vwGwXUQ&bvm=bv.45512109,d.dmQ&cad=rja; consulted: March, 2013.
Angarita, A. 1995. Síntomas, detección y control de virus y viroides en crisantemo. p. 114. In: Memorias II simposio nacional de crisantemo, plagas y enfermedades. Asocolflores, Rionegro, Colombia.
Antignus, Y, M. Lapidot, N. Ganaim, J. Cohen, O. Lachman, M. Pearlsman, B. Raccah, and A. Gera. 1997. Biological and molecular characterization of Tomato spotted wilt virus in Israel. Phytoparasitica 25(4), 319-330.
Bautista, R., R. Mau, J. Cho, and D. Custer. 1995. Potencial of Tomato spotted wilt virus Tospovirus plant host in Hawai as reservoirs for transmisión by Frankliniella occidentalis (Thysanoptera: Thripidae). Phytopathology 85(9), 953-958.
Bennison, J., K. Maulden, I. Barker, J. Morris, N. Boonham, P. Smith, and N. Spence. 2002. Reducing spread of TSWV on ornamentals by biological control of western flower thrips. pp. 157-162. In: Proceedings of the seventh International Symposium on Thysanoptera, Reggio Calabria.
Cayon, D. and A. Mendoza. 1989. Manual de semillas de malezas. Convenio ICA; SENA , Ibagué, Colombia.
Cho, J., R. Mau, T. German, R. Hartmann, L. Yudin, D. Gonsalves, and R. Prowldentl. 1989. A multidisciplinary approach to management of Tomato spotted wilt virus in Hawaii. Plant Dis. 73(5), 375-383.
Cho, J., R. Mau, R. Hamssak, and D. Gonsalves. 1988. Detection of Tomato spotted wilt virus in individual thrips by enzymelinked immunosorbent assay. Phytopathology 78(10), 1348-1351.
Cho, J., R. Mau, S. Pang, M. Wang, C. Gonsalves, J. Watterson, D. Custer, and D. Gonsalves. 1998. Approaches for controlling Tomato spotted wilt virus. pp. 547-564. In: Hadidi, A., R.K. Khetarpal, and H. Koganezawa (eds.). Plant virus disease control. APS Press, St. Paul, MN.
Corredor, C. 1999. Determinación de las principales características de la transmisión biológica del virus TSWV vía el vector Frankliniella occidentalis. M.Sc. thesis. Faculty of Agronomy, Universidad Nacional de Colombia, Bogota.
De Avila, A., P. De Haan, R. Kormelink, R. Resende, R. Goldbach, and D. Peters. 1993. Classification of Tospoviruses based on phylogeny of nucleoprotein. J. Gen. Virol. 74, 153-159.
De Avila, A.C., C. Huuenot, R. Resende, E. Kitajima, R. Goldbach, and D. Peters. 1990. Serological differentiation of 20 isolates of Tomato spotted wilt virus. J. Gen. Virol. 71, 2801-2807.
Duffus, J. 1971. Role of weeds in the incidence of virus diseases. Ann. Rev. Phytopathol. 9, 319-340.
Encuesta Nacional Agropecuaria. 2011. Oferta agropecuaria. Cifras. In: Ministerio de Agricultura Desarrollo Rural, http://www.agronet.gov.co/htm3b/public/ENA /ENA _2010.pdf; consulted: March, 2013.
EPP O, European and Mediterranean Plant Protection Organization. 2002. Diagnostic protocols for regulated pests: Frankliniella occidentalis. Bull. EPP O 32, 241-243.
Farooq, A. and A.M. Akanda. 2007. Symptoms and prevalence of Tomato spotted wilt virus (TSWV) infection in Bangladesh. Intl. J. Sustain. Crop Prod. 2(5), 51-58.
Funderburk, J., S. Diffie, J. Sharma, A. Hodges, and L. Osborne. 2007. Thrips of ornamentals in the Southeastern US . ENY -845 (IN 754). Entomology & Nematology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, Gainesville, FL.
Gonsalves, D. and E. Trujillo. 1986. Tomato spotted wilt virus in papaya and detection of the virus by ELISA . Plant Dis. 70(6), 501-506.
Hogenhout, S.A., El-D. Ammar, A.E. Whitfield, and M.G. Redinbaugh. 2008. Insect vector interactions with persistently transmitted viruses. Ann. Rev. Phytopathol. 46, 327-359.
Huguenot, C., G. Van Den Dobbelsteen, P. de Haan, C.A. Wagemakers, G.A. Drost, A.D.M.E. Osterhaus, and D. Peters. 1990. Detection of Tomato spotted wilt virus using monoclonal antibodies and riboprobes. Arch. Virol. 110, 47-62.
Kirk, W.D.J. 2001. The pest and vector from the west: Frankliniella occidentalis. pp. 33-42. In: Proceedings of the 7th International Symposium on Thysanoptera, Australian National Insect Collection; CSIR O, Canberra, Australia.
Kritzman, A., A. Gera, B. Raccah, J. Lent, and D. Peters. 2002. The route of Tomato spotted wilt virus inside the thrips body in relation to transmission efficiency. Arch. Virol. 147, 2143-2156.
Lucena, E. 1992. Estudio de la detección del marchitamiento moteado del tomate (TSWV) en el cultivo del pompón (Chrysantemun morifolium), en malezas y trips asociados. M.Sc. thesis. Faculty of Agronomy, Universidad Nacional de Colombia, Bogota.
Maris, P., N. Joosten, R. Goldbach, and D. Peters. 2004. Tomato spotted wilt virus infection improves host suitability for its vector Frankliniella occidentalis. Phytopathology 94(7), 706-711.
Matsuura, S., S. Hoshino, H. Hayashi, T. Kohguchi, K. Hagiwara, and T. Omura. 2002. Effects of latent infection of stock plants and abundance of thrips on the occurrence of tomato spotted wilt virus in Chrysantemun fields. J. Gen. Plant Pathol. 68, 99-102.
Medeiros, R., R. Resende, and A. De Ávila. 2004. The plant virus Tomato Spotted Wilt Tospovirus activates the immune system of its main insect vector, Frankliniella occidentalis. J. Virol. 78(10), 4976-4982.
Mitidieri, M., I. De Mitidieri, and E. DalBó. 1996. Evaluation of tomato hybrids resistant to TSWV under greenhouse conditions in Argentina. Acta Hort. 559, 775-779.
Momol, M.T., J. Funderburk, S. Olson, and J. Stavisky. 2002. Management of tomato Spotted wilt Tospovirus (TSWV) on tomatoes with UV-reflective mulch and Acibenzolar-S-methyl. pp. 111-116. In: Proceedings of the 7th International Symposium on Thysanoptera, Australian National Insect Collection, Canberra, Australia.
Morris, J. 2004. Diagnostic protocol for the Tospoviruses Tomato spotted wilt virus (TSWV), Impatiens necrotic spot virus (INS V) and Watermelon silver mottle virus (WSMoV). Protocol for the diagnosis of quarantine organisms. Bolletin OEPP / EPP O 34, 271-279.
Morse, J. and M. Hoddle. 2006. Invasion biology of thrips. Annu. Rev. Entomol. 51, 67-89.
Mound, L. 2005. Plants, thrips, Tospoviruses the enigmatic triad. In: VIII International Symposium on Thysanoptera and Tospoviruses. CSIR O Entomology, Canberra, Australia.
Mound, L. and K. Geoffrey. 1998. Thisanoptera an identification guide. CAB International, Wallingford, The Netherlands. Mound, L. and P. Gillespie. 1997. Identification guide to thrips associated with crops in Australia. NSW Agriculture, Orange & CSIR O Entomology, Canberra, Australia.
Mound, L.A. 1996. The Thysanoptera vector species of Tospoviruses. Acta Hort. 431, 298-306.
Mound, L. and R. Marullo. 1996. The thrips of central and South America an Introduction (Insecta: Thysanoptera). Mem. Entomol. Intl. 6, 487.
Mound, L. and D. Teulon. 1995. Thysanoptera as phytophagous opportunists. pp. 3-20. In: Parker, B.L., M. Skinner, and T. Lewis (eds.). Thrips biology and management. Plenum, New York, NY .
Murray, T. 2001. The pest and vector from the East: Thrips palmi. pp. 19-32. In: Proceedings of the 7th International Symposium on Thysanoptera, Australian National Insect Collection, Canberra, Australia.
Nagata, T., A. Almeida, R. Resende, and A. De Ávila. 2004. The competence of four thrips species to transmit and replicate four Tospoviruses. Plant Pathol. 53, 136-140.
Nagata, T., A. Inoue-Nagata, J. Lent, R. Goldbach, and D. Peters. 2002. Factors determining vector competence and specificity for transmission of Tomato spotted wilt virus. J. Gen. Virol. 83, 663-671.
Ohnishi, J., H. Katsuzaki, and S. Tsuda. 2006. Frankliniella cephalica, a new vector for Tomato spotted wilt virus. Plant Dis. 90(5), 685.
Okazaki, S., M. Okuda, K. Komi, H. Yoshimatsu, and T. Iwanami. 2007. Overwintering viruliferous Frankliniella occidentalis (Thysanoptera: Thripidae) as an infection source of Tomato spotted wilt virus in green pepper fields. Plant Dis. 91, 842-84.
Persley, D., M. Sharman, J. Thomas, L Kay, S. Heisswolf, and L. Michael. 2007. Thrips and Tospoviruses. A management guide. Department of Primary Industries and Fisheries. Queensland, Australia.
Rodríguez, M., N .Niño, C. Carranza, O. Lanchero, D. Miranda, and S. Magnitskiy. 2008. Diseminación de virus y patógenos de suelo en las principales áreas productoras de tomate (Solanum lycopersicum L.) de los municipios de Fómeque, Fusagasugá Y Villa de Leyva (Colombia). In: Book of abstract, Simposio Internacional de Tomate en el Trópico. Sociedad Colombiana de Ciencias Hortícolas, Villa de Leiva, Colombia.
Sakimura, K. 1969. Frankliniella occidentalis (Thysanoptera: Thripidae), a vector of the Tomato spotted wilt virus, with special reference to the color forms. Ann. Entomol. Soc. Amer. 55, 387-89.
Sakurai, T. 2004. Transmission of Tomato spotted wilt virus by de dark form of Frankliniella schultzei (Thysanoptera: Thripidae) originating in tomato fields in Paraguay. Appl. Entomol. Zool. 39(1), 189-194.
Sang-Hoon, S., B. Mcnulty, G. Kennedy, and J. Moyer. 2005. Viral genetic determinants for thrips transmission of Tomato spotted wilt virus. PNAS 102(14), 5168-5173.
Sherwood, J.L., M.R. Sanborn, G.C. Keyser, and L.D. Myers. 1989. Use of monoclonal antibodies in detection of tomato spotted wilt virus. Phytopathology 79, 61-64.
Tamayo, P. and P. Jaramillo. 2006. Enfermedades del tomate, pimentón, ají y berenjena en Colombia guía para su diagnóstico y manejo. Corpoica, Rionegro, Colombia.
Ullman, D.E. 1996. Thrips and Tospoviruses: advances and future directions. Acta Hort. 431, 310-332.
Ullman, D., T. German, J. Sherwood, D. Westcot, and F. Cantone. 1993. Tospovirus replication in insect vector cells: Immunocytochemical evidence that the nonstructural protein encoded by the S RNA of Tomato spotted wilt Tospovirus is present in thrips vector cells. Phytopathology 83, 456-463.
Van De Wetering, F., R. Golbach, and D. Peters. 1996. Tomato spotted wilt virus ingestion by first instar larvae of Frankliniella occidentalis is a prerequisite for transmission. Phytopathology 86(9), 900-905.
Vásquez, S. 1996. Purificación, producción de un antisuero policlonal y evaluación de la técnica de ELISA indirecto para la detección del virus del marchitamiento moteado del tomate (TSWV) y virus de la mancha necrótica del impatiens (INS V) en el cultivo del crisantemo. M.Sc. thesis. Faculty of Agronomy, Universidad Nacional de Colombia, Bogota.
Wang, M. and D. Gonsalves. 1990. ELISA detection of various Tomato spotted wilt virus isolates using specific antisera to structural proteins of the virus. Plant Dis. 75, 154-158.
Wijkamp, I., J. Lent, R. Kormelink, R. Goldbach, and D. Peters. 1993. Multiplication of Tomato spotted wilt virus in its insect vector, Frankliniella occidentalis. J. Gen. Virol. 74, 341-349.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
Article abstract page views
Downloads
License
Copyright (c) 2013 Agronomía Colombiana

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
© Centro Editorial de la Facultad de Ciencias Agrarias, Universidad Nacional de Colombia
Reproduction and quotation of material appearing in the journal is authorized provided the following are explicitly indicated: journal name, author(s) name, year, volume, issue and pages of the source. The ideas and observations recorded by the authors are their own and do not necessarily represent the views and policies of the Universidad Nacional de Colombia. Mention of products or commercial firms in the journal does not constitute a recommendation or endorsement on the part of the Universidad Nacional de Colombia; furthermore, the use of such products should comply with the product label recommendations.
The Creative Commons license used by Agronomia Colombiana journal is: Attribution - NonCommercial - ShareAlike (by-nc-sa)

Agronomia Colombiana by Centro Editorial of Facultad de Ciencias Agrarias, Universidad Nacional de Colombia is licensed under a Creative Commons Reconocimiento-NoComercial-CompartirIgual 4.0 Internacional License.
Creado a partir de la obra en http://revistas.unal.edu.co/index.php/agrocol/.