Physicochemical characterization and nutritional composition analysis of pineapple guava at three different ripening stages
Caracterización fisicoquímica y análisis de la composición nutricional de la feijoa en tres diferentes estadios de madurez
DOI:
https://doi.org/10.15446/agron.colomb.v34n2.56030Palabras clave:
Acca sellowiana, chemical composition, vitamins, postharvest (en)Acca sellowiana, composición química, vitaminas, poscosecha (es)
La feijoa (Acca sellowiana [O. Berg] Burret) es una fruta con potencial productivo y de exportación en Colombia, sin embargo existen pocos reportes de su composición en función al comportamiento fisiológico a través de diferentes estadios de madurez. Frente a esto, fue propuesto un estudio para evaluar las propiedades fisicoquímicas y la composición nutricional en frutos de feijoa en tres estadios de madurez determinados por las semanas transcurridas después de la antesis, siendo verde para la semana 17 (W17), maduro para la semana 20 (W20) y sobre maduro para la semana 21 (W21). A las frutas se les realizó una caracterización preliminar, se sometieron a un análisis bromatológico y se estableció su contenido en vitamina A y C. Los resultados muestran un contenido significativo de fibra cruda y carbohidratos totales en los tres estadios evaluados. También se evidencia un descenso en el contenido de fibra detergente neutra (NDF) del 38% entre W17 y W21. El mayor nivel de vitamina C se registró en la semana W17 (67.82 mg ácido ascórbico/g muestra seca), al igual que para vitamina A (12.65 mg β-caroteno/g muestra seca). Respecto a la caracterización fisicoquímica, es posible la existencia de un comportamiento fisiológico particular en la feijoa atribuible al desarrollo en tamaño y masa del fruto luego de superar su estado de madurez fisiológica. Además, el contenido de calcio y carbohidratos reductores puede llegar a estar asociado al fenómeno de pardeamiento de la pulpa. Claramente, la feijoa es una fruta promisoria gracias a sus propiedades nutricionales, de acuerdo a las tendencias de consumo actuales.
Doi: https://doi.org/10.15446/agron.colomb.v34n2.56030
Physicochemical characterization and nutritional composition analysis of pineapple guava at three different ripening stages
Caracterización fisicoquímica y análisis de la composición nutricional de la feijoa en tres diferentes estadios de madurez
Lina María García-Rivera1, Henry Alexander Váquiro-Herrera1, and José Fernando Solanilla-Duque1
1 Faculty of Agronomy Engineering, Universidad del Tolima. Ibague (Colombia). jfsolanilla@ut.edu.co
Received for publication: 29 March, 2016. Accepted for publication: 30 June, 2016.
ABSTRACT
Pineapple guava (Acca sellowiana [O. Berg] Burret) is a fruit with export and production potential in Colombia. However, there are few reports about its composition concerning physiological behavior throughout the different ripening phases. Intending to confront this situation, a study was proposed in order to evaluate the physicochemical properties and the nutritional composition of pineapple guava fruits in three different phases of ripening, determined by the weeks elapsed after anthesis, considering it undeveloped for week 17 (W17), ripe for week 20 (W20) and overripe from week 21 (W21). Pineapple guava fruits were preliminarily characterized, they underwent a bromatological analysis and their content of vitamin A and C content was established. The results showed a significant content of crude fiber and total carbohydrates in the three evaluated phases. There was also a decrease in the neutral detergent fiber of 38% between W17 and W21. The highest level of vitamin C was reported in W17 (67.82 mg ascorbic acid/g dry sample), as well as for vitamin A (12.65 mg β-carotene/g dry sample). In a physical-chemical characterization, the existence of a particular physiological behavior is possible because of the development in size and mass of the fruit after physiological ripening. Additionally, the calcium and reducing carbohydrate content can be associated with the pulp browning phenomenon. Clearly, pineapple guava is a promising fruit thanks to its nutritional properties, according to the current consumption tendencies.
Key words: Acca sellowiana, chemical composition, vitamins, postharvest.
RESUMEN
La feijoa (Acca sellowiana [O. Berg] Burret) es una fruta con potencial productivo y de exportación en Colombia, sin embargo existen pocos reportes de su composición en función al comportamiento fisiológico a través de diferentes estadios de madurez. Frente a esto, fue propuesto un estudio para evaluar las propiedades fisicoquímicas y la composición nutricional en frutos de feijoa en tres estadios de madurez determinados por las semanas transcurridas después de la antesis, siendo verde para la semana 17 (W17), maduro para la semana 20 (W20) y sobre maduro para la semana 21 (W21). A las frutas se les realizó una caracterización preliminar, se sometieron a un análisis bromatológico y se estableció su contenido en vitamina A y C. Los resultados muestran un contenido significativo de fibra cruda y carbohidratos totales en los tres estadios evaluados. También se evidencia un descenso en el contenido de fibra detergente neutra (NDF) del 38% entre W17 y W21. El mayor nivel de vitamina C se registró en la semana W17 (67.82 mg ácido ascórbico/g muestra seca), al igual que para vitamina A (12.65 mg β-caroteno/g muestra seca). Respecto a la caracterización fisicoquímica, es posible la existencia de un comportamiento fisiológico particular en la feijoa atribuible al desarrollo en tamaño y masa del fruto luego de superar su estado de madurez fisiológica. Además, el contenido de calcio y carbohidratos reductores puede llegar a estar asociado al fenómeno de pardeamiento de la pulpa. Claramente, la feijoa es una fruta promisoria gracias a sus propiedades nutricionales, de acuerdo a las tendencias de consumo actuales.
Palabras clave: Acca sellowiana, composición química, vitaminas, poscosecha.
Introduction
Colombian tropical fruits, such as pineapple guava, nowadays, present a great export and productive potential, according to Arias et al. (2006), which has permitted a meaningful consolidation in external market niches, especially in developed countries, where the demand has been increasing.
Pineapple guava (Acca sellowiana [O. Berg] Burret) is native from South America (from Brazil and northeast Uruguay to western Paraguay and northeastern Argentina) and was introduced in Colombia in regions with agroecological conditions for its growth, such as Cundinamarca (1,800 m a.s.l., 13°C annual mean temperature) and Boyaca (2,600 m a.s.l., 21°C annual mean temperature) (Perea et al., 2010). This crop is highly influenced by crossed pollination (Fischer, 2003), which in some fields guarantees a bigger size in the fruit (Harman, 1987).
The fruit is a berry with a smooth crust, wrinkly in appearance, with an intense dark green color, ovoid shape and grained, white pulp, nice smell and bittersweet flavor (Perea et al., 2010), characteristics that can vary according to the grown genotype (Fischer et al., 2003). Just the same, it presents aromatic qualities attributed to ethyl and methyl benzoates (Bontempo et al., 2007), bioactive properties (Weston, 2010), an antioxidant potential related to the phenol content (Beyhan et al., 2010), polyphenols and ascorbic acid (Clerici and Carvalho, 2011). It is a good source of vitamin A, E and C (Perea et al., 2010). It also reports antimicrobial, anti-inflammatory and immunestimulating properties (Weston, 2010).
Ripening process in climacteric fruits is mainly due to the continuance of physiological processes, even in posthar-vest stages, which allows for the development of desirable organoleptic properties of the fruit and at the same time makes it prone to deterioration, reducing its shelf-life (Mogollón et al., 2010). Thus, it is necessary to carry out different research studies to reach a greater understanding in the particular behaviors of fruits such as pineapple guava, during its ripening process in postharvest stage.
The objective of this study was to evaluate physicochemical properties and analyze the nutritional composition of pineapple guava fruit in three different ripening stages at postharvest.
Materials and methods
Plant material
The pineapple guava fruits were obtained from a crop field located in the municipality of La Vega (Cundinamarca, Colombia), located at 4°55'51.4" N and 74°19'25.5" W, at 2,050 m a.s.l., 18°C mean annual temperature, and 85% mean annual relative humidity. Trees were selected at random in the same orchard, and at least 10 fruit were harvested from each tree. The fruits were immediately classified according to the weeks elapsed after anthesis as a parameter of ripening state. After classification, the fruits were packed in perforated bags, transported to the laboratory in expanded polystyrene cooling containers with ice packs wrapped in newspaper (Rodríguez et al., 2006), they were stored in a cooling chamber with temperature control at 2°C at 12 h following the recommendations of Valderrama et al. (2005). Before each test, the fruits were disinfected by immersion in a solution of 200 mg L-1 of sodium hypochlorite for 5 min.
The ripening stages selected for the study correspond to fruits harvested at 17 (W17), 20 (W20) and 21 (W21) weeks after anthesis.
Preliminary characterization of fruits
As soon as the samples were prepared for storage, an initial characterization was carried out by taking the physical and chemical features of the fruit in order to know its postharvest conditions. Determinations were performed three times using batches of 20 fruits for each ripening stage to guarantee the feasibility of collected data.
In the physical characterization, the longitudinal diameter (ΦL) and transversal diameter (ΦT) were measured using a Vernier (HX190306, Hopex Solingen Werkzeuge, Solingen, Germany). Mass was measured per fruit for each sample batch, using a free-dish scale balance (EW 2200-2NM, Kern, Germany), with which the average of the mass was calculated for every ripening stage. For the firmness measurement, a penetrometer (FT-327, Bertuzzi, Facchini, Italy) was used to get a coherent average of the firmness of all the fruits (Milošević et al. 2015).
For the preliminary chemical characterization, three randomly selected fruits were processed in a juice extractor (JE-1500, Black and Decker, Towson, MD). To the juice, free of seeds and skin, total soluble solids, pH and titratable acidity were determined. Total soluble solids were measured using a refractometer (Brix 35HP, Reichert Analytical Instrument, Buffalo, NY) and pH was read on a potentiometer (Handylab pH11/pH14, Schott instruments, Mainz, Germany). Titratable acidity was determined by titration with 0.1 N NaOH. The chemical characterization was assessed by triplicate, repeating the juice extraction for each replicate.
Bromatological analysis
The fruits were cut in slabs and dried in a stove (TH53, Thermolab, DIES, Medellin, Colombia) at 50±3°C for 12 h. After that, dried fruits were ground using a steel mortar, sieved and kept in hermetic bags.
Moisture content, dry matter, ash, ethereal extract, crude fiber, protein, nitrogen and nitrogen-free extract were determined by AOAC methods (AOAC, 1997). The mineral content was valued by atomic absorption spectrophotometry (for Ca, Mg, K, Na, Cu, Zn, Fe and Mn) and UV-visible (for S, P and B), previously conditioning simple ashes with 10% HCl solution and distilled water, preparing later specific dissolutions for every mineral. The analysis of total, reducing and non-reducing carbohydrate contents were based on the methodologies proposed by Goldman and Green (2015), thus determining total carbohydrates by anthrone method and reducing carbohydrates by DNS (3.5-dinitrosalicilic acid) method (Najmus and Whitney, 2011). The contents of acid detergent fiber (ADF) and neutral detergent fiber (NDF) were determined by using Cetyl-trimethyl-ammonium at acid pH (AOAC, 1997) and Sodium Lauril Sulfate at neutral pH (Ali et al., 2012). The solubility of ashes, in both water and acid, was evaluated by ash lixiviation, followed by gravimetric and drying methods.
Vitamin content
The vitamin C and A contents were determined in the whole fruit from lyophilized samples (LS). Prior to analysis, samples were kept in hermetic bags wrapped in foil paper and stored at 4°C for 12 h. The purpose of the sample lyophilization was to obtain a dry and porous sample, enabling the extraction by solvents and guarantee functional constituent preservation and secondary metabolites (Marques et al., 2009).
The content of vitamin A was determined according to the methodology proposed by Sanusi and Adebiyi (2009) and it was calculated from a standard (3-carotene curve in mg of (β-carotene acid per g of LS.
The ascorbic acid quantification in LS was determined using the colorimetric method reported by García et al. (2010), with some modifications. LS extracts were prepared in 0.15% oxalic acid and treated with 0.16% 2-nitroaniline, 0.08% sodium nitrite, 97% ethanol and 10% NaOH. The absorbance was measured at 540 nm and the content of ascorbic acid, in mg of ascorbic acid per g of LS, was calculated from a standard ascorbic acid curve.
Statistical analysis
The data were processed using one-way analysis of variance at the 95% confidence level to compare the mean values of each parameter among the different ripening stages (Statgraphics Centurion XV, StatPoint Technologies, Warrenton, VA). A multiple range test was used to determine which means were significantly different from the others along with Fisher's least significant difference (LSD) procedure.
Results and discussion
Preliminary characterization of fruits
Longitudinal diameter, transversal diameter, firmness and mass
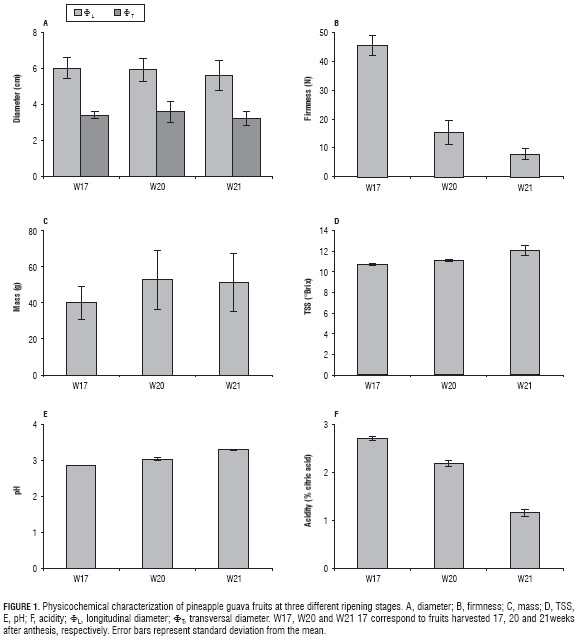
The significant differences (P≤0.05) between longitudinal (ΦL) and transversal (ΦT) diameters for pineapple guava fruits in three ripening stages (Fig. 1A) were in agreement with the typically ovoid geometry of the fruit, related structurally with radiated cells that surround sclereids and with the tangential enlargement of hypodermic cells at the end of the transversal development of the fruit (Rodríguez et al., 2010). Just the same, as the ripening stage goes on, longitudinal diameter (ΦL) has not changed considerably (α = 0.21 cm), something that can be attributed to the protective factor that the epidermis has against dehydration (Rodríguez et al., 2010), avoiding a drastic contraction of the fruit. In this way, it is possible to indicate that after the filling of the fruit in the crop field, the subsequent ripening process does not influence the significant loss of volume.
The ΦL, as much as ΦT (Fig. 1A), agreed with the ones published by Combariza et al. (2007), who also reported variations in ΦL between 3.93 to 6.60 cm and in ΦT between 2.51 to 4.49 cm for pineapple guava from La Vega (Cundinamarca, Colombia).
Figure 1B shows how fruit firmness reduced in response to cellular deterioration and internal structural changes that it suffers as its ripening process advances. This drastic reduction was due to the fact that the placental parenchyma and the septa become pulp and present a soft and juicy consistence when the fruit is ripe (Esemann-Quadros et al., 2008; Rodríguez et al., 2010). Just the same, Fig. 1C shows that an increase in the fruit mass between W17 and W20, followed by a small decrease in the W21 samples. The particularity of this behavior can show traces of some differential metabolic process in pineapple guava, possibly attributed to intrinsic characteristics of the fruit, which could be related to the sigmoidal growth and development model of the fruit described by Rodríguez et al. (2006). This appreciation makes it easy to consider that in weeks 20 and 21 after anthesis, there are influential processes both metabolic and catabolic in the model, which allows for the supposition that it might be double-sigmoidal type in pineapple guava fruits harvested at W20 (Rodríguez et al., 2006; Parra and Fischer, 2013; Parra-Coronado et al., 2015). The behavior of mass and firmness between W17 and W21 differed substantially (P≤0.05), as described above.
Experiment data provided by Romero et al. (1994) show the mass (31.7±3.43 g), firmness (32.36±4.11 N), ΦL (4.6±0.24 cm) and Ot (3.6±0.14 cm) of pineapple guava grown in North Spain. These values are approximate to the ones listed by Lim (2012) for ΦL from 5.00 to 8.00 cm and ΦTfrom 3.00 to 6.00 cm. Thus, it is possible to observe that the pineapple guava of this study had a higher mass and, even so, a larger size (if ΦL and ΦT are considered), conditions that might be related to the optimal development of the fruit after blossoming, due to either agroecological conditions. Additionally, it must be considered that, in pineapple guava, blossoming, pollination and fruit filling improve if there ise free exposure to sunlight, and not o dry conditions and low temperatures (Combariza et al., 2007).
Total soluble solids, pH and titratable acidity
The data in Figs. 1D, 1E and 1F show typical changes of fruits during their ripening. A small increase in pineapple guava pH can be observed as ripening stages advance, matching the reduction of citric acid. This is coherent if it is considered that the increase of pH indicates a tendency to alkalinity, being proportional in the decreasing of the predominant acid content of the fruit. Therefore, there were meaningful differences for pH and acidity among the three ripening stages (P≤0.05). Thus, the decrease of citric acid in pineapple guava throughout its ripening stages is due to the use of the content of organic acids in the fruit as respiratory substrate and carbon skeletons for new compounds (Valderrama et al., 2005).
The increase in total soluble solids (TSS), evidenced in Fig. 1D, is related to sugar synthesis throughout ripening, since these represent the highest proportion of TSS and, along with organic acids, are responsible of aromatic properties of the fruit (Beckles, 2012). Such behavior is attributed to the hydrolysable starch that pineapple guava contains, which leads to saccharose synthesis and oxidation of acids, consumed during respiration, which finally increases TSS of the fruit (Valderrama et al., 2005). Regarding TSS, there was no meaningful difference between W17 and W20, whereas the difference between W20 and W21 was statistically significant.
Bromatological analysis
Moisture content, crude fiber and ethereal extract
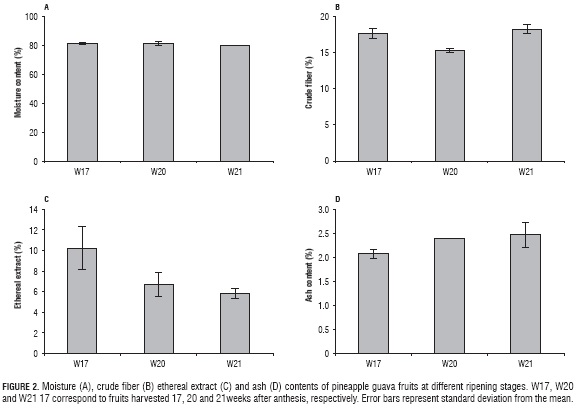
Moisture content of pineapple guava decreased 1.77% between W17 and W21 (Fig. 2A), given that this fruit presents characteristics of resistance to dehydration during final ripening stages. Even so, the high moisture content is an important factor affecting perishability, which suggests postharvest methods that reduce as much as possible any damage that may affect the quality of the fruit are required (Ramírez et al., 2005; Valderrama et al., 2005).
Crude fiber in the food gets to represent 0.5 to 4.0% (Kro-ntveit et al., 2014) and is composed of fibrous elements of the cell wall, which include cellulose, lignin, pectins, and gums, among others (Smiechowska and Dmowski, 2006). Therefore, as fruits lose these components, a decrease in firmness begins, indicating variations in the ripening stage. As a result, ripening of the fruit is eventually associated to the breakage of the structure of the cell wall, which causes softening of tissues and accumulation of sugar and nutrients (Goñi et al., 2010). So the decrease of crude fiber in pineapple guava between W17 and W20 (Fig. 2B) was coherent with the degradative behavior of the cell as its physiological processes. However, the increase of 2.82 percentage points in crude fiber content between W20 and W21 (Fig. 2B) could be a result of non-structural fiber accumulation due to metabolic processes, given that acid fruits tend to be an important source of carbohydrates, including polysaccharides, such as cellulose, starch, hemicellulose and pectins (Repo and Encina, 2008), representative compounds in cellular secretions during ripening. Compared with other fruits of the Myrtaceae family, pineapple guava presents 44% greater content of crude fiber than lyophilized guava (Psidium guajava L.) (12.7% crude fiber) (Moreno et al., 2014), which makes it potentially adequate within fiber-rich diets since they favor the reduction of problems such as constipation, diabetes, cardiovascular diseases and obesity (López-Vargas et al., 2014).
Concerning ethereal extract, there were not significant differences between W17, W20 and W21. However, this tended to decrease around 4.41 percentage points as ripening progressed (Fig. 2C), which is equivalent to 43% of total ethereal extract of W17. Results indicate that liposoluble compounds of fruit, such as pro-vitamin A, could be affected during the ripening process.
Ash and mineral contents
The proportion of ash in pineapple guava increased gradually between every ripening stage (Fig. 2D), with a significant (P≤0.05) statistical difference between them, given that such components are not sensitive to physical or thermal processes; they can only migrate from the fruit by lixiviation. Besides, it is feasible to preliminarily estimate the content of minerals in the fruits, considering possible that in every ripening stage there are certain conditions that favor the metabolism of some specific minerals (Santoni et al., 2014).
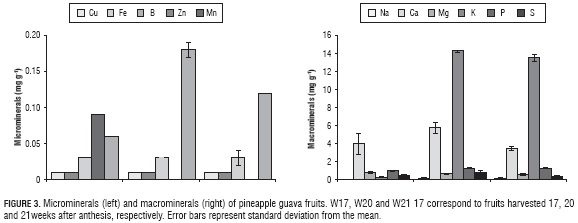
The micromineral contents, such as iron, copper and zinc, did not present significant variations throughout the tested ripening stages (P>0.05); however, boron differred statistically from the other minerals (P≤0.05) (Fig. 3A). Concerning the values reported by Romero et al. (1994) for copper, iron, zinc and manganese content (0.001, 0.003, 0.001 y 0.002 mg g-1 of fresh sample, respectively), it is possible to observe that there were higher concentrations of these minerals in pineapple guava grown in La Vega (Cundinamarca), compared to the ones grown in North Spain. Such information can be related with the different agroecological conditions and the implemented farming techniques, such as fertilizer applications and organic compost that can improve the quality of the soil and, hence, fruits (Marzouk and Kassem, 2011).
Within the macronutrients (Fig. 3B), it was evident that there is not a homogeneous behavior. On one hand, the sodium, potassium and phosphorous increased their proportion in the sample, while magnesium decreased gradually as ripening progresses. Besides, the data revealed that there is a low amount of calcium in pineapple guava (between 0.04 and 0.14 mg g-1 dry sample), which can be related to the high degree of browning appreciated in this fruit; concerning this phenomenon, in some studies, it has been stated that a calcium deficiency in fruits directly affects the integrity and firmness of cell tissue, which unchains internal processes of decomposition that result in a higher degree of browning in the pulp of the fruit (Hofman et al., 2002; Manganaris et al., 2007).
The calcium, potassium and sodium contents were higher than reported by Romero et al. (1994) (0.14, 1.33 and 0.049 mg g-1 of fresh sample, respectively) and were in the ranges reported for these macrominerals by Schotsmans et al. (2011). The large difference in calcium content between the two pineapple guava fruits could be associated with the supply of fertilizers and compost, irrigation and vegetative/ productive balance (Hofman et al., 2002), which, for the fruit tested on this study, could favor calcium absorption during its growth.
The amount of micro and macrominerals identified in pineapple guava, makes of this fruit a potential source of such elements. Just the same, it shows that its mineral content in the fruit may vary according to the origin of the plant, its degree of ripening, soil conditions, climate and farming practices (Konczak and Roulle, 2011).
Nitrogen and protein determination
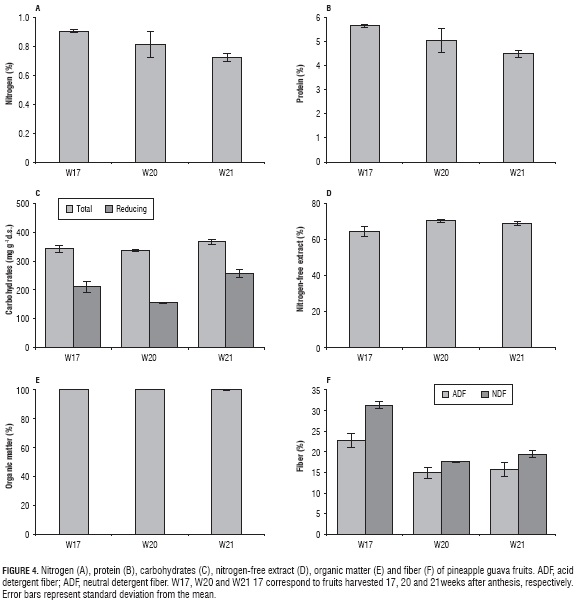
The protein content did not surpass 5.63%, reducing as the ripening stage advanced (Fig. 4), hinting at the possibility of a complex behavior of proteins present in pineapple guava, which can be supported based on the fact that some of the proteins present in fruits express functional properties during ripening, associated to the activation or deactivation of metabolic routes (Toledo et al., 2012).
Determination of total and reducing carbohydrates
The total carbohydrates in the pineapple guava presented a slight increase between W17 and W21 (Fig. 4C), referable to the low content of starch that the fruit has. Therefore, the development of sugars such as fructose, saccharose and glucose, predominant in the pineapple guava (Rodríguez et al., 2006) is not very significant; despite this, the total content of carbohydrates is high compared to other fruits such as bitter guava (unpublished data), probably due to the effect of temperature during development in the growth, just as reported for fruits such as strawberry (Wang and Camp, 2000). This way, the typical low temperature at the crop location (∼18°C) could have influenced the conservation of carbohydrates since, at higher temperatures, metabolic processes increase and, therefore, their consumption since they are an energy reserve for the fruit.
The reducing carbohydrates were noticeably high (Fig. 4C) which can be associated with the characteristic browning of pineapple guava pulp, which, additional to calcium effect, can be influenced by the action of free radical precursors in oxidative processes, such as aldehyde functional groups of free ketones, typical of reducing carbohydrates (Rivera, 2005).
Additionally, there was a decrease of both total and reducing carbohydrates between W17 and W20 (Fig. 4C), which might indicate that the fruit still presents a slight physiological process of the development and formation, given that there is still consumption of metabolic reserves and no significant accumulations of sugar reserve have started to appear (Pimienta et al., 2004). This behavior could be supported with the data reported for size and mass on this same study and with the behavior of mineral synthesis.
The statistical analysis showed that there are significant differences (P≤0.05) between W17 and W21 for both total and reducing carbohydrates.
Determination of nitrogen-free extract and organic matter
The content of nitrogen-free extract (Fig. 4D) and organic matter (Fig. 4E) and gross energy differed significantly (P≤0.05) between W17 and W21. The percentage of the nitrogen-free extract reported in Fig. 4D represents and approximate of cellulose-free carbohydrates (reducing and non-reducing sugars, hemicellulose, gums and part of lignin) (Mora, 2007); this way, the drastic raise evidenced between W17 and W21 indicates that such compounds increase inside the fruit, as an answer to metabolic processes of ripening.
Despite the changes that pineapple guava experienced in composition throughout the three studied stages of ripening, a significant reduction was not perceived in the gross energy of the fruit, indicating that the metabolic processes associated with such changes only produced a reduction of 7.02% of starting gross energy in W17 (3.7274 ±0.0791 kcal g-1 dry sample), this means, approximately 0.2617 kcal g-1 dry sample were consumed in ripening.
Determination of acid detergent fiber (ADF) and neutral detergent fiber (NDF)
Content of neutral detergent fiber (NDF) in pineapple guava decreased substantially by 11.92 percentile points between W17 and W21 (Fig. 4F). This decrease means a loss of structure in the cell wall, attributed to a deterioration of lignin and, therefore, to the release of cellulose and hemicellulose, which is exposed to the metabolic processes of the fruit, increasing its digestibility (Robles, 2009). For the ADF and NDF, significant differences (P<0.05) were found between W17 and W20.
Vitamin content
Determination of vitamin C (ascorbic acid)
As was expected, the content of vitamin C in pineapple guava is higher in W17 than in the other two ripening stages since, in green fruits, the concentration of ascorbic acid is higher. Just the same, the reduction presented in this fruit between W17 and W20 is coherent with the rise in degradation of fruit tissue as the ripening stages occur (Tavarini et al., 2008), which seems analogous to the neutral detergent fiber data reported on this paper, given that the NDF content decreases approximately 43.83% between W17 and W20. Significant differences were found among the three ripening stages (P≤0.05) for the content of vitamin C.
According to the classification of fruits in function of the ascorbic acid content mentioned by Ríos et al. (2012), pineapple guava could be considered as an excellent source of vitamin C.
However, other authors have stated that the quantities of ascorbic acid vary among the different ripening stages, in other studies, such as carried out on the strawberry, proved a totally opposed behavior, where the content of this vitamin increased through ripening; leading to the conclusion that the genotype, growth and post-harvest handling factors, specific for each fruit, could be determined the behavior of vitamin C content (Pineli et al., 2011).
Determination of vitamin A (β-carotene)
The content of (β-carotene in W17 and W21 decreased by 2.56 mg g-1 of dry sample. This was possibly due to the metabolic processes that the fruit suffers during the ripening process. Different factors influence this process, the time of harvesting, handling and storage that can have an effect on conservation of the fruit (Tavarini et al., 2008). Significant differences were found among the three ripening stages (P≤0.05) for the content of vitamin A.
Conclusions
Pineapple guava is a promising fruit within Colombian agricultural production thanks to its nutritional properties, making the high levels of pro-vitamin A, vitamin C and fiber stand out, something that is compatible with current consumption trends. The contents of crude fiber and total carbohydrates were significant in the three evaluated ripening stages, whereas the levels of vitamin C and vitamin A were highest in W17.
For the physicochemical characterization, the development in size and mass of the pineapple guava after surpassing the physiological ripening state might be attributed to the specific physiological behavior. Just the same, it is important to highlight that agroecological conditions could influence the properties and the metabolic processes of the fruit during the ripening. Besides, the high degree of browning in the pulp can be correlated with the low level of calcium and the high content of carbohydrates reported in this study.
Acknowledgements
The main author expresses sincere thanks to the Administrative Department of Science, Technology and Innovation of Colombia for the support given through the program "Jóvenes Investigadores e Innovadores de Colciencias, Generación del Bicentenario - 2012". Also, thanks are in order to Eng. Fabio Barrero for his collaboration in the acquisition of the vegetative material used in this study.
Literature cited
Ali, M., M.R. Weisbjerg, J.W. Cone, G. Van Duinkerken, M.C. Blok, M. Bruinenberge, and W.H. Hendriks. 2012. Postruminal degradation of crude protein, neutral detergent fibre and starch of maize and grass silages in dairy cows. Anim. Feed Sci. Tech. 177, 172-179. Doi: 10.1016/j.anifeedsci.2012.08.015.
AOAC. 1997. Official methods of analysis of AOAC International. 16th ed. Association of Official Analytical Chemists, Gaithersburg, MD.
Arias, F., L. Tamara, and F. Arbeláez. 2006. Apuesta exportadora agropecuaria 2006-2020. Ministerio de Agricultura y Desarrollo Rural de Colombia, Bogotá.
Beckles, D.M. 2012. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Tec. 63, 129-140. Doi: 10.1016/j. postharvbio.2011.05.016.
Beyhan, Ö., M. Elmastas, and F. Gedikli. 2010. Total phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of pineapple guava (Acca sellowiana, Myrtaceae). J. Med. Plants Res. 4, 1065-1072.
Bontempo, P., L. Mita, M. Miceli, A. Doto, A. Nebbioso, F. De Bellis, and F.A.M. Molinari. 2007. Pineapple guava sellowiana derived natural flavone exerts anti-cancer action displaying HDAC inhibitory activities. Int. J. Biochem. Cell Biol. 39, 1902-1914. Doi: 10.1016/j.biocel.2007.05.010.
Clerici, M.T.P.S. and L.B. Carvalho-Silva. 2011. Nutritional bioactive compounds and technological aspects of minor fruits grown in Brazil. Food Res. Int. 44, 1658-1670. Doi: 10.1016/j. foodres.2011.04.020.
Combariza, L.F., C. Neira, G. Fischer, G. Corredor, and O.C. Quintero. 2007. Crecimiento, producción y calidad de fruta en feijoa (Acca sellowiana (O. Berg) Burret) en respuesta al nitrato de potasio, fosfato de potasio y ethephon. Rev. Colomb. Cienc. Hortic. 1, 170-181. Doi: 10.17584/rcch.2007v1i2.1158.
Esemann-Quadros, K., A.P. Mota, G.B. Kerbauy, M.P. Guerra, J.P.H.J. Ducroquet, and R. Pescador. 2008. Estudo anatômico do crescimento do fruto em Acca sellowiana Berg. Rev. Bras. Frutic. 30, 296-302. Doi: 10.1590/S0100-29452008000200005.
Fischer, G. 2003. Ecofisiología, crecimiento y desarrollo de la feijoa. pp. 9-26. In: Fischer, G., D. Miranda, G. Cayón, and M. Mazorra (eds.). Cultivo, poscosecha y exportación de la feijoa (Acca sellowiana Berg). Produmedios, Bogotá.
García, M., J. Rugel, E. Rodríguez S., and E.M. Vargas S. 2010. Aprovechamiento de cilantro (Coriandrum sativum) y perejil (Petrosilenum crispum) aplicando procesos combinados de deshidratación. Xpress Estudio Gráfico y Digital, Bogotá.
Goldman, E. and L.H. Green. 2015. Practical handbook of microbiology. 2nd ed. CRC Press, New York. NY.
Goñi, O., M.T. Sánchez-Ballesta, C. Merodio, and M.I. Escribano. 2010. Ripening-related defense proteins in Annona fruit. Postharvest Biol. Tec. 55, 169-173. Doi: 10.1016/j. postharvbio.2009.11.001.
Harman, J.E. 1987. Pineapple guava fruit: growth and chemical composition during development. N. Z. J. Exp. Agric. 15, 209215. Doi: 10.1080/03015521.1987.10425561.
Hofman, P.J., S. Vuthapanich, A.W. Whiley, A. Klieber, and D.H. Simons. 2002. Tree yield and fruit minerals concentrations influence 'Hass' avocado fruit quality. Sci. Hortic. 92, 113-123. Doi: 10.1016/S0304-4238(01)00286-2.
Konczak, I. and P. Roulle. 2011. Nutritional properties of commercially grown native Australian fruits: lipophilic antioxidants and minerals. Food Res. Int. 44, 2339-2344. Doi: 10.1016/j. foodres.2011.02.023.
Krontveit, R.I., E.A. Bendiksen, and A. Aunsmo. 2014. Field monitoring of feed digestibility in Atlantic salmon farming using crude fiber as an inert marker. Aquaculture 426-427, 249-255. Doi: 10.1016/j.aquaculture.2014.02.015.
Lim, T.K. 2012. Acca sellowiana. Edible medicinal and non-medicinal plants. Springer, The Netherlands. pp. 601-608. Doi: 10.1007/978-94-007-2534-8_81.
López-Vargas, J.H., J. Fernández-López, J.A. Pérez-Álvarez, and M. Viuda-Martos. 2014. Quality characteristics of pork burger added with albedo-fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co-products. Meat Sci. 97, 270-276. Doi: 10.1016/j.meatsci.2014.02.010.
Manganaris, G.A., M. Vasilakakis, G. Diamantidis, and I. Mig-nani. 2007. The effect of postharvest calcium application on tissue calcium concentration, quality attributes, incidence of flesh browning and cell wall physicochemical aspects of peach fruits. Food Chem. 100, 1385-1392. Doi: 10.1016/j. foodchem.2005.11.036.
Marques, L.G., M.M. Prado, and J.T. Freire. 2009. Rehydration characteristics of freeze-dried tropical fruits. LWT-Food Sci. Technol. 42, 1232-1237. Doi: 10.1016/j.lwt.2009.02.012.
Marzouk, H.A. and H.A. Kassem. 2011. Improving fruit quality, nutritional value and yield of Zaghloul dates by the application of organic and/or mineral fertilizers. Sci. Hortic. 127, 249-254. Doi: 10.1016/j.scienta.2010.10.005.
Milošević, T., N. Milošević, and P. Maskovic. 2015. Do the rootstocks determine tree growth, productivity and fruit quality of pears, which grow on typical heavy and acidic soil? Erwerbs- Obstbau 57, 125-134. Doi: 10.1007/s10341-015-0239-5.
Mogollón, C.G., K.I.C. Regno, and S.D. Sarria. 2010. Evaluación poscosecha y estimación de vida útil de guayaba fresca utilizando el modelo de Weibull. Acta Agron. 59, 347-355.
Mora B., I. 2007. Aspectos generales sobre nutrición animal. pp. 11-24. In: Nutrición animal. Universidad Estatal a distancia San Jose de Costa Rica, San Jose.
Moreno, M.A., I.C. Zampini, M. Costamagna, J.E. Sayago, R.M. Ordoñez, and M.I. Isla. 2014. Phytochemical composition and antioxidant capacity of Psidium guajava fresh fruits and flour. Food Nutr. Sci. 5, 725-732. Doi: 10.4236/fns.2014.58082.
Najmus S., A.A. and P.J. Whitney. 2011. Differential behaviour of the dinitrosalicylic acid (DNS) reagent towards mono- and di-saccharide sugars. Biomass Bioenergy 35, 4748-4750. Doi: 10.1016/j.biombioe.2011.09.013.
Parra, A. and G. Fischer. 2013. Maduración y comportamiento poscosecha de la feijoa (Acca sellowiana (O. Berg) Burret). Una revision. Rev. Colomb. Cienc. Hortic. 7, 98-110. 10.17584/rcch.2013v7i1.2039.
Parra-Coronado, A., G. Fischer, and J.H. Camacho. 2015. Development and quality of pineapple guava fruit in two locations with different altitudes in Cundinamarca, Colombia. Bragantia 74(3), 359-366. Doi: 10.1590/1678-4499.0459.
Perea D., M., G. Fischer, and D. Miranda. 2010. Feijoa Acca sellowiana Berg. pp. 330-349. In: Perea D., M., L.P. Matallana R., and A. Tirado P. (eds.). Biotecnología aplicada al mejoramiento de los cultivos de frutas tropicales. Facultad de Ciencias, Universidad Nacional de Colombia, Bogotá.
Pimienta, E., S. Mena, C. Robles, and E. Pimienta. 2004. Relaciones entre carbohidratos de reserva, azúcares reductores, crecimiento y fenología del pitayo de querétaro [Stenocereus que-retaroensis (Weber) Buxbaum]. Rev. Fitotec. Mex. 27, 101-106.
Pineli, L., C.L. Moretti, M.S. Dos Santos, A.B. Campos, A.V. Brasileiro, A.C. Córdova, and M.D. Chiarello. 2011. Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. J. Food Comp. Anal. 24, 11-16. Doi: 10.1016/j.jfca.2010.05.004.
Ramírez, J.M., J.A. Galvis, and G. Fischer. 2005. Maduración poscosecha de la feijoa (Acca sellowiana Berg) tratada con CaCl2 en tres temperaturas de almacenamiento. Agron. Colomb. 23, 117-127.
Repo, R. and C.R. Encina Z. 2008. Determinación de la capacidad antioxidante y compuestos bioactivos de frutas nativas peruanas. Rev. Soc. Quím. Perú 74, 108-124.
Ríos S., V., P.A. Pimenta P., F. Queiroz, S.V. Borges, and J.D. Souza C. 2012. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 134, 381-386. Doi: 10.1016/j.foodchem.2012.02.191.
Rivera, E.I.V. 2005. Prácticas de bioquímica descriptiva: reacciones características para la identificación de carbohidratos. Vol. 51. Editorial UniSon, Hermosillo, Mexico.
Robles R., J.M. 2009. Digestión del forraje íntegro y de las paredes celulares en los pastos Pennisetum purpureum CT 115, Brachiaria humidicola y en Saccharum officinarum. MSc thesis. Specialization in Livestock, Colegio de Postgraduados. Montecillo, Mexico.
Rodríguez, M., H.E. Arjona, and H.A. Campos. 2006. Caracterización fisicoquímica del crecimiento y desarrollo de los frutos de feijoa (Acca sellowiana Berg) en los clones 41 (Quimba) y 8-4. Agron. Colomb. 24, 54-61.
Rodríguez, M., H. Arjona, G. Fischer, H. Campos, and M. Chaparro. 2010. Aspectos anatómicos del desarrollo del fruto de feijoa [Accasellowiana (O.Berg) Burret]. Rev. Fac. Nal Agr. Medellín 63, 5267-5273.
Romero R., M.A., M.L. Vázquez O., J. López H., and J. Simal L. 1994. Composition of babaco, pineapple guava, passionfruit and tamarillo produced in Galicia (North-west Spain). Food Chem. 49(1): 23-27. Doi: 10.1016/0308-8146(94)90227-5.
Santoni, F., J. Paolini, T. Barboni, and J. Costa. 2014. Relationships between the leaf and fruit mineral compositions of Actinidia deliciosa var. Hayward according to nitrogen and potassium fertilization. Food Chem. 147, 269-271. Doi: 10.1016/j.foodchem.2013.09.154.
Sanusi, R.A. and A.E. Adebiyi. 2009. Beta carotene content of commonly consumed foods and soups in Nigeria. Pakistan J. Nut. 8, 1512-1516. Doi: 10.3923/pjn.2009.1512.1516
Schotsmans, W.C., A. East, G. Thorp, A.B. Woolf, and E.M. Yahia. 2011. Pineapple guava (Acca sellowiana [Berg] Burret). pp. 115133. In: Yahia, E.M. (ed.). Postharvest biology and technology of tropical and subtropical fruits. Vol. 3: Cocona to mango, Woodhead Publishing, Cambridge, UK.
Śmiechowska, M. and P. Dmowski. 2006. Crude fibre as a parameter in the quality evaluation of tea. Food Chem. 94, 366-368. Doi: 10.1016/j.foodchem.2004.11.026.
Tavarini, S., E. Degl'Innocenti, D. Remorini, R. Massai, and L. Guidi. 2008. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 107, 282-288. Doi: 10.1016/j. foodchem.2007.08.015.
Toledo, T.T., S.B. Nogueira, B.R. Cordenunsi, F.C. Gozzo, E.J. Pilau, F.M. Lajolo, and J. R.O. do Nascimento. 2012. Proteomic analysis of banana fruit reveals proteins that are differentially accumulated during ripening. Postharvest Biol. Tecnol. 70, 51-58. Doi: 10.1016/j.postharvbio.2012.04.005.
Valderrama, J.K., G. Fischer, and M.S. Serrano. 2005. Fisiología poscosecha en frutos de dos cultivares de feijoa (Acca sellowiana O. Berg Burret) sometidos a un tratamiento cuarentenario de frío. Agron. Colomb. 23, 276-282.
Wang, S.Y. and M.J. Camp. 2000. Temperatures after bloom affect plant growth and fruit quality of strawberry. Sci. Hortic. 85, 183-199. Doi: 10.1016/S0304-4238(99)00143-0.
Weston, R.J. 2010. Bioactiveproducts from fruit of the feijoa (Feijoa sellowiana, Myrtaceae): A review. Food Chem. 121, 923-926. Doi: 10.1016/j.foodchem.2010.01.047.
Referencias
Ali, M., M.R. Weisbjerg, J.W. Cone, G. Van Duinkerken, M.C. Blok, M. Bruinenberge, and W.H. Hendriks. 2012. Postruminal degradation of crude protein, neutral detergent fibre and starch of maize and grass silages in dairy cows. Anim. Feed Sci. Tech. 177, 172-179. Doi: 10.1016/j.anifeedsci.2012.08.015.
AOAC. 1997. Official methods of analysis of AOAC International. 16th ed. Association of Official Analytical Chemists, Gaithersburg, MD.
Arias, F., L. Tamara, and F. Arbeláez. 2006. Apuesta exportadora agropecuaria 2006-2020. Ministerio de Agricultura y Desarrollo Rural de Colombia, Bogotá.
Beckles, D.M. 2012. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Tec. 63, 129-140. Doi: 10.1016/j. postharvbio.2011.05.016.
Beyhan, Ö., M. Elmastas, and F. Gedikli. 2010. Total phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of pineapple guava (Acca sellowiana, Myrtaceae). J. Med. Plants Res. 4, 1065-1072.
Bontempo, P., L. Mita, M. Miceli, A. Doto, A. Nebbioso, F. De Bellis, and F.A.M. Molinari. 2007. Pineapple guava sellowiana derived natural flavone exerts anti-cancer action displaying HDAC inhibitory activities. Int. J. Biochem. Cell Biol. 39, 1902-1914. Doi: 10.1016/j.biocel.2007.05.010.
Clerici, M.T.P.S. and L.B. Carvalho-Silva. 2011. Nutritional bioactive compounds and technological aspects of minor fruits grown in Brazil. Food Res. Int. 44, 1658-1670. Doi: 10.1016/j. foodres.2011.04.020.
Combariza, L.F., C. Neira, G. Fischer, G. Corredor, and O.C. Quintero. 2007. Crecimiento, producción y calidad de fruta en feijoa (Acca sellowiana (O. Berg) Burret) en respuesta al nitrato de potasio, fosfato de potasio y ethephon. Rev. Colomb. Cienc. Hortic. 1, 170-181. Doi: 10.17584/rcch.2007v1i2.1158.
Esemann-Quadros, K., A.P. Mota, G.B. Kerbauy, M.P. Guerra, J.P.H.J. Ducroquet, and R. Pescador. 2008. Estudo anatômico do crescimento do fruto em Acca sellowiana Berg. Rev. Bras. Frutic. 30, 296-302. Doi: 10.1590/S0100-29452008000200005.
Fischer, G. 2003. Ecofisiología, crecimiento y desarrollo de la feijoa. pp. 9-26. In: Fischer, G., D. Miranda, G. Cayón, and M. Mazorra (eds.). Cultivo, poscosecha y exportación de la feijoa (Acca sellowiana Berg). Produmedios, Bogotá.
García, M., J. Rugel, E. Rodríguez S., and E.M. Vargas S. 2010. Aprovechamiento de cilantro (Coriandrum sativum) y perejil (Petrosilenum crispum) aplicando procesos combinados de deshidratación. Xpress Estudio Gráfico y Digital, Bogotá.
Goldman, E. and L.H. Green. 2015. Practical handbook of microbiology. 2nd ed. CRC Press, New York. NY.
Goñi, O., M.T. Sánchez-Ballesta, C. Merodio, and M.I. Escribano. 2010. Ripening-related defense proteins in Annona fruit. Postharvest Biol. Tec. 55, 169-173. Doi: 10.1016/j. postharvbio.2009.11.001.
Harman, J.E. 1987. Pineapple guava fruit: growth and chemical composition during development. N. Z. J. Exp. Agric. 15, 209215. Doi: 10.1080/03015521.1987.10425561.
Hofman, P.J., S. Vuthapanich, A.W. Whiley, A. Klieber, and D.H. Simons. 2002. Tree yield and fruit minerals concentrations influence 'Hass' avocado fruit quality. Sci. Hortic. 92, 113-123. Doi: 10.1016/S0304-4238(01)00286-2.
Konczak, I. and P. Roulle. 2011. Nutritional properties of commercially grown native Australian fruits: lipophilic antioxidants and minerals. Food Res. Int. 44, 2339-2344. Doi: 10.1016/j. foodres.2011.02.023.
Krontveit, R.I., E.A. Bendiksen, and A. Aunsmo. 2014. Field monitoring of feed digestibility in Atlantic salmon farming using crude fiber as an inert marker. Aquaculture 426-427, 249-255. Doi: 10.1016/j.aquaculture.2014.02.015.
Lim, T.K. 2012. Acca sellowiana. Edible medicinal and non-medicinal plants. Springer, The Netherlands. pp. 601-608. Doi: 10.1007/978-94-007-2534-8_81.
López-Vargas, J.H., J. Fernández-López, J.A. Pérez-Álvarez, and M. Viuda-Martos. 2014. Quality characteristics of pork burger added with albedo-fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co-products. Meat Sci. 97, 270-276. Doi: 10.1016/j.meatsci.2014.02.010.
Manganaris, G.A., M. Vasilakakis, G. Diamantidis, and I. Mig-nani. 2007. The effect of postharvest calcium application on tissue calcium concentration, quality attributes, incidence of flesh browning and cell wall physicochemical aspects of peach fruits. Food Chem. 100, 1385-1392. Doi: 10.1016/j. foodchem.2005.11.036.
Marques, L.G., M.M. Prado, and J.T. Freire. 2009. Rehydration characteristics of freeze-dried tropical fruits. LWT-Food Sci. Technol. 42, 1232-1237. Doi: 10.1016/j.lwt.2009.02.012.
Marzouk, H.A. and H.A. Kassem. 2011. Improving fruit quality, nutritional value and yield of Zaghloul dates by the application of organic and/or mineral fertilizers. Sci. Hortic. 127, 249-254. Doi: 10.1016/j.scienta.2010.10.005.
Milošević, T., N. Milošević, and P. Maskovic. 2015. Do the rootstocks determine tree growth, productivity and fruit quality of pears, which grow on typical heavy and acidic soil? Erwerbs- Obstbau 57, 125-134. Doi: 10.1007/s10341-015-0239-5.
Mogollón, C.G., K.I.C. Regno, and S.D. Sarria. 2010. Evaluación poscosecha y estimación de vida útil de guayaba fresca utilizando el modelo de Weibull. Acta Agron. 59, 347-355.
Mora B., I. 2007. Aspectos generales sobre nutrición animal. pp. 11-24. In: Nutrición animal. Universidad Estatal a distancia San Jose de Costa Rica, San Jose.
Moreno, M.A., I.C. Zampini, M. Costamagna, J.E. Sayago, R.M. Ordoñez, and M.I. Isla. 2014. Phytochemical composition and antioxidant capacity of Psidium guajava fresh fruits and flour. Food Nutr. Sci. 5, 725-732. Doi: 10.4236/fns.2014.58082.
Najmus S., A.A. and P.J. Whitney. 2011. Differential behaviour of the dinitrosalicylic acid (DNS) reagent towards mono- and di-saccharide sugars. Biomass Bioenergy 35, 4748-4750. Doi: 10.1016/j.biombioe.2011.09.013.
Parra, A. and G. Fischer. 2013. Maduración y comportamiento poscosecha de la feijoa (Acca sellowiana (O. Berg) Burret). Una revision. Rev. Colomb. Cienc. Hortic. 7, 98-110. 10.17584/rcch.2013v7i1.2039.
Parra-Coronado, A., G. Fischer, and J.H. Camacho. 2015. Development and quality of pineapple guava fruit in two locations with different altitudes in Cundinamarca, Colombia. Bragantia 74(3), 359-366. Doi: 10.1590/1678-4499.0459.
Perea D., M., G. Fischer, and D. Miranda. 2010. Feijoa Acca sellowiana Berg. pp. 330-349. In: Perea D., M., L.P. Matallana R., and A. Tirado P. (eds.). Biotecnología aplicada al mejoramiento de los cultivos de frutas tropicales. Facultad de Ciencias, Universidad Nacional de Colombia, Bogotá.
Pimienta, E., S. Mena, C. Robles, and E. Pimienta. 2004. Relaciones entre carbohidratos de reserva, azúcares reductores, crecimiento y fenología del pitayo de querétaro [Stenocereus que-retaroensis (Weber) Buxbaum]. Rev. Fitotec. Mex. 27, 101-106.
Pineli, L., C.L. Moretti, M.S. Dos Santos, A.B. Campos, A.V. Brasileiro, A.C. Córdova, and M.D. Chiarello. 2011. Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. J. Food Comp. Anal. 24, 11-16. Doi: 10.1016/j.jfca.2010.05.004.
Ramírez, J.M., J.A. Galvis, and G. Fischer. 2005. Maduración poscosecha de la feijoa (Acca sellowiana Berg) tratada con CaCl2 en tres temperaturas de almacenamiento. Agron. Colomb. 23, 117-127.
Repo, R. and C.R. Encina Z. 2008. Determinación de la capacidad antioxidante y compuestos bioactivos de frutas nativas peruanas. Rev. Soc. Quím. Perú 74, 108-124.
Ríos S., V., P.A. Pimenta P., F. Queiroz, S.V. Borges, and J.D. Souza C. 2012. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 134, 381-386. Doi: 10.1016/j.foodchem.2012.02.191.
Rivera, E.I.V. 2005. Prácticas de bioquímica descriptiva: reacciones características para la identificación de carbohidratos. Vol. 51. Editorial UniSon, Hermosillo, Mexico.
Robles R., J.M. 2009. Digestión del forraje íntegro y de las paredes celulares en los pastos Pennisetum purpureum CT 115, Brachiaria humidicola y en Saccharum officinarum. MSc thesis. Specialization in Livestock, Colegio de Postgraduados. Montecillo, Mexico.
Rodríguez, M., H.E. Arjona, and H.A. Campos. 2006. Caracterización fisicoquímica del crecimiento y desarrollo de los frutos de feijoa (Acca sellowiana Berg) en los clones 41 (Quimba) y 8-4. Agron. Colomb. 24, 54-61.
Rodríguez, M., H. Arjona, G. Fischer, H. Campos, and M. Chaparro. 2010. Aspectos anatómicos del desarrollo del fruto de feijoa [Accasellowiana (O.Berg) Burret]. Rev. Fac. Nal Agr. Medellín 63, 5267-5273.
Romero R., M.A., M.L. Vázquez O., J. López H., and J. Simal L. 1994. Composition of babaco, pineapple guava, passionfruit and tamarillo produced in Galicia (North-west Spain). Food Chem. 49(1): 23-27. Doi: 10.1016/0308-8146(94)90227-5.
Santoni, F., J. Paolini, T. Barboni, and J. Costa. 2014. Relationships between the leaf and fruit mineral compositions of Actinidia deliciosa var. Hayward according to nitrogen and potassium fertilization. Food Chem. 147, 269-271. Doi: 10.1016/j.foodchem.2013.09.154.
Sanusi, R.A. and A.E. Adebiyi. 2009. Beta carotene content of commonly consumed foods and soups in Nigeria. Pakistan J. Nut. 8, 1512-1516. Doi: 10.3923/pjn.2009.1512.1516
Schotsmans, W.C., A. East, G. Thorp, A.B. Woolf, and E.M. Yahia. 2011. Pineapple guava (Acca sellowiana [Berg] Burret). pp. 115133. In: Yahia, E.M. (ed.). Postharvest biology and technology of tropical and subtropical fruits. Vol. 3: Cocona to mango, Woodhead Publishing, Cambridge, UK.
Śmiechowska, M. and P. Dmowski. 2006. Crude fibre as a parameter in the quality evaluation of tea. Food Chem. 94, 366-368. Doi: 10.1016/j.foodchem.2004.11.026.
Tavarini, S., E. Degl'Innocenti, D. Remorini, R. Massai, and L. Guidi. 2008. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 107, 282-288. Doi: 10.1016/j. foodchem.2007.08.015.
Toledo, T.T., S.B. Nogueira, B.R. Cordenunsi, F.C. Gozzo, E.J. Pilau, F.M. Lajolo, and J. R.O. do Nascimento. 2012. Proteomic analysis of banana fruit reveals proteins that are differentially accumulated during ripening. Postharvest Biol. Tecnol. 70, 51-58. Doi: 10.1016/j.postharvbio.2012.04.005.
Valderrama, J.K., G. Fischer, and M.S. Serrano. 2005. Fisiología poscosecha en frutos de dos cultivares de feijoa (Acca sellowiana O. Berg Burret) sometidos a un tratamiento cuarentenario de frío. Agron. Colomb. 23, 276-282.
Wang, S.Y. and M.J. Camp. 2000. Temperatures after bloom affect plant growth and fruit quality of strawberry. Sci. Hortic. 85, 183-199. Doi: 10.1016/S0304-4238(99)00143-0.
Weston, R.J. 2010. Bioactiveproducts from fruit of the feijoa (Feijoa sellowiana, Myrtaceae): A review. Food Chem. 121, 923-926. Doi: 10.1016/j.foodchem.2010.01.047.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Yatheesharadhya Bylappa, Anish Nag. (2025). A statistical approach to study anatomical changes of pink guava cultivar (Psidium guajava L. cv Arka Kiran) during its ripening at the room temperature storage. Kuwait Journal of Science, 52(1), p.100295. https://doi.org/10.1016/j.kjs.2024.100295.
2. Andrea Milena Sánchez-Riaño, José Fernando Solanilla-Duque, Jonh Jairo Méndez-Arteaga, Henry Alexander Váquiro-Herrera. (2020). Bioactive potential of Colombian feijoa in physiological ripening stage. Journal of the Saudi Society of Agricultural Sciences, 19(4), p.299. https://doi.org/10.1016/j.jssas.2019.05.002.
3. Duke G Omayio, George O Abong’, Michael W Okoth, Charles K Gachuiri, Agnes W Mwangombe. (2022). Physicochemical and Processing Qualities of Guava Varieties in Kenya. International Journal of Fruit Science, 22(1), p.329. https://doi.org/10.1080/15538362.2022.2039342.
4. Adriana María Castro, Luis Eduardo Díaz, Maria Ximena Quintanilla-Carvajal, Edgar Yesid Mayorga, Fabián Leonardo Moreno. (2023). Convective drying of feijoa (Acca sellowiana Berg): A study on bioactivity, quality, and drying parameters. LWT, 186, p.115209. https://doi.org/10.1016/j.lwt.2023.115209.
5. Diana Cristina Sinuco León, Darling Katherine Rubio Ortíz, Diego Fernando Jaimes González. (2020). Sensory approach and chiral analysis for determination of odour active compounds from feijoa (Acca sellowiana). Food Chemistry, 317, p.126383. https://doi.org/10.1016/j.foodchem.2020.126383.
6. Jacqueline Oseko, Andrew East, Julian Heyes. (2022). Recent advances in the postharvest technology of feijoa. Scientia Horticulturae, 297, p.110969. https://doi.org/10.1016/j.scienta.2022.110969.
7. Shafa Nayab, Kashif Razzaq, Sami Ullah, Ishtiaq Ahmad Rajwana, Muhammad Amin, Ambreen Naz. (2020). Harvest Maturity Influences Fruit Quality of Carambola (Averrhoa carambola L.). Journal of Horticultural Science & Technology, , p.109. https://doi.org/10.46653/jhst2034109.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2016 Agronomía Colombiana

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
© Centro Editorial de la Facultad de Ciencias Agrarias, Universidad Nacional de ColombiaSe autoriza la reproducción y citación del material que aparece en la revista, siempre y cuando se indique de manera explícita: nombre de la revista, nombre del autor(es), año, volumen, número y páginas del artículo fuente. Las ideas y afirmaciones consignadas por los autores están bajo su responsabilidad y no interpretan necesariamente las opiniones y políticas de la Universidad Nacional de Colombia. La mención de productos o firmas comerciales en la revista no implica una recomendación o apoyo por parte de la Universidad ni de la Facultad; el uso de tales productos debe ceñirse a las recomendaciones de las etiquetas.
La licencia Creative Commons utilizada por Agronomía Colombiana es la siguiente: Reconocimiento – NoComercial – CompartirIgual (by-nc-sa)

Agronomia Colombiana by Centro Editorial of Facultad de Ciencias Agrarias, Universidad Nacional de Colombia is licensed under a Creative Commons Reconocimiento-NoComercial-CompartirIgual 4.0 Internacional License.
Creado a partir de la obra en http://revistas.unal.edu.co/index.php/agrocol/
Los autores que publican sus artículos en Agronomía Colombiana ceden de manera indefinida, todos los derechos patrimoniales, es decir, transformación, reproducción, comunicación pública, y distribución, y son otorgados sin ninguna limitación en cuanto a territorio se refiere al Centro Editorial de la Facultad de Ciencias Agrarias, Universidad Nacional de Colombia