Mejoramiento de la germinación, control de la hiperhidricidad y formación de brotes en Vasconcellea stipulata Badillo
Enhancement of germination, hyperhydricity control and in vitro shoot formation of Vasconcellea stipulata Badillo
DOI:
https://doi.org/10.15446/rev.colomb.biote.v17n2.43611Palabras clave:
dormancy, germination, embreo culture, hyperhydricity, embryo culture, germinación, cultivo de embriones, hiperhidricidad (es)Título en ingles: Enhancement of germination, hyperhydricity control and in vitro shoot formation of Vasconcellea stipulata Badillo
Vasconcellea stipulata posee una gran importancia comercial debido a su actividad enzimática y como fuente para el mejoramiento genético de papaya, debido a su resistencia al virus de la mancha anular de esta especie. Sin embargo, debido a su baja regeneración por semillas y al limitado conocimiento de sus propiedades genéticas y farmacéuticas, esta especie no es cultivada ampliamente. La propagación a través del cultivo in vitro de semillas se ha usado para contrarrestar este tipo de problema, pero la hiperhidricidad, un trastorno fisiológico, expresado principalmente en los ejes embrionarios en desarrollo y asociado específicamente a esta especie, es una restricción significativa. Con el fin de obtener material de élite para el cultivo de V. stipulata, el objetivo de este trabajo fue incrementar la germinación, controlar la hiperhidricidad en embriones y evaluar el potencial para inducir respuestas morfogénicas, es decir, la formación de brotes. Nuestros resultados mostraron que es posible aumentar la germinación hasta un 53% en condiciones in vitro, dentro de un período más corto en presencia de peróxido de hidrógeno. Además, la hiperhidricidad se redujo significativamente (50%) en condiciones in vitro cuando se incluyó ácido giberélico en bajas concentraciones en el medio 1/2 Nitsch y Nitsch. Esto permitió recuperar hasta aproximadamente el 80% de plántulas viables. Finalmente, otros reguladores de crecimiento vegetal evaluados, indujeron la formación de brotes en yemas axilares y la formación de callos en secciones de hoja derivadas de plántulas.
Vasconcellea stipulata has great commercial importance because of its enzymatic activity and as a source for genetic improvement of papaya since it is resistant to the papaya ringspot virus. However, due to its low regeneration by seeds and limited knowledge of its genetic and pharmaceutical properties, this species is not widely cultivated. For propagation, in vitro culture of seeds has been used to address this problem, but hyperhydricity, a physiological disorder, mainly expressed in the developing embryonic axis and specifically associated with this species, is a significant constraint. In order to obtain elite material for culture of V. stipulata, the aim of this work was to increase germination, to control hyperhydricity in embryos and to evaluate the potential to induce morphogenic responses, i.e., shoot formation. Our results showed that it is possible to increase germination up to 53% under in vitro conditions within a short period in the presence of hydrogen peroxide. In addition, hyperhydricity was significantly reduced (50%) in vitro when gibberellic acid concentrations were included on a 1/2 Nitsch and Nitsch nutrient medium, resulting in approximately 80% recovery of viable seedlings. Finally, other plant growth regulators were evaluated and found to trigger shoot formation in axillary buds as well as induce the formation of callus in leaf sections derived of seedlings.
Key words: germination, embryo culture, hyperhydricity.
DOI: https://doi.org/10.15446/rev.colomb.biote.v17n2.43611
ARTÍCULO DE INVESTIGACIÓN
Enhancement of germination, hyperhydricity control and in vitro shoot formation of Vasconcellea stipulata Badillo
Mejoramiento de la germinación, control de la hiperhidricidad y formación de brotes en Vasconcellea stipulata Badillo
Diego Paúl Vélez-Mora*, Rosa Armijos González**, Miguel Jordán Zimmermann***
* Master in Characterization and Conservation of Biodiversity, Departamento de Ciencias Naturales. Universidad Técnica Particular de Loja. P.C. 1101608. San Cayetano Alto, Ecuador. dpvelez@utpl.edu.ec
** Environmental engineer, Ph.D student. Departamento de Ciencias Naturales. Universidad Técnica Particular de Loja. P.C. 1101608. San Cayetano Alto, Ecuador. rearmijos@utpl.edu.ec
*** Ph.D in Plant Physiology and Plant Biotechnology Instituto de Biotecnología. Universidad Mayor. Camino La Pirámide 5750, Huechuraba, Chile. mjordanz@gmail.com
Recibido: octubre 15 de 2014 Aprobado: septiembre 28 de 2015
Abstract
Vasconcellea stipulata has great commercial importance because of its enzymatic activity and as a source for genetic improvement of papaya since it is resistant to the papaya ringspot virus. However, due to its low regeneration by seeds and limited knowledge of its genetic and pharmaceutical properties, this species is not widely cultivated. For propagation, in vitro culture of seeds has been used to address this problem, but hyperhydricity, a physiological disorder, mainly expressed in the developing embryonic axis and specifically associated with this species, is a significant constraint. In order to obtain elite material for culture of V. stipulata, the aim of this work was to increase germination, to control hyperhydricity in embryos and to evaluate the potential to induce morphogenic responses, i.e., shoot formation. Our results showed that it is possible to increase germination up to 53% under in vitro conditions within a short period in the presence of hydrogen peroxide. In addition, hyperhydricity was significantly reduced (50%) in vitro when gibberellic acid concentrations were included on a 1/2 Nitsch and Nitsch nutrient medium, resulting in approximately 80% recovery of viable seedlings. Finally, other plant growth regulators were evaluated and found to trigger shoot formation in axillary buds as well as induce the formation of callus in leaf sections derived of seedlings.
Key words: germination, embryo culture, hyperhydricity.
Resumen
Vasconcellea stipulata posee una gran importancia comercial debido a su actividad enzimática y como fuente para el mejoramiento genético de papaya, debido a su resistencia al virus de la mancha anular de esta especie. Sin embargo, debido a su baja regeneración por semillas y al limitado conocimiento de sus propiedades genéticas y farmacéuticas, esta especie no es cultivada ampliamente. La propagación a través del cultivo in vitro de semillas se ha usado para contrarrestar este tipo de problema, pero la hiperhidricidad, un trastorno fisiológico, expresado principalmente en los ejes embrionarios en desarrollo y asociado específicamente a esta especie, es una restricción significativa. Con el fin de obtener material de élite para el cultivo de V. stipulata, el objetivo de este trabajo fue incrementar la germinación, controlar la hiperhidricidad en embriones y evaluar el potencial para inducir respuestas morfogénicas, es decir, la formación de brotes. Nuestros resultados mostraron que es posible aumentar la germinación hasta un 53% en condiciones in vitro, dentro de un período más corto en presencia de peróxido de hidrógeno. Además, la hiperhidricidad se redujo significativamente (50%) en condiciones in vitro cuando se incluyó ácido giberélico en bajas concentraciones en el medio 1/2 Nitsch y Nitsch. Esto permitió recuperar hasta aproximadamente el 80% de plántulas viables. Finalmente, otros reguladores de crecimiento vegetal evaluados, indujeron la formación de brotes en yemas axilares y la formación de callos en secciones de hoja derivadas de plántulas.
Palabras clave: germinación, cultivo de embriones, hiperhidricidad.
Introduction
Vasconcellea stipulata B. (toronche) represents a genetic resource to be used for the improvement of common papaya due to its resistance to the papaya ringspot virus disease (Magdalita et al., 1997; Drew et al., 1998). This and other different species and varieties of the genus Vasconcellea are also important due to their potential to confer desirable characteristics to babaco (Vasconcellea × heilbornii 'Babaco'), i.e. phytosanitary problems, cold tolerance and organoleptic characteristics (Guerrero & Castro, 1999) of interest to the nutritional and pharmaceutical industries.
Furthermore, this species has commercial importance as a source of papain (Guerrero & Castro, 1999). This proteolytic enzyme, present in the Caricaceae, shows in V. stipulata enzymatic activity up to 17 times higher than that reported in Carica papaya (Scheldeman, 2002). However, despite its attributes this species is threatened by habitat loss due to deforestation and the conversion of native forest to cropland or pasture (IUCN, 2003).
The generative form in several Vasconcellea species shows limited responses due to seed dormancy, low germination rates and long-term germination up to 240 days with high variability of germination responses between sites (Jiménez et al., 1998; Scheldeman, 2002). Additionally, losses due to vulnerability of the sarcotesta to insects, pathogens and fungal attack are significant (Badillo, 1993). Therefore the establishment of efficient protocols is necessary for multiplication of new individuals, germplasm conservation and production of seedlings with better agronomic traits.
In this report, the specific aims were 1) To present some alternative protocols to increase the percentage of germination with the use of pre-germination treatments, 2) To reduce hyperhydricity tissue by modifying the culture medium and the application of gibberellins, 3) To stimulate shoot regeneration using growth regulators. The latter concerning sprouting of V. stipulata under in vitro conditions is reported here for the first time.
Materials and methods
Determination of viability. The presence and viability of embryos were compared from three provenances: Loja, El Oro (Ecuador), and Ayabaca (Perú), using the triphenyl tetrazolium salt test (TZ) (ISTA, 2005).
In vitro germination. To evaluate the effect of pre-germination treatments, seeds from Loja were used due to the proximity and availability of plant material. The elimination and/or weakening of the sclerotesta were assessed by the application of different concentrations of chemicals and exposure times: hydrogen peroxide (10, 50, 100 % for 20, 30 and 40 min), sulfuric acid (20, 40, 80 % for 10, 15 and 20 min) and sodium hypochlorite (1, 3, 4 % for 15, 20 and 30 min). The concentrations of each chemical are not equal, because the effect of each is different. A total of 27 treatments and a control were applied. Table 2 shows the nine best results of all treatments in the results section. The seeds were then washed to remove residues of each of these substances by submerging them in water for 24 hours and were disinfected with 70% ethanol for 20 sec, followed by 1% sodium hypochlorite for 5 min, and then cultured in MS (Murashige and Skoog, 1962) medium under a photoperiod of 12 hours and photon flux density of 57 µmol-2 s-1 (white fluorescent light 40-W General Electric F40D-EX) and temperature of 21±2 °C, for a period of six months.
Control of hyperhydricity in germinated embryos. During germination, a high percentage of embryonic axes and seedlings showed hyperhydricity symptoms; this caused a substantial loss of plant material. In order to avoid or reduce this constraint, the embryos were extracted from seeds submerged for 24 h in water and then cultured in vitro. To disinfect the seeds the same procedure as for in vitro germination was performed. Embryo extraction was followed by liquid immersion in H2O2, (10 Vol.) for 1 min., and embryos were then cultured in MS and NN (Nitsch and Nitsch, 1969) nutrient media at different concentrations plus gibberellic acid (GA3) to evaluate embryo hyperhydration. Both media contained 2% sucrose and were solidified with 0.7% agar (Bacto TM Agar, BD). The light regime, lamps and photon flux density were the same as for in vitro cultured seeds.
Morphogenic responses. Buds, leaf explants and hypocotyl explants were isolated from six-month old seedlings (approx. 6 cm) and cultured on NN medium with different combinations of plant growth regulators, including 0.54-1 µM of α-naphthaleneacetic acid (NAA) or 6.8 µM of 2.4-dichlorophenoxyacetic acid (2.4-D) in combination with 0.5-2.2 µM of 6-benzylaminopurine (BAP).
Statistical analysis. A factorial design was established for statistical analysis. Each treatment for germination, hyperhydricity and regeneration included five units (individual), five repetitions (flasks) and three replicates (total re-design). Data were registered periodically every five days. Variance analysis (ANOVA) was followed by comparison of the groups' means using the Duncan test at the p = 0.05 level using the R software (R Development Core Team 2012).
Results and discussion
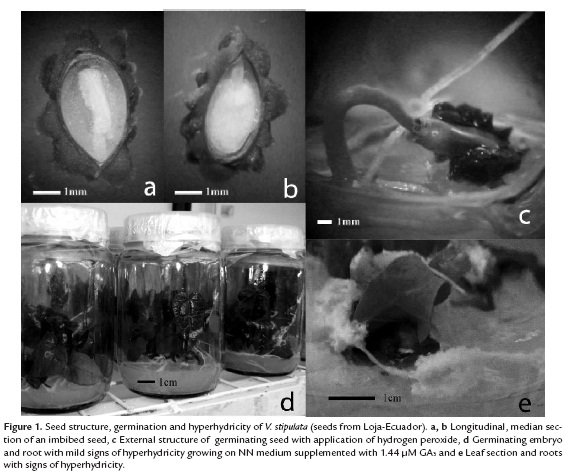
Presence of embryos and viability. Seeds from Ayabaca showed the highest percentages of embryos and viability compared to those from El Oro and Loja (figure 1 a, b). Significant differences in the amount of empty seeds (without embryo) were found in the material collected at Loja, El Oro and Ayabaca (table 1). The high percentage of empty seeds may be due to the existing hybridization in the Vasconcellea genus, according to Kyndt et al. (2005 a, b) who stated that hybridization is common among species of the genus Vasconcellea, with evidence of introgression of V. cundinamarcensis into V. stipulata, both under natural conditions, in the province of Loja (Horovitz & Jiménez, 1967) and in controlled conditions in Venezuela (De Zerpa, 1980). The material from Loja evidenced the greatest amount of fruit with incomplete and non-viable seeds, possibly because this is the center of diversification and hybridization of highland papayas (Scheldeman, 2002).
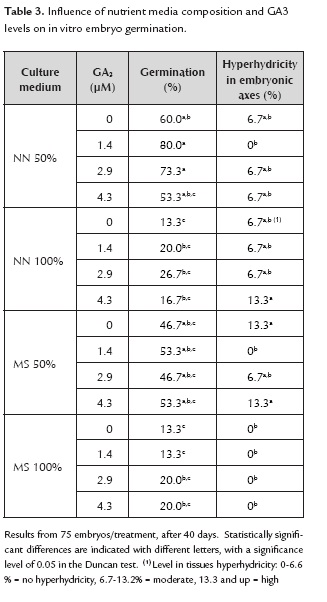
Germination. The use of hydrogen peroxide increased the germination rate (figure 1c), evidencing significant differences in comparison with the other pre-germination treatments (sulfuric acid and sodium hypochlorite) (table 2). According to Scheldeman (2002), the presence of sulfuric acid affects the structure of the embryo and the sarcotesta, limiting responses. By contrast, hydrogen peroxide enabled maximum germination, equivalent to 52.6% by placing the seeds at a concentration of 100% for 30 minutes, after 40 days. This percentage was significantly higher than the control treatment and the results reported by Jiménez et al. (1998) and Scheldeman (2002) where germination was about 0-5% and 32% over 174 days, respectively. The application of hydrogen peroxide has been used to promote germination in several species (Dolatabadian & Modarres-Sanavy, 2008). Although its mechanism is not understood (Klein et al., 2008), it has been mainly attributed to a strong oxidant effect on organic matter (Moreno et al., 2007) coupled with the destruction of some inhibitors present in the outer layers of the seed, including phenols and other compounds, thus enabling gases and moisture to reach the embryo.
According to our results and several other studies, the low germination rates in the species of this genus is not a result of viability loss due to storage or to origin sites of V. stipulata; instead, it would be more attributable to the high site-specific variability existing in the genus (Horovitz & Jiménez, 1967). Carica and Vasconcellea seeds are very similar in structure (Badillo, 2000) and both groups require treatment to promote germination. In Vasconcellea the often-irregular germination could be improved by removal of the sarcotesta and application of GA3 (Scheldeman, 2002). In Carica papaya the sarcotesta and inhibitors present in the fruit can prevent germination, but the effect can be reduced by the application of gibberellic acid and/or potassium nitrate (Pérez et al., 1980; Yahiro & Oryoji, 1980).
Embryo germination and hyperhydricity control. Despite having obtained a relatively high in vitro germination percentage in full seeds by the use of hydrogen peroxide, this was surpassed by isolated embryo culture. The highest germination percentage (80%) was obtained with ½ strength of NN salts in the culture medium in the presence of 1.44 µM GA3 (figure 1d). This is consistent with other reports about the application of GA3 promoting the germination of intact seeds in V. stipulata, V. cundinamarcensis and V. x heilbornii (Scheldeman, 2002). Pérez et al. (1980) also reported that GA3 significantly promoted germination in C. papaya seeds accelerating the transport of nutrients via the endosperm.
Hyperhydricity produces an abnormal anatomy in seedlings of many species, especially in young leaves and hypocotyls that appear swollen and translucent (Acram et al., 1996; Ziv, 1991) and is extremely frequent in seedlings and tissues of V. stipulata (figure 1e). Many factors cause this effect, including a high concentration of nutrient salts or nitrates (Ziv & Ariel, 1992; Ivanova & Standen, 2008). Thus, in C. papaya and some Vasconcellea species, the use of ½ MS salts (De Winnaar, 1988) and/or media with low nitrate levels as NN and woody plant medium (Jordán, 1986; Jordán and Piwanski, 1997) allowed survival and good quality of the plants, the same as in V. pubescens. Current results in V. stipulata apparently showed a lower hyperhydricity in the seedlings developed in presence of both, full MS nutrient medium and in more diluted media (NN, ½ NN salts), reaching levels of hyperhydricity between 6.7 to 13.3%. Another factor that can influence hyperhydricity is the type of agar (Ziv, 1991). In this case the AgarTM Bacto helped to keep hyperhydricity low, with only 13.3% of tissue affected (table 3). A similar result has been reported by Marga et al. (1997) and Ascencio et al. (2008), although the responses depend mainly on agar concentration as well as its preparation (Loreti & Pasqualetto, 1986).
Therefore, to establish a protocol for tissue culture in this species, it is recommended to use seeds from Ayabaca, Peru and El Oro, Ecuador, due to their high percentage of viability and germination. In addition, to start growing from seed or embryo, 1/2 NN with low concentrations of gibberellins can be used, as this favors a high percentage of germination and maintains controlled tissue hyperhydricity. These results could be used not only for growing V. stipulata but also for the in vitro culture of C. papaya, since the latest results show that even this crop has high percentages of hyperhydricity in leaves and roots of material from the somatic embryo's mature cotyledon (Clarindo et al., 2008, Koehler 2013).
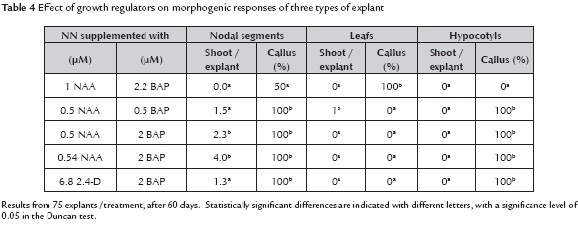
Morphogenic responses. The morphogenic responses of the various organs of V. stipulata are summarized in table 4. A wide range of inductive responses was observed in nodal segments: sprouting of axillary buds with up to 4 new buds per explant and profuse induction of callus. A relatively low level of NAA (on the order of 0.5 µM) and a similar concentration of BAP induced indirect shoot regeneration from leaves. According to results for C.papaya (De Winnaar, 1988) and in C. pubescens, the multiplication from axillary buds is similarly achieved in the presence of 0.1 mg l-1 BAP, 0.1 mg l-1 GA3 and 126 mg l-1 phloroglucinol (Jordán, 1992).
Reports on babaco showed shoot formation in leaf explants, although not on the leaf lamina but predominantly on the pre-existing nodular structures arranged in the central venation of the leaf (Jordán & Piwanski, 1997). However, for V. stipulata (this work) the nodular structures did not express any morphogenic responses under the various plant growth regulator combinations tested. The shoots exhibited a compressed structure, as it has also been reported for other Caricaceae (Mondal et al., 1994). Growth can be triggered subsequently by subculture on media with the application of 1.44 µM GA3 only.
Callus formation and regeneration of new tissues were low in leaf tissues. The opposite occurred with the hypocotyl where dedifferentiation of tissues was evident in most combinations of growth regulators, but these were not morphogenic. In other species and hybrids of the same family the formation of embryogenic callus was reported when cultured in medium with high concentrations of 2.4-D (Chen et al., 1991; Fitch, 1993).
Conclusion
Significant differences in V. stipulata seed viability between the three provenances were observed. The highest percentage of viability was observed in seeds from Ayabaca. The evaluation of different pre-germination treatments, determined that hydrogen peroxide at 100% promotes seed germination up to 53%. The culture of isolated embryos in 1/2 NN medium increases germination and controls hyperhydricity. Finally, to promote the production of shoots, nodal segments should be grown in 1/2 NN medium supplemented with NAA and BAP.
Acknowledgments. We thank Diana Ochoa for her assistance in this study. We especially thank the authorities of the Universidad Técnica Particular de Loja for their support of this research and all members of the Departamento de Ciencias Naturales/UTPL and Instituto de Biotecnología/Universidad Mayor. We also thank Mrs. Andrée Goreux for correction of the manuscript.
References
Acram, M. T., Richard, R. W. & Warren, H. S. (1996). Comparative anatomy of four rare Australian plants grown in vitro. Botanic Gardens Micropropagation News, 2(2), 20-25.
Ascencio, A., Gutiérrez, H., Rodríguez, B. & Gutiérrez, A. (2008). Plant regeneration of Carica papaya L. through somatic embryo-genesis in response to light quality, gelling agent and phlorid-zin. Scientia Horticulturae, 118(1), 155-160.
Badillo, V. (1993). Caricaceae, Segundo Esquema. Revista de la Facultad de Agronomía de la Universidad Central de Venezuela, 43(1), 1-111.
Badillo, V. (2000). Carica L. vs. Vasconcellea St. Hill (Caricaceae) con la rehabilitación de este último. Ernstia, 10(1), 74-79.
Chen, M. H., Chen, C. C., Wang, D. N. & Chen, F. C. (1991). Somatic embryogenesis and plant regeneration from immature embryos of Carica papaya x Carica cauliflora cultured in vitro. Canadian Journal of Botany, 69(19), 1913-1918.
Clarindo, W.R., Carvalho, C. R., Araújo, F. S., Abreu, I. S. & Otoni, W. C. (2008). Recovering polyploid papaya in vitro regenerants as screened by flow cytometry. Plant Cell, Tissue and Organ Culture, 92(1), 207-214.
De Winnaar, W. (1988). Clonal propagation of papaya in vitro. Plant Cell, Tissue and Organ Culture, 12(1), 305-310.
De Zerpa, D. M. (1980). Comportamiento meiótico de la descendencia híbrida producida al transferir el carácter bisexual de Carica pubescens a Carica stipulata. Revista Facultad de Agronomía (Maracay), 11(1), 5-47.
Drew, R., O'Brien, C. & Magdalita, P. (1998). Development of Carica interspecific hybrids. Acta Horticulturae, 461(1), 285-292.
Dolatabadian, A. & Modarres-Sanavy, S. (2008). Effect of the ascorbic acid, pyridoxine and hydrogen peroxide treatments on germination, catalase activity, protein and malondialdehyde content of three oil seeds. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 36(2), 61-66.
Fitch, M. M. M. (1993). High frequency somatic embryogenesis and plant regeneration from papaya hypocotyl callus. Plant Cell, Tissue and Organ Culture, 32(1), 205-212.
Guerrero, D. & Castro, S. (1999). Cultivo de babaco en Loja. Universidad Nacional de Loja. Proyecto VLIR. Pp 10-30.
Horovitz, S. & Jiménez, H. (1967). Cruzamientos interespecíficos e intergenéricos en Caricaceas y sus implicaciones fitotécnicas. Agronomía Tropical, 17(1), 323-243.
ISTA (International Seed Testing Association), (2005). International Rules for Seed Testing. Bassersdorf, CH-Switzerland.
IUCN. (2003). The IUCN red list of threatened species. IUCN Species Survival Comisión. IUCN, Gland, Switzerland, and Cambridge, UK.
Ivanova, M. & Van Staden, J. (2008). Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla. Plant Cell, Tissue and Organ Culture, 92(1), 227-231.
Jiménez, Y., Romero, J., & Scheldeman, X. (1998). Colección, caracterización y descripción de Carica X heilbornii nm. pentagona B.; Carica pubescens (A.DC) Solms-Laub y Carica stipulata B., en la provincia de Loja. Revista de Difusión Técnica y Científica de la Facultad de Ciencias Agrícolas de la Universidad Nacional de Loja, 29(1), 43-54.
Jordán, M. (1986). Somatic embryogenesis from cell suspension cultures in Carica candamarcensis.Plant Cell, Tissue and Organ Culture, 7(1), 257-251.
Jordán, M. & Piwanski, D. (1997). Regeneration of babaco [Carica pentagona (Heilborn) Badillo] using leaf explants and shoot-tip culture. ΦYTON, 61(1/2), 109-115.
Jordán, M. (1992). Micropropagation of Papaya (Carica sp). In: Bajaj Y. P. S. 1992. Biotechnology in Agriculture and Forestry. Springer, Berlin. Verlag. 18, 418-459.
Klein, J. D., Wood, L.A. & Geneve, R.L. (2008). Hydrogen peroxide and color sorting improves germination and vigor of eastern gamagrass (Tripsacum dactyloides) seed. Acta Horticulturae,782(1), 93-98.
Koehler, A. D., Carvalho, C. R. & Abreu, I. S. (2013). Somatic embryo-genesis from leaf explants of hermaphrodite Carica papaya: a new approach for clonal propagation. African Journal of Biotechnology, 12(18), 2386-2391.
Kyndt, T., Romeijn, E., Van Droogenbroeck, B., Romero, J., Gheysen, G. & Goetghebeur, P. (2005a). Species relationships in the genus Vasconcellea (Caricaceae) based on molecular and morphological evidence. American Journal of Botany, 92(6), 1033-1044.
Kyndt, T., Van Droogenbroeck, B., Romeijn, E., Romero, J., Scheldeman, X., Goetghebeur, P., Van Damme, P. & Gheysen, G. (2005b). Molecular phylogeny and evolution of Caricaceae based on rDNA internal transcribed spacer (ITS) and chloroplast sequence data. Molecular Phylogenetics and Evolution, 37(1) 442-459.
Loreti, F. & Pasqualetto, P. (1986). Vitrification of plants culture in vitro. Proceedings of the International Plant Propagators Society, 36(1), 66-71.
Magdalita, P., Drew, R., Adkins, S. & Godwin, I. (1997). Morphological, molecular and cytological analysis of Carica papaya X C. cauliflora interspecific hybrids. Theoretical and Applied Genetics,95(1), 224-229.
Marga, F., Vebret, L. & Morvan, H. (1997). Agar fractions could protect apple shoots cultured in liquid media against hyperhydricity. Plant Cell, Tissue and Organ Culture, 49(1), 1-5.
Mondal, M., Sukumar, G. & Banran, B. (1994). Callus culture and plantlet production in Carica papaya (Var. Honey Dew). Plant Cell Reports,13(1), 390-393.
Moreno, J., Sarria, V., Polo, A. & Giraldo, L. (2007). Evaluación del peróxido de hidrógeno en la oxidación de fenol con hierro soportado sobre tela de carbón activado. Información Tecnológica, 18(2), 67-72.
Murashige, T. & Skoog, F. (1962). A revised medium for rapid growth at bioassays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473-97.
Nitsch, J. P. & Nitsch, C. (1969). Haploid plants from pollen grains. Science, 163(3863), 85-87.
Pérez, A., Reyes, M. & Cuevas, J. (1980). Germination of two papaya varieties: effect of seed aeration, K-treatment, removing of the sarcotesta, high temperature, soaking in distilled water and age of seeds. Journal of Agriculture of the University of Puerto Rico,64(1), 164-172.
R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Scheldeman, X. (2002). Distribution and potential of cherimoya (Annona cherimola Mill.) and highland papayas (Vasconcellea spp.) in Ecuador. PhD Thesis. Faculty of Agricultural and applied Biological Sciences, University Ghent. Belgium. p 165.
Yahiro, M. & Oryoji, Y. (1980). Effects of gibberellin and cytokinin treatments on the promotion of germination in papaya, Carica papaya L., seeds. Memoris of the Faculty of Agriculture, 16(1), 45-51.
Ziv, M. (1991). Quality of micropropagated plants - vitrification. In Vitro Cellular & Developmental Biology-Plant, 27(1), 64–69.
Ziv, M. & Ariel, T. (1992). On the relation between vitrification and stomatal cell wall deformity in carnation. Acta Horticulturae,314(1), 121-129.
Referencias
Acram, M. T., Richard, R. W. & Warren, H. S. (1996). Comparative anatomy of four rare Australian plants grown in vitro. Botanic Gardens Micropropagation News, 2(2), 20-25.
Ascencio, A., Gutiérrez, H., Rodríguez, B. & Gutiérrez, A. (2008). Plant regeneration of Carica papaya L. through somatic embryogenesis in response to light quality, gelling agent and phloridzin. Scientia Horticulturae, 118(1), 155-160.
Badillo, V. (1993). Caricaceae, Segundo Esquema. Revista de la Facultad de Agronomía de la Universidad Central de Venezuela, 43(1), 1-111.
Badillo, V. (2000). Carica L. vs. Vasconcellea St. Hill (Caricaceae) con la rehabilitación de este último. Ernstia, 10(1), 74-79.
Chen, M. H., Chen, C. C., Wang, D. N. & Chen, F. C. (1991). Somatic embryogenesis and plant regeneration from immature embryos of Carica papaya x Carica cauliflora cultured in vitro. Canadian Journal of Botany, 69(19), 1913-1918.
Clarindo, W.R., Carvalho, C. R., Araújo, F. S., Abreu, I. S. & Otoni, W. C. (2008). Recovering polyploid papaya in vitro regenerants as screened by flow cytometry. Plant Cell, Tissue and Organ Culture, 92(1), 207-214.
De Winnaar, W. (1988). Clonal propagation of papaya in vitro. Plant Cell, Tissue and Organ Culture, 12(1), 305-310.
De Zerpa, D. M. (1980). Comportamiento meiótico de la descendencia híbrida producida al transferir el carácter bisexual de Carica pubescens a Carica stipulata. Revista Facultad de Agronomía (Maracay), 11(1), 5-47
Drew, R., O’Brien, C. & Magdalita, P. (1998). Development of Carica interspecific hybrids. Acta Horticulturae, 461(1), 285-292.
Dolatabadian, A. & Modarres-Sanavy, S. (2008). Effect of the ascorbic acid, pyridoxine and hydrogen peroxide treatments on germination, catalase activity, protein and malondialdehyde content of three oil seeds. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 36(2), 61-66.
Fitch, M. M. M. (1993). High frequency somatic embryogenesis and plant regeneration from papaya hypocotyl callus. Plant Cell, Tissue and Organ Culture, 32(1), 205-212.
Guerrero, D. & Castro, S. (1999). Cultivo de babaco en Loja. Universidad Nacional de Loja. Proyecto VLIR. Pp 10-30.
Horovitz, S. & Jiménez, H. (1967). Cruzamientos interespecíficos e intergenéricos en Caricaceas y sus implicaciones fitotécnicas. Agronomía Tropical, 17(1), 323-243.
ISTA (International Seed Testing Association), (2005). International Rules for Seed Testing. Bassersdorf, CH-Switzerland.
IUCN. (2003). The IUCN red list of threatened species. IUCN Species Survival Comisión. IUCN, Gland, Switzerland, and Cambridge, UK.
Ivanova, M. & Van Staden, J. (2008). Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla. Plant Cell, Tissue and Organ Culture, 92(1), 227-231.
Jiménez, Y., Romero, J., & Scheldeman, X. (1998). Colección, caracterización y descripción de Carica x heilbornii nm. pentagona B.; Carica pubescens (A.DC) Solms-Laub y Carica stipulata B., en la provincia de Loja. Revista de Difusión Técnica y Científica de la Facultad de Ciencias Agrícolas de la Universidad Nacional de Loja, 29(1), 43-54.
Jordán, M. (1986). Somatic embryogenesis from cell suspension cultures in Carica candamarcensis. Plant Cell, Tissue and Organ Culture, 7(1), 257-251.
Jordán, M. & Piwanski, D. (1997). Regeneration of babaco [Carica pentagona (Heilborn) Badillo] using leaf explants and shoot-tip culture. YTON, 61(1/2), 109-115.
Jordán, M. (1992). Micropropagation of Papaya (Carica sp). In: Bajaj Y. P. S. 1992. Biotechnology in Agriculture and Forestry. Springer, Berlin. Verlag. 18, 418-459.
Klein, J. D., Wood, L.A. & Geneve, R.L. (2008). Hydrogen peroxide and color sorting improves germination and vigor of eastern gamagrass (Tripsacum dactyloides) seed. Acta Horticulturae, 782(1), 93-98.
Koehler, A. D., Carvalho, C. R. & Abreu, I. S. (2013). Somatic embryogenesis from leaf explants of hermaphrodite Carica papaya: a new approach for clonal propagation. African Journal of Biotechnology, 12(18), 2386-2391.
Kyndt, T., Romeijn, E., Van Droogenbroeck, B., Romero, J., Gheysen, G. & Goetghebeur, P. (2005a). Species relationships in the genus Vasconcellea (Caricaceae) based on molecular and morphological evidence. American Journal of Botany, 92(6), 1033-1044.
Kyndt, T., Van Droogenbroeck, B., Romeijn, E., Romero, J., Scheldeman, X., Goetghebeur, P., Van Damme, P. & Gheysen, G. (2005b). Molecular phylogeny and evolution of Caricaceae based on rDNA internal transcribed spacer (ITS) and chloroplast sequence data. Molecular Phylogenetics and Evolution, 37(1) 442-459.
Loreti, F. & Pasqualetto, P. (1986). Vitrification of plants culture in vitro. Proceedings of the International Plant Propagators Society, 36(1), 66-71.
Magdalita, P., Drew, R., Adkins, S. & Godwin, I. (1997). Morphological, molecular and cytological analysis of Carica papaya x C. cauliflora interspecific hybrids. Theoretical and Applied Genetics, 95(1), 224-229.
Marga, F., Vebret, L. & Morvan, H. (1997). Agar fractions could protect apple shoots cultured in liquid media against hyperhydricity. Plant Cell, Tissue and Organ Culture, 49(1), 1-5.
Mondal, M., Sukumar, G. & Banran, B. (1994). Callus culture and plantlet production in Carica papaya (Var. Honey Dew). Plant Cell Reports, 13(1), 390-393.
Moreno, J., Sarria, V., Polo, A. & Giraldo, L. (2007). Evaluación del peróxido de hidrógeno en la oxidación de fenol con hierro soportado sobre tela de carbón activado. Información Tecnológica, 18(2), 67-72.
Murashige, T. & Skoog, F. (1962). A revised medium for rapid growth at bioassays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473-97.
Nitsch, J. P. & Nitsch, C. (1969). Haploid plants from pollen grains. Science, 163(3863), 85-87.
Pérez, A., Reyes, M. & Cuevas, J. (1980). Germination of two papaya varieties: effect of seed aeration, K-treatment, removing of the sarcotesta, high temperature, soaking in distilled water and age of seeds. Journal of Agriculture of the University of Puerto Rico, 64(1), 164-172.
R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Scheldeman, X. (2002). Distribution and potential of cherimoya (Annona cherimola Mill.) and highland papayas (Vasconcellea spp.) in Ecuador. PhD Thesis. Faculty of Agricultural and applied Biological Sciences, University Ghent. Belgium. p 165.
Yahiro, M. & Oryoji, Y. (1980). Effects of gibberellin and cytokinin treatments on the promotion of germination in papaya, Carica papaya L., seeds. Memoris of the Faculty of Agriculture, 16(1), 45-51.
Ziv, M. (1991). Quality of micropropagated plants - vitrification. In Vitro Cellular & Developmental Biology-Plant, 27(1), 64–69.
Ziv, M. & Ariel, T. (1992). On the relation between vitrification and stomatal cell wall deformity in carnation. Acta Horticulturae, 314(1), 121-129.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Andrea P. Loayza, Patricio García-Guzmán, Giovanni Carozzi-Figueroa, Danny E. Carvajal. (2023). Dormancy-break and germination requirements for seeds of the threatened Austral papaya (Carica chilensis). Scientific Reports, 13(1) https://doi.org/10.1038/s41598-023-44386-y.
2. Carloalberto Petti. (2020). Phloroglucinol Mediated Plant Regeneration of Ornithogalum dubium as the Sole “Hormone-Like Supplement” in Plant Tissue Culture Long-Term Experiments. Plants, 9(8), p.929. https://doi.org/10.3390/plants9080929.
3. Yoiner K. Lapiz Culqui, osé J. Tejada Alvarado, Jegnes B. Meléndez Mori, Nuri C. Vilca Valqui, Eyner Huaman-Huaman, Segundo M. Oliva Cruz. (2021). Establecimiento y multiplicación in vitro de papayas de montaña: Vasconcellea chachapoyensis Y Vasconcellea x Heilbornii. Bioagro, 33(2), p.135. https://doi.org/10.51372/bioagro332.7.
4. Adriana Aguirre-Rodríguez, Rodrigo Duarte-Casar, Marlene Rojas-Le-Fort, Juan Carlos Romero-Benavides. (2024). Food uses, functional activities, and bioactive compounds of three Ecuadorian Vasconcellea fruits: Bibliometric analysis and review. Journal of Agriculture and Food Research, 17, p.101244. https://doi.org/10.1016/j.jafr.2024.101244.
5. Ahmed Madi Waheed Al-Mayahi. (2024). Effect of Ancymidol and Phloroglucinol on the Number and the Quality of Shoots in the Micropropagation of Date Palm (Phoenix dactylifera L.). Journal of Horticultural Research, 32(2), p.47. https://doi.org/10.2478/johr-2024-0013.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2015 Revista Colombiana de Biotecnología

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Esta es una revista de acceso abierto distribuida bajo los términos de la Licencia Creative Commons Atribución 4.0 Internacional (CC BY). Se permite el uso, distribución o reproducción en otros medios, siempre que se citen el autor(es) original y la revista, de conformidad con la práctica académica aceptada. El uso, distribución o reproducción está permitido desde que cumpla con estos términos.
Todo artículo sometido a la Revista debe estar acompañado de la carta de originalidad. DESCARGAR AQUI (español) (inglés).