Impacts of different coffee systems on soil microbial populations at different altitudes in Villavicencio (Colombia)
Impactos de diferentes sistemas de café sobre las poblaciones microbiales del suelo a diferentes altitudes en Villavicencio (Colombia)
DOI:
https://doi.org/10.15446/agron.colomb.v34n2.55420Keywords:
Actinomycete, bacteria, Coffea arabica L., fungi, soil quality, soil diversity. (en)Actinomiceto, bacteria, calidad del suelo, Coffea arabica L., hongo, diversidad del suelo (es)
Downloads
El café en Colombia presenta favorables características para prácticas agroforestales, los agricultores tradicionalmente cultivan el café bajo diferentes tipos de sistemas de café orgánico, principalmente dominados por sistemas de café asociado bajo sombra con especies de árboles tipo leguminoso, siendo uno de los ecosistemas importantes para la vida de microorganismos. Esta investigación fue desarrollada en la región cafetera de Puente Abadía, Villavicencio, Colombia. El objetivo fue evaluar la población de organismos heterotróficos aeróbicos en Coffea arábica var. Castilla de bacterias (BP), hongos (FP) y actinomicetos (AP), debido al efecto combinado de tres factores. El factor A fue relacionado con dos zonas bioclimáticas: zona de baja altitud < 700 msnm (Z1) y zona de alta altitud >700 msnm (Z2); el factor B correspondió a dos sistemas de café siendo que fue sistema de monocultivo sin sombra (S1) y sistema asociado con sombra (S2), y un tratamiento control de sistema de frutales (S3). Por otra parte, el factor C fue referido a dos profundidades de muestreo, correspondiendo a una profundidad de 10-20 cm (D1) y a una profundidad de 20 a 30 cm (D2). Diferencias significativas (P≤0.05) entre la interacción de factores Z2*S2*D2, causó las más altas de BP, pero también de AP; a su vez la variable actinomicetos también fue positivamente influenciada por las interacciones Z2*S2*D1, Z1*S2*D2 y Z1*S2*D1 (P≤0.05). Las poblaciones de hongos (FP) fueron afectados positivamente por las interacciones Z1*S2*D2 y Z1*S2*D1. El sistema con sombra (S2) tuvo ventajas comparativas sobre la población de microorganismos del suelo comparado con S1 y S3.
Doi: https://doi.org/10.15446/agron.colomb.v34n2.55420
Impacts of different coffee systems on soil microbial populations at different altitudes in Villavicencio (Colombia)
Impactos de diferentes sistemas de café sobre las poblaciones microbiales del suelo a diferentes altitudes en Villavicencio (Colombia)
Carlos Hernando Colmenares P1, Amanda Silva P1, and Ángela María Mogollón O.1
1 Grupo de Investigación "Innovación en Sistemas Agrícolas y Forestales ISAF", Faculty of Agricultural Sciences and Natural Resources, Universidad de los Llanos (Unillanos). Villavicencio (Colombia). asilvap@unillanos.edu.co
Received for publication: 27 January, 2016. Accepted for publication: 30 June, 2016.
ABSTRACT
Coffee in Colombia has favorable characteristics for agroforestry practices; farmers traditionally grow coffee under various types of organic coffee systems, mainly dominated by associated systems coffee with shade leguminous tree species, making it one of the essential ecosystems for microorganisms life. This research was developed in the coffee region of Puente Abadia, Villavicencio, Colombia; the objective was to evaluate the heterotrophic aerobic microbial population in Coffea arábica var. Castilla of bacteria (BP), fungi (FP) and actinomycetes (AP), due to the combined effect of three factors. Factor A was related to two bioclimates zones: Low altitude zone <700 m a.s.l. (Z1) and high altitude zone >700 m a.s.l. (Z2); factor B corresponded to two coffee systems: coffee monoculture system (unshaded) (S1), associated coffee system (shaded) (S2) and control treatment of fruit monoculture system (S3). On the other hand, factor C referred to two sampling depth, corresponding to a 0-20 cm depth (D1) and 20-30 cm depth (D2). Significant differences (P≤0.05) between the Z2*S2*D2 factor interaction caused the highest (BP) and (AP); (AP) also was positively influenced by the Z2*S2*D1, Z1*S2*D2 and Z1*S2*D1 interactions (P≤0.05). The (FP) was affected positively by the Z1*S2*D2 and Z1*S2*D1 interactions. The (S2) (shade) had comparative advantages for the soil microbial population, as compared with (S1) and (S3).
Key words: Actinomycete, bacteria, Coffea arabica L., fungi, soil quality, soil diversity.
RESUMEN
El café en Colombia presenta favorables características para prácticas agroforestales, los agricultores tradicionalmente cultivan el café bajo diferentes tipos de sistemas de café orgánico, principalmente dominados por sistemas de café asociado bajo sombra con especies de árboles tipo leguminoso, siendo uno de los ecosistemas importantes para la vida de microorganismos. Esta investigación fue desarrollada en la región cafetera de Puente Abadía, Villavicencio, Colombia. El objetivo fue evaluar la población de organismos heterotróficos aeróbicos en Coffea arábica var. Castilla de bacterias (BP), hongos (FP) y actinomicetos (AP), debido al efecto combinado de tres factores. El factor A fue relacionado con dos zonas bioclimáticas: zona de baja altitud < 700 msnm (Z1) y zona de alta altitud >700 msnm (Z2); el factor B correspondió a dos sistemas de café siendo que fue sistema de monocultivo sin sombra (S1) y sistema asociado con sombra (S2), y un tratamiento control de sistema de frutales (S3). Por otra parte, el factor C fue referido a dos profundidades de muestreo, correspondiendo a una profundidad de 10-20 cm (D1) y a una profundidad de 20 a 30 cm (D2). Diferencias significativas (P≤0.05) entre la interacción de factores Z2*S2*D2, causó las más altas de BP, pero también de AP; a su vez la variable actinomicetos también fue positivamente influenciada por las interacciones Z2*S2*D1, Z1*S2*D2 y Z1*S2*D1 (P≤0.05). Las poblaciones de hongos (FP) fueron afectados positivamente por las interacciones Z1*S2*D2 y Z1*S2*D1. El sistema con sombra (S2) tuvo ventajas comparativas sobre la población de microorganismos del suelo comparado con S1 y S3.
Palabras clave: Actinomiceto, bacteria, calidad del suelo, Coffea arabica L., hongo, diversidad del suelo.
Introduction
Worldwide, coffee systems are important natural resources that need to be preserved (Souza et al., 2012; Evizal et al., 2012; Srivastava et al., 2014; Cardona and Sadeghiankh, 2005).
Soil microorganisms control the functioning of coffee ecosystems through decomposition and nutrient cycling, which in turn play a vital role in microbial conversion of complex organic compounds into simple inorganic compounds (Evizal et al., 2012; Nigussie and Kissi, 2012), so it could improve its productive capacity (Haggar et al., 2011; Evizal et al., 2012; Bagyaraj et al, 2015; Salgado et al., 2006).
It has been found that the bioclimatic characteristics of a region can influence the dynamics of soil microorganisms (Criquet et al., 2004; Srivastava et al., 2014). Generally, bacteria are the dominant group followed by actinomycetes and fungi are the least dominant among the three groups of microorganisms (Bagyaraj et al., 2015). Srivastava et al. (2014) reported higher microbial populations in a sub-humid (moist) zone than in subhumid (dry) and semi-arid zones.
On the other hand, depending on the coffee systems, the type can result in different substrate availabilities that affect the establishment of different microbial groups. Studies have revealed that the agroforestry coffee systems are more effective in promoting soil microbial population than conventional monoculture (unshaded) coffee systems (Evizal et al., 2012; Notaro et al., 2014; Bagyaraj et al, 2015).
The denomination of associated coffee production mainly involves agroforestry based systems, where coffee grows beneath various shade trees (mainly tree legumes), and are well suited for sustainable production compared with conventional (unshaded) coffee monoculture systems (Salgado et al., 2006; Haggar et al., 2011; Nigussie and Kissi, 2012; Bagyaraj et al., 2015).
Agroforestry coffee systems can increase microbial populations and soil nutrient availability that are characterized by a complex and diverse interrelationship among and between the microorganisms (Evizal et al., 2012; Souza et al., 2012); affecting directly the beneficial soil populations of fungi, bacteria and actinomycetes (Beer et al., 1998).
The result of the vertical distribution of bacteria, fungi and actinomycetes in coffee systems was observed by Notaro et al. (2014). The surface soils (0-30 cm) recorded higher bacteria (41.3%), actinomycetes (38.4%) and fungi (26.1%) as compared to the subsurface horizons (Srivastava et al., 2014).
Understanding that worldwide the coffee ecosystems need to be preserved, the aim of this study was to research the microbial population of bacteria (BP), fungi (FP) and actinomycete (AP) that can be explained by a combination of coffee system types, soil and climatic factors in Puente Abadia-Villavicencio (Colombia).
Methodology
The study site was in the Puente Abadia region, which is part of Villavicencio (4°08'33" N, 73°37'46" W), Colombia. The area is a tropical rainforest (Holdridge, 1947). The research factors were as follows: Two bioclimatic zones were defined with cartography in Puente Abadia, Villavicencio municipality corresponding to factor A.
Low zone of Puente Abadia (Z1): This area is characterized by an altitude of < 700 m a.s.l., temperature >24°C, humidity of 76%, average annual rainfall of 2,080 mm, located on the alluvial plain of the Guaitiquia River, which is less susceptible to erosion problems.
High zone of Puente Abadia (Z2): This area is characterized by an altitude > 700 m a.s.l., temperature 18-20°C, humidity of 85%, average annual rainfall of 3,663 mm, slopes greater than 30% and high susceptibility to erosion, soils characterized by layers of silt alluvium.
Two types of coffee production used in organic coffee producing systems, monoculture coffee system (40%) and associated coffee system (60%), were found in both zones, together with a monoculture fruit system, that was next to the production fields which constituted a control. The system types were as follows:
Coffee monoculture system (S1): The cultivation of coffee was the conventional type (unshaded), the variety is the Coffea arabica 'Castilla', with a spacing of 2.5 m between plants, made up of six-year-old coffee trees . Conventional tillage only in the first year.
Associated coffee system (S2): The cultivation of coffee had been associated type (shaded), the plants responsible for the shading are banana (Musa paradisiaca), guamo (Inga spp.), legumes (Leucaena leucocephala) and native trees of the region, even citrus; made up of six-year-old coffee trees. Without planning shading with coffee trees. Zero tillage.
Monoculture fruit system (S3): This area was investigated for comparative purposes and served as the control treatment. The area was made up of guamo (Inga spp.), citrus plants (Citrus sinensis and Citrus latifolia), guava (Psidium guajava) and other fruit trees typically found in the zone. In addition, systems of the same age as S1 and S2 were selected.
In all evaluated systems predominate the application of organic matter, formed by the pulp of C. arabica and residues of Musa paradisiaca (banana), being the major constituents of organic amendments, as implemented in most coffee areas of Colombia (Cardona and Sadeghiankh, 2005). Farmers usually do not apply agrochemicals in the S2 and S3 systems.
In each zone, three farms were selected including the three researched systems (S1, S2, S3); in 1,000 m2 sub-areas,10 soil sub-samples were extracted from the first 0-20 cm (D1) and 20-30 cm of depth (D2), which were mixed until obtaining a homogeneous sample of the soil, this procedure was performed in triplicate for microbiological and physico-chemical soil analysis purposes (Tab. 1). The D1 and D2 corresponding to factor C.
Statistical analysis corresponded to a factorial design (2 zones x 3 systems x 2 depth) in a random arrangement unrestrictedly, with three replications for a total of 36 experiment units, composite soil samples were stored in a deep freezer to control microbial activities for soil dilution, plating and biological analysis. A microbial analysis was carried out within 48 to 72 h.
The variables analyzed were bacteria, fungi and actinomycetes population (BP, FP, AP), determined by serial dilution plate count method (Notaro et al., 2014). Soil dilution (10-1) prepared by 10 g of soil sample was transferred into 90 mL sterile water and mixing the solution for few seconds. Then, 1 mL of dilution was transferred into 9 mL of sterile peptonized water test tube (10-2) and a soil serial dilution was prepared, up to 10-5.
Spread 0.1 mL of diluted soil suspension from each serial dilution (10-6 for Bacteria, 10-4 for fungi, 10-5 for actinomycetes) on different selective media, nutrient agar (NA) medium for bacteria, potato dextrose agar (PDA) medium for fungi and starch ammonia (SA) medium for actinomycetes and the plates in triplicate were incubated at optimum temperature between 25°C and 30°C at the duration of 5-7 d for fungi, 1-2 d for bacteria, 12-14 d for actinomycetes. For determine fungal growth was added chloramphenicol 30 mg L-1. The colony forming units are expressed as CFU 10n/g of soil on a moisture basis.
The number of microorganisms were used in the following formula: Population = number of colonies x dilution factor x 10. Expressed as colony forming units per gram of soil (CFU/g soil) (Notaro et al., 2014).
All the experimental data were analyzed using INFOSTAT. The variations among microbial population by effect of impact of different coffee systems were analyzed by one way ANOVA test. The treatment means were compared using Fisher'test (comparison of means least significant difference LSD) at 5% level of confidence.
Results and discussion
Bacterial population (BP)
The BP was significantly higher (P≤0.05) in S2, 98.0 x 106 CFU/g, than in S1 and S3, accounting for 51.0 x 106 CFU/g and 9.57 x 106 CFU/g, respectively (Fig. 1). It demonstrated that (BP) in associated coffee system (shaded) has a role in nutrient cycling and soil fertility through biochemical processes which preserve the source of soil mineral nutrients (Garcia et al., 2010; Evizal et al., 2012; Bagyaraj et al., 2015).
On the other hand, these differences in (BP) in (S1) and (S3) may be driven, in part, by differences in agriculture intensification and anthropogenic activities that affected the soil quality, as demonstrated also by Ademir et al.(2009) (Fig. 1).
Additionally, (BP) showed significant variation due to sampling depth (P≤0.05), it was higher in (D2) than in (D1) accounting for 71.18 x 106 CFU/g compared with 40.44 x 106 CFU/g (Fig. 1). This response coincided with the results obtained by Srivastava et al. (2014), while the maximum microbial communities were restricted at 0-30 cm depth (surface horizon).
Higher populations of heterotrophic (BP) in (S2) can be attributed to their higher tolerance and adaptation to (Z2), accounting for 141.73 x 106 CFU/g (Fig. 2) probably can be due to the availability of soil for C accumulation occasioned in high humidity and low temperature of the site, as stated by Bardgett et al. (2005), as suggested also by Criquet et al. (2004). The (BP) was more or less same in humid and sub-humid dry bioclimates humid zones directly influences higher soil humid for movement and nutrient acquisition of (BP) through of soil profile (Srivastava et al. 2014).
The maximum (BP) in (S2) was restricted to (D2) (188.0 x 106 CFU/g), the (BP) of this study is similar to that (BP) found by Muhammed et al. (2015) in a culture with different coffee systems. However, a similar concept of sustainability of the systems was applied. The low amount of (BP) found in the (S2) at the 0-20 cm depth (D1) (7.07 x 106 CFU/g) was similar when compared to data found by Notaro et al. (2014) in biodynamic coffee plantations (BS) at 0-10 cm depth (8.1 x 106 CFU/g).
The (BP) had a decline in (S1) and (S3) with an increase in D1 (P≤0.05) (Fig. 2), possibly related with the deficiency of soil nutrients through of soil profile in more simplified coffee production systems (unshaded), as demonstrated by Notaro et al. (2014).
The (BP) had a significant difference among the various factors (P≤0.05). The (Z2) cultivated with coffee under the shade of fruit trees (S2), resulted in a high (BP) at a 20-30 cm depth (D2) (280 x 106 CFU/g).
The increase in (BP) may be explained by a combination of high additions of SOM in the (S2) in surface soil, mulching with fruit and coffee residues, leaves, nourishes the rhizosphere and positively influences the (BP), making this an important practice for maintaining high levels of soil carbon (Cardona and Sadeghiankh, 2005), improving the soil fertility and soil microbial activity, as supported by Beer et al. (1998); Notaro et al. (2014); Bagyaraj et al. (2015).
But also, (BP) may benefit more from elevated precipitation and soil moisture, because they are more abundant in surface soils (Srivastava et al. 2014).
Actinomycetes population (AP)
The (AP) increased from (S3) to (S2) accounting for 11.80 x 105 CFU/g; (S3) and (S1) were statistically similar (P≤0.05) accounting for 1.60 and 1.90 x 105 CFU/g (P≤0.05), respectively (Fig. 3). This study proved that (S2) increased the (AP) as demonstrated by Bagyaraj et al. (2015). Biological indicators are extremely sensitive when attempting to detect changes in soil quality, as showed in (S3) and (S1) (Fig. 3).
The increase in (AP) of (S2) in Z1 and Z2 can be attributed to their higher tolerance and adaptation to wide variations of climate and soil in tropical conditions, accounting for 12.27 and 11.3 x 105 CFU/g respectively, with equal significance (P≤ 0.05) (Fig. 4) which was different to conditions found in temperate zones (Srivastava et al., 2014).
Reduction in soil organic matter (SOM) in monoculture systems regardless of the area in which it is, may have had a negative effect on (AP) as showed for the Z1*S3, Z1*S1, Z2*S1, Z2*S3 interactions that recorded the lowest count of 2.87, 1.93, 1.53 and 1.27 x 105 CFU/g, respectively, with no statistically differences (P≤0.05) (Fig. 4).
The content of (AP) in the (S2) at (D1) and (D2) was not different (13.93 and 9.67 x 105 CFU/g) (P≤0.05) (Fig. 4). On the other hand, the S3*D2, S1*D1, S3*D1, S1*D2 interactions can affect the establishment of (AP) (3, 1.87, 1.40 and 1.33 x 105 CFU/g), respectively (Fig. 4). The surface soils recorded higher (AP) compared to the subsurface horizons (Srivastava et al. 2014; Hartmann et al. 2009).
The results of (AP) in the Z1*S2*D1 and Z2*S2*D2 interactions responsible for 16.40, 11.47, 11.47 and 11.20 x 105 CFU/g, respectively, with no significant difference (P>0.05) between them, but different to the other interactions (P≤0.05) which demonstrate the similarity of (S2) between the areas and soil depths studied.
The increase in (AP) under associated coffee systems may be explained by a combination of system types than can markedly benefit the activity of soil microorganisms and their diversity (Evizal et al., 2012; Notaro et al., 2014) and climatic factors (Bagyaraj et al., 2015).
Fungus population (FP)
Our (S2) maintained an almost consistent (FP) (39.50 x 104 CFU/g) than (S1) (13.83 x 104 CFU/g), but the lowest (FP) count was recorded in (S3) accounting for 4.50 x 104 CFU/g (Fig. 5). All of the systems were statistically different (P≤ 0.05). Various coffee management systems can result in different substrate availabilities that affect the establishment of a fungal population (Notaro et al., 2014).
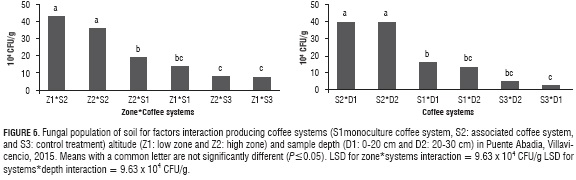
A higher (FP) was observed in (S2) of the Z1 and Z2 (43 and 36 x 104 CFU/g) respectively (Fig. 6), indicating a similar trend with (AP). The lowest (FP) was observed in (S3) of Z2 and Z1, but equal to the (S1) of the (Z1), in turn this did not have a significant difference with the (S1) of the (Z2) (P≤ 0.05) (Fig. 6). Our results no corroborate with the findings of Srivastava et al. (2014) and Mogollón and Martinez (2009); due to the fact that (FP) was more tolerant to variations of weather conditions.
Soil fungi can extend hyphae through air-filled pore spaces to access moisture and nutrients and can translocate these resources to water and nutrient-limited cells within their mycelia network (Muleta et al., 2007; Srivastava et al., 2014).
Comparatively, (S2), which are better managed, are rich in (FP) showing more diversity through the soil profile (39.67 and 39.63 x 104 CFU/g) indicate a significant difference than in the poorly managed systems (S1) and (S3) (P<0.01) (Figure 6).
The (S1) and (S3) require different adaptation strategies of (FP) to soil edaphic factors with emphasis on the rhizosphere; biological indicators are extremely sensitive when attempting to detect changes in soil quality (Bardgett et al., 2005; Muleta et al., 2007). Reduction in soil carbon, may have had a negative effect on (FP).
The Z2*S2*D2 and Z1*S2*D1 interactions had a higher (FP) accounting for 46.67 and 39.33 x 104 CFU/g, respectively; confirming that there has been a growing interest in the (S2). (S2) is based on principles of diversification, recycling, biological processes and the imitation of natural habitats, as demonstrated by Beer et al. (1998); Notaro et al. (2014); Evizal et al. (2012); Garcia et al. (2010) and Cardona and Sadeghiankh (2005).
Conclusions
The data so generated on microbial population of (S1), (S2) and (S3) can be considered part of the soil information for monitoring the soil quality and sustainability of coffee systems in Puente Abadia, Villavicencio.
The (Z2) in Puente Abadia are zones that are highly susceptible to environmental degradation (erosion), and can be used for the (S2) because it potentiated a greater number of (BP), and (AP) to a 20-30 cm depth (D2); that stimulates growth of (S2) in (Z1), which generated major (AP) and (FP) at both depths evaluated.
Agroforestry can be an interesting alternative for improving soil quality in marginal coffee areas in Colombia, due to the increase in soil microbial activity.
literature cited
Ademir, A., L. Leite., V. Santos, and R. Carneiro. 2009. Soil microbial activity in conventional and organic agricultural systems. Sustainability 1, 268-276. Doi: 10.3390/su1020268.
Bagyaraj, D.J., G. Thilagar, C. Ravisha, C.G. Kushalappa, K.N. Krishnamurthy, and P. Vaast. 2015. Below ground microbial diversity as influenced by coffee agroforestry systems in the Western Ghats, India. Agric. Ecosyst. Environ. 202, 198-202. Doi: 10.1016/j.agee.2015.01.015.
Bardgett, R.D., W.D. Bowman., R. Kaufmann, and S.K. Schmidt. 2005. A temporal approach to linking aboveground and belowground ecology. Ecol. Evol. 20, 634-641. Doi: 10.1016/j.tree.2005.08.005.
Beer, J., R. Muschler, D. Kass, and E. Somarriba. 1998. Shade management in coffee and cacao plantations. Agrof. Syst. 38, 139-164. Doi: 10.1023/A:1005956528316.
Cardona, D.A. and S. Sadeghiankh. 2005. Evaluación de propiedades físicas y químicas de suelo establecidas con café bajo sombra y a plena exposición solar. Cenicafe 56, 348-364.
Criquet, S., A.M. Farnet, E. Ferre, and J. Le Petit. 2004. Annual dynamics of phosphatase activities in an evergreen oak litter: influence of biotic and abiotic factors. Soil Biol. Biochem. 36, 1111-1118. Doi: 10.1016/j.soilbio.2004.02.021.
Evizal, R., I.D. Tohari, J.W. Prijambada, and D. Widianto. 2012. Soil bacterial diversity and productivity of coffee shade tree agro-ecosystem. J. Trop. Soils 17, 181-187.
Garcia, C.A., S.A. Bhagwat, J. Ghazoul, C.D. Nath, K.M. Nanaya, C. G. Kushalappa, Y. Raghuramulu, R. Nasi, and P. Vaast. 2010. Changelles and opportunities of coffee agroforests in the Western Ghats, India. Conserv. Boil. 24, 479-488. Doi: 10.1111/j.1523-1739.2009.01386.x.
Haggar, J.P., M. Barrios, M. Bolanos, M. Merlo, P. Moraga, R. Munguia, A. Ponce, S. Romero, G. Soto, C. Staver, and E.M. De Virginio. 2011. Coffee agroecosystem performance under full sun, shade, conventional and organic management regimes in Central America. Agroforest. Syst. 82, 285-301. Doi: 10.1007/s10457-011-9392-5.
Hartmann, M., S. Lee, S.J. Hallam, and W.W. Mohn. 2009. Bacterial, archaeal and eukaryal community structures throughout soil horizons of harvested and naturally disturbed forest stands. Environ. Microbiol. 11, 3045-3062. Doi: 10.1111/j.1462-2920.2009.02008.x.
Holdridge, L. 1947. Determination of world plant formations from simple climatic data. Science 105, 367-368. Doi: 10.1126/science.105.2727.367.
Mogollón, J. and A. Martinez. 2009. Variation of soil biological activity in an altitudinal transect of la sierra de San Luis, Falcon state. Agron. Trop. 59, 469-479.
Muhammed, U.P.F.B., P.V., Sindhu, K.S. Gopal, and C.G. Thomas. 2015. Influence of mulches on rhizosphere microflora, yield and weed competition in okra Abelmoschus esculentus (L.) Moench. J. Trop. Agric. 53, 70-74.
Muleta, D., F. Assefa, S. Nemomissa, and U. Granhall. 2007. Composition of coffee shade tree species and density of indigenous arbuscular mycorrhizal fungi (AMF) spores in Bonja natural coffee forest Southwestern Ethiopia. For. Ecol. Manage. 241, 145-154. Doi: 10.1016/j.foreco.2007.01.021.
Nigussie, A. and E. Kissi. 2012. The contribution of coffee agroecosystem to soil fertility in Southwestern Ethiopia. Afr. J. Agric. Res. 7, 74-81. Doi: 10.5897/ajar11.1566.
Notaro, K., E.V. De Medeiros., G. Duda., A. Silva, and P. De Moura. 2014. Agroforestry systems, nutrients in litter and microbial activity in soils cultivated with coffee at high altitude. Sci. Agric. 71, 87-95.
Salgado, B.G., R.L.G. Macedo., M.I.N. Alvarenga, and N. Venturin. 2006. Evaluation of soil fertility in agroforest systems with coffee trees (Coffea arabica L.) in Lavras, MG. Rev. Árvore 30, 343-349. Doi: 10.1590/S0100-67622006000300004.
Souza, H.N.D., R.G. de Goede, L. Brussaard, I.M. Cardoso, E.M. Duarte, R.B.A. Fernandes, and M.M. Pulleman. 2012. Protective shade, tree diversity and soil properties in coffee agroforestry systems in the Atlantic rainforest biome. Agric. Ecosyst. Environ. 146, 179-196. Doi: 10.1016/j.agee.2011.11.007.
Srivastava, A.K., K. Velmourougane, T. Bhattacharyya, D. Sarkar, D. K. Pal, J. Prasad, G.S. Sidhu, K.M. Nair, A.K. Sahoo, T.H. Das, R.S. Singh, R. Srivastava, T.K. Sen, S. Chatterji, P. Chandran, S.K. Ray, N.G. Patil, G.P. Obireddy, S.K. Mahapatra, and K.S. Kumar. 2014. Impacts of agro-climates and land use systems on culturable microbial population in soils ofthe Indo-Gangetic Plain, India. Current Sci. 107, 1464-1469.
References
Ademir, A., L. Leite., V. Santos, and R. Carneiro. 2009. Soil microbial activity in conventional and organic agricultural systems. Sustainability 1, 268-276. Doi: 10.3390/su1020268.
Bagyaraj, D.J., G. Thilagar, C. Ravisha, C.G. Kushalappa, K.N. Krishnamurthy, and P. Vaast. 2015. Below ground microbial diversity as influenced by coffee agroforestry systems in the Western Ghats, India. Agric. Ecosyst. Environ. 202, 198-202. Doi: 10.1016/j.agee.2015.01.015.
Bardgett, R.D., W.D. Bowman., R. Kaufmann, and S.K. Schmidt. 2005. A temporal approach to linking aboveground and belowground ecology. Ecol. Evol. 20, 634-641. Doi: 10.1016/j.tree.2005.08.005.
Beer, J., R. Muschler, D. Kass, and E. Somarriba. 1998. Shade management in coffee and cacao plantations. Agrof. Syst. 38, 139-164. Doi: 10.1023/A:1005956528316.
Cardona, D.A. and S. Sadeghiankh. 2005. Evaluación de propiedades físicas y químicas de suelo establecidas con café bajo sombra y a plena exposición solar. Cenicafe 56, 348-364.
Criquet, S., A.M. Farnet, E. Ferre, and J. Le Petit. 2004. Annual dynamics of phosphatase activities in an evergreen oak litter: influence of biotic and abiotic factors. Soil Biol. Biochem. 36, 1111-1118. Doi: 10.1016/j.soilbio.2004.02.021.
Evizal, R., I.D. Tohari, J.W. Prijambada, and D. Widianto. 2012. Soil bacterial diversity and productivity of coffee shade tree agro-ecosystem. J. Trop. Soils 17, 181-187.
Garcia, C.A., S.A. Bhagwat, J. Ghazoul, C.D. Nath, K.M. Nanaya, C. G. Kushalappa, Y. Raghuramulu, R. Nasi, and P. Vaast. 2010. Changelles and opportunities of coffee agroforests in the Western Ghats, India. Conserv. Boil. 24, 479-488. Doi: 10.1111/j.1523-1739.2009.01386.x.
Haggar, J.P., M. Barrios, M. Bolanos, M. Merlo, P. Moraga, R. Munguia, A. Ponce, S. Romero, G. Soto, C. Staver, and E.M. De Virginio. 2011. Coffee agroecosystem performance under full sun, shade, conventional and organic management regimes in Central America. Agroforest. Syst. 82, 285-301. Doi: 10.1007/s10457-011-9392-5.
Hartmann, M., S. Lee, S.J. Hallam, and W.W. Mohn. 2009. Bacterial, archaeal and eukaryal community structures throughout soil horizons of harvested and naturally disturbed forest stands. Environ. Microbiol. 11, 3045-3062. Doi: 10.1111/j.1462-2920.2009.02008.x.
Holdridge, L. 1947. Determination of world plant formations from simple climatic data. Science 105, 367-368. Doi: 10.1126/science.105.2727.367.
Mogollón, J. and A. Martinez. 2009. Variation of soil biological activity in an altitudinal transect of la sierra de San Luis, Falcon state. Agron. Trop. 59, 469-479.
Muhammed, U.P.F.B., P.V., Sindhu, K.S. Gopal, and C.G. Thomas. 2015. Influence of mulches on rhizosphere microflora, yield and weed competition in okra Abelmoschus esculentus (L.) Moench. J. Trop. Agric. 53, 70-74.
Muleta, D., F. Assefa, S. Nemomissa, and U. Granhall. 2007. Composition of coffee shade tree species and density of indigenous arbuscular mycorrhizal fungi (AMF) spores in Bonja natural coffee forest Southwestern Ethiopia. For. Ecol. Manage. 241, 145-154. Doi: 10.1016/j.foreco.2007.01.021.
Nigussie, A. and E. Kissi. 2012. The contribution of coffee agroecosystem to soil fertility in Southwestern Ethiopia. Afr. J. Agric. Res. 7, 74-81. Doi: 10.5897/ajar11.1566.
Notaro, K., E.V. De Medeiros., G. Duda., A. Silva, and P. De Moura. 2014. Agroforestry systems, nutrients in litter and microbial activity in soils cultivated with coffee at high altitude. Sci. Agric. 71, 87-95.
Salgado, B.G., R.L.G. Macedo., M.I.N. Alvarenga, and N. Venturin. 2006. Evaluation of soil fertility in agroforest systems with coffee trees (Coffea arabica L.) in Lavras, MG. Rev. Árvore 30, 343-349. Doi: 10.1590/S0100-67622006000300004.
Souza, H.N.D., R.G. de Goede, L. Brussaard, I.M. Cardoso, E.M. Duarte, R.B.A. Fernandes, and M.M. Pulleman. 2012. Protective shade, tree diversity and soil properties in coffee agroforestry systems in the Atlantic rainforest biome. Agric. Ecosyst. Environ. 146, 179-196. Doi: 10.1016/j.agee.2011.11.007.
Srivastava, A.K., K. Velmourougane, T. Bhattacharyya, D. Sarkar, D. K. Pal, J. Prasad, G.S. Sidhu, K.M. Nair, A.K. Sahoo, T.H. Das, R.S. Singh, R. Srivastava, T.K. Sen, S. Chatterji, P. Chandran, S.K. Ray, N.G. Patil, G.P. Obireddy, S.K. Mahapatra, and K.S. Kumar. 2014. Impacts of agro-climates and land use systems on culturable microbial population in soils ofthe Indo-Gangetic Plain, India. Current Sci. 107, 1464-1469.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Yu Ge, Fengying Zhang, Chun Xie, Peng Qu, Kuaile Jiang, Huabo Du, Meng Zhao, Yunfeng Lu, Butian Wang, Xuedong Shi, Xuejun Li, Chuanli Zhang. (2023). Effects of Different Altitudes on Coffea arabica Rhizospheric Soil Chemical Properties and Soil Microbiota. Agronomy, 13(2), p.471. https://doi.org/10.3390/agronomy13020471.
2. Paulo Prates Júnior, Tomás Gomes Reis Veloso, Marliane de Cássia Soares da Silva, José Maria Rodrigues da Luz, Sabrina Feliciano Oliveira, Maria Catarina Megumi Kasuya. (2021). Quality Determinants In Coffee Production. Food Engineering Series. , p.101. https://doi.org/10.1007/978-3-030-54437-9_3.
3. Carmenza Pérez Fagua, Ángela Yaneth Landínez-Torres , Amanda Silva Parra. (2023). Carbono orgánico y su dinámica en suelos tropicales: una revisión. Cultura Científica, (21), p.1. https://doi.org/10.38017/1657463X.820.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2016 Agronomía Colombiana

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
© Centro Editorial de la Facultad de Ciencias Agrarias, Universidad Nacional de Colombia
Reproduction and quotation of material appearing in the journal is authorized provided the following are explicitly indicated: journal name, author(s) name, year, volume, issue and pages of the source. The ideas and observations recorded by the authors are their own and do not necessarily represent the views and policies of the Universidad Nacional de Colombia. Mention of products or commercial firms in the journal does not constitute a recommendation or endorsement on the part of the Universidad Nacional de Colombia; furthermore, the use of such products should comply with the product label recommendations.
The Creative Commons license used by Agronomia Colombiana journal is: Attribution - NonCommercial - ShareAlike (by-nc-sa)

Agronomia Colombiana by Centro Editorial of Facultad de Ciencias Agrarias, Universidad Nacional de Colombia is licensed under a Creative Commons Reconocimiento-NoComercial-CompartirIgual 4.0 Internacional License.
Creado a partir de la obra en http://revistas.unal.edu.co/index.php/agrocol/.