TGF-b y un inhibidor específico de TGF-b regulan pericentrina B y MYH9 en células de glioma
TGF-b and a specific TGF-b inhibitor regulate pericentrin B and MYH9 in glioma cell lines
Palabras clave:
DIGE, proteomica, glioma, TGF-P, espectrometría de masas, miosina no muscular, pericentrina B, proteomics, mass spectrometry, non muscle myosin, pericentrin B. (es)|
|
||
|
Rev. Colomb. Biotecnol. Vol. VIII N° 1 Julio 2006 48-56
|
||
|
|
||
|
TGF-p and a specific TGF-p inhibitor regúlate pericentrin B
and MYH9 in glioma cell lines
TGF-p y un inhibidor específico de TGF-p regulan pericentrina B y MYH9 en células de glioma
Óscar Álzate*, Cristina Osorio**, Michael H. Herbstreith***, Mark Hjelmeland****, Robert Buechler*****, Dong Ning He*****, Shideng Bao*******, Jeremy N. R¡cri********
|
||
|
|
||
|
ABSTRACT
Malignant gliomas are heterogeneous, highly invasive vascular tumours. The multifunctional cytokine, transforming growth factor-beta (TGF-P), is expressed by grade III/IV gliomas and promotes tumour angiogenesis, invasión and immune escape. It has been shown previously that a small TGF-P receptor type I (TGF-(3-RI) molecule inhibitor (SB-431542) blocks TGF-(3-mediated signal transduction, induction of angiogenic factor expression and cellular motility. As glioma cell lines display differential sensitivity to TGF-P, it was expected that they would also be differentially impacted by disruption of TGF-P signalling. Differential in gel expression (DIGE) analysis and mass spectrometry was used in this work for determining protein regulation effects of both TGF-P and SB-431542 on human glioma cell lines. It was found that pericentrin B and non muscle myosin were differentially expressed in fragments which likely resulted from protease activation by the tumour growth mechanism. These results suggest that both pericentrin B and non-muscle myosin might be potential glioma biomarkers.
Key words: DIGE, proteomics, glioma, TGF-P, mass spectrometry, non muscle myosin, pericentrin B.
RESUMEN
Los gliomas malignos son tumores vasculares heterogéneos altamente invasivos. El factor de transformación de crecimiento P (TGF-P) es una citoquina multifuncional que es expresada por gliomas de grado III /IV y promueve angiogenesis de tumores, invasión y escape inmunológico. Recientemente se demostró que una pequeña molécula inhibidora (SB-431542) del receptor de TGF-P tipo I (TGF-P-RI), bloquea la señal de transducción mediada por TGF-P, la inducción del factor angiogénico de expresión y la movilidad celular. Ya que las líneas celulares de gliomas muestran sensitividad diferencial a TGF-P, se esperaba que también mostrarían impacto diferencial por el bloqueo de la señal de TGF-p. En el presente trabajo se usó un análisis diferencial en gel (DIGE, por sus siglas en inglés: Differential in gel electrophoresis) y espectrometría de masas para determinar los efectos sobre regulación de proteínas por TGF-
Recibido: diciembre 04 de 2005 Aceptado: mayo 02 de 2006
|
||
|
|
||
|
* Ph. D. Assistant Professor. Duke Neuroproteomics Center. Department of Neurobiology. 258 Bryan Research Building,
DUMC Box 3209. Duke University Medical Center. Durham, NC. 27710. USA. Correo electrónico: alzate@neuro.duke.edu.
Phone: +1 919 681 5855. Scientific advisor: Parque Tecnológico de Antioquia, Medellín, Colombia. ** B. Se. Duke Neuroproteomics Center. Department of Neurobiology. 258 Bryan Research Building, DUMC Box 3209. Duke
University Medical Center. Durham, NC. 27710. USA. *** B. Se. Duke Neuroproteomics Center. Department of Medicine, División of Neurology. 258 Bryan Research Building, DUMC
Box 3209. Duke University Medical Center. USA.
**** BSc. Department of Medicine. Bryan Research Building, DUMC, Box 2900. Duke University Medical Center. USA. ***** Student. Duke Neuroproteomics Center. Department of Neurobiology. 258 Bryan Research Building, DUMC Box 3209.
Duke University Medical Center. USA. ****** M. Se. Duke Neuroproteomics Center. Department of Neurobiology. 258 Bryan Research Building, DUMC Box 3209. Duke
University Medical Center. USA. ******* Ph. D. Department of Medicine. División of Neuro Oncology. 225 Bryan Research Building, DUMC Box 3813. Duke University
Medical Center. USA. ******** M. D. Department of Medicine. División of Neurology. 225 Bryan Research Building, DUMC Box 2900. Duke University
Medical Center. USA.
|
||
|
|
||
|
48
|
||
|
|
||
|
|
|||
|
PROTEOMICS OF GLIOMA CELL UNES
|
|||
|
|
|||
|
(3 y SB-431542 en células de gliomas humanos. Se encontró que pericentrina B y miosina no muscular fueron expresadas diferencialmente en fragmentos, los cuales pueden ser el resultado de la activación de proteasas por el mecanismo de crecimiento del tumor. Estos resultados sugieren que tanto pericentrina B como miosina no muscular, podrían ser usadas como bio-marcadores potenciales de gliomas.
Palabras clave: DIGE, proteomica, glioma, TGF-P, espectrometría de masas, miosina no muscular, pericentrina B.
|
|||
|
|
|||
|
INTRODUCTION
Malignant gliomas: The number of new patients in 2003 suffering from primary malignant brain tumours was estimated to be 18,300 in the USA alone, leadingtol3,100 deaths (Jemal etál., 2003). Malignant gliomas remain almost universally fatal despite máximum therapy being provided. One of the most interesting pathways playing a critical role in malignant gliomas is transforming growth factor á (TGF-P), a multifunctional cytokine frequently expressed at high levéis in múltiple types of malignant brain tumour (Rich, 2003).
TGF-p acts as a tumour suppressor through growth inhibition in normal epithelial tissues; however, in advanced epithelial cancers, through inducing tumour invasión and neoangiogenesis combined with suppression of the immune response, TGF-p promotes tumour growth (Chang et ál., 1993; Rich, 2003). Malignant glioma cell lines are not affected by TGF-p-mediated growth inhibition and keep expressing the cognate receptors and SMADS, essential elements of TGF-p signal transduction pathways (Jennings et ál., 1991; Kjellman et ál., 2000; Rich et ál., 2003). TGF-p expression in gliomas is usually associated with advanced tumours and poor patient outcome (Rich, 2003). TGF-á causes cell cycle arrest of astrocytes associated with inducing cyclin-dependent kinase inhibitor, p15INK4B (Hjelmeland et ál., 2004). While p15INK4B is a frequent deletion target in malignant gliomas, it is expected that other mechanisms contribute towards glioma resistance to TGF-p-mediated growth inhibition.
TGF-p activation and gene regulation targeting are critically determined by the cellular context. TGF-P family members are organised into subsets of closely related factors: transforming growth factors
|
P, activins, growth differentiation factors, Mullerian inhibitory substance and bone morphogetic proteins (BMPs) (Kingsley, 1994). TGF-p and BMP family members play critical roles in brain development and response to injury including determining cell lineage and survival regulation (Bottner et ál., 2000; Munoz-Sanjuan and Brivanlou, 2002; Zhao and Schwartz, 1998). The TGF-p super-family regulates numerous cell properties, including growth, differentiation, angiogenesis, extracellular interactions, invasión and immune system function regulation (Blobe et ál., 2000; Dang et ál., 1995; Diebold et ál., 1995; Geiser etál., 1993; Kehrletál., 1986a; Kehrletál., 1986b; Rich, 2003).
TGF-P's mode of action is outlined as follows. TGF-p ligands bind to specific cell surface receptors initiating the formation of an activated heterodimeric Ser/Thr kinase receptor complex. The type I receptor is phosphorylated and activated by type II receptor, initiating the intracellular signalling cascade from the cytoplasm to the nucleus by phos-phorylating intracellular mediators (predominantly SMADs). The signáis induced by TGF-p are spe-cifically mediated by SMAD2 and SMAD3. Following phosphorylation, these SMADs are released from the receptor, then alter their auto-inhibitory folding and bind SMAD4, leading to translocation to the nucleus where transcription regulation is initiated (Rich, 2003). Complexity in TGF-p signalling, by which TGF-p may either act to promote or inhibit specific target transcription, is derived from regula-tory sequences to which SMAD-containing complexes bind and on the other members of the transcriptional complex, from non-SMAD pathway activation and from the interaction of other signal transduction pathways at receptor level (Rich, 2003).
2D-DIGE-based proteomics for identifying TGF-p and SB-431542 regulated proteins in human
|
||
|
|
|||
|
49
|
|||
|
|
|||
|
|
|||
|
Rev. Colomb. Biotecnol. Vol. VIII N° 1 Julio 2006 48-56
|
|||
|
|
|||
|
glioma cell tissue cultures is presented here. The major experimental approaches were 2-dimensio-nal differential in gel electrophoresis (2D-DIGE) to determine quantitative differential protein expression and matrix-assisted láser desorption/ionisation (MALDI) time of flight (TOF) in tándem mass spectrometry (MS/MS). Two proteins were identified whose fragments were affected, both in expression and post-translational modifications, due to the presence of TGF-p and SB-431542. It is thus proposed that these protein fragments should provide a target for human glioma biomarker development.
MATERIALS AND METHODS
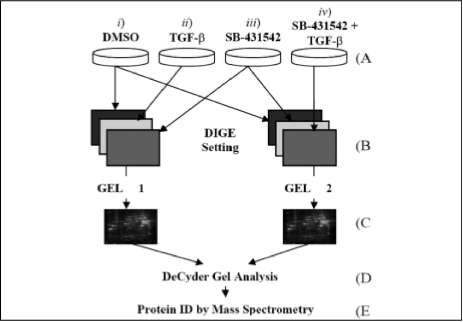
Cell cultures. The general experimental methodology is outlined in figure 1. D54MG is Duke University's A-172 glioma cell subline (Hjelmeland etál., 2004). Cellswere maintained in zinc médium supplemented with 10% foetal bovine serum and glutamine (Invitrogen, Carlsbad, California). The TGF-á inhibitor was purchased from Tocris (Ellisville, MO). SB-431542 was dissolved in 100% DMSO to a final working concentration of 10 mmol/L. Four experimental conditions were selected: i) control samples in which cells were cultured in a médium containing DMSO, /'/) cells cultured in the presence of 100 pmol/L TGF-p, ///) cells cultured in the presenceoftheTGF-p¡nhibitorSB-431542(1 mmol/ L) and iv) cells cultured in a médium supplemented with TGF-p (100 pmol/L) and SB-431542 (1 mmol/ L). Cells were plated in 6-cm plates at a density of 1.5x105 cells per well, then treated as described above (Hjelmeland et ál., 2004).
Protein preparation. Cells were harvested after 72 hours, by centrifuging, and suspended in 200 u.L lysis buffer (8M urea, 2M thiourea, 4% CHAPS, 20mM Tris, pH 7.5, supplemented with protease inhibitor complete (Roche) and NaVO4). Cells were gently ground in 1.5 mL Eppendorf vials, vortexed for 5 min at 4°C and sonicated in mild conditions twice in ice, for 30 sec each (Fisher model 100 sonicator, output power 4). The resulting mixture was vortexed for 5 min at room temperature. Samples were then centrifuged for 20 min, 4°C, 14,000 rpm. The resulting supernatant (protein lysate) was used for protein analysis. Protein lysates were cleaned to remove debris and non-proteinaceous material with the 2D-clean up kit (GE
|
Healthcare, Piscataway, NJ) following the manufacturéis instructions. The final pellet was suspended in focusing buffer (8M urea, 4% CHAPS, 30mM tris-HCl, pH 8.5). Protein concentration was determined with the 2D-Quant kit (GE Healthcare).
Protein labelling with fluorescent dyes. The
internal control methodology was followed for determining differential protein expression (Alban et ál., 2003; Friedman et ál., 2004). 120 u.g total protein was used for each sample (cells in DMSO, cells in the presence of TGF-p, cells supplemented with SB-43 1542 and cells in SB-431542/TGF-p combination). Each sample was labelled with 200 pmol Cy dyes, as indicated in figure 1. Proteins were labelled in ice for 30 min, in the dark. The labelling reaction was stopped by adding 1 u.L 10mM lysine for 10 min in ice, in the dark. Labelled samples were mixed following the scheme displayed in figure 1. The resulting mixture volume was determined and an equal volume of 2X sample buffer (8M urea, 4% CHAPS, 20 mg/mL DTT, 2% VA/ IPG buffer 3-10 (GE Healthcare)) was added and left in ice for 15 min. The resulting solution was brought up to final 250 u.L volume by adding rehydration buffer (8M urea, 4% CHAPS, 2mg/mL DTT, 1% VA/ IPG Buffer 3-10).
2-dimensional gel electrophoresis (2D-PAGE). Labelled samples were loaded onto the rehydration trays and covered with 13 cm immobilised pH gradient (IPG) strips (pH range, 3-10; GE Healthcare). Strips were submitted to active rehydration at 30 V for 14 h, followed by isoelectric focusing using an IPGphor II unit (GE Healthcare) to a total of 26 kVh (step 500 V for 1 h, step 1000 V for 1 h, step 8000 V to 26 kVh total). After isoelectric focusing, disulfide bridges were reduced by submerging the strips in 20 mL equilibration buffer (6M urea, 50mM tris, pH 8.8, 30% glycerol, 2% SDS) supplemented with 5 mg/mL DTT for 10 min. The strips were then incubated for 10 min in freshly prepared equilibration buffer supplemented with 45 mg/mL iodoacetamide (BioRad, Hércules, CA). IPG strips were transferred onto 12% polyacrylamide gels (4% stacking zone). SDS-PAGE gels were prepared using low fluorescence glass plates (13 cm, GE Healthcare) previously treated with bind-silane (GE Healthcare). Each gel was run at 9 mA for 16 h using a Hoeffer SE-600 Ruby system (GE Healthcare). Individual images
|
||
|
|
|||
|
50
|
|||
|
|
|||
|
|
|||
|
PROTEOMICS OF GLIOMA CELL UNES
|
|||
|
|
|||
 |
|||
|
|
|||
|
Figure 1. General experimental approach: A) Cell cultures were grown in four experimental conditions: i) cells in media containing DMSO, to be used as control, i i) cells in media supplemented with TGF-p, iii) cells in media containing SB-431542, and cells in a médium containing both TGF-p and SB-431542. B) Protein lysates were produced by grinding cell pellets, followed by sonication and centrifuging. Each protein lysate was labelled as indicated. Control (DMSO) was labelled with Cy2 (blue) and loaded on both gels, intemal control sample was labelled with Cy3 (green), and test sample for each gel was labelled with Cy5 (red). C) Samples were pooled and fractionated by 2D-PAGE. D) Resulting 2D-gels were analysed using DeCyder and differentially expressed proteins were isolated with the Ettan spot picker. E) Isolated proteins of interest were identified by mass spectrometry.
|
|||
|
|
|||
|
of proteins labelled Cy2, Cy3 and Cy5 in each gel were obtained by scanning on a Typhoon 9410 (GE Healthcare) with 480/530 nm excitation/emission wavelengths for Cy2, 520/590 nm for Cy3, and 620/ 680 nm for Cy5. After imaging, gels were stained with colloidal Coomassie (BioRad).
DIGE analysis: DeCyder 6.0 software (GE Healthcare) was used for determining differential protein expression. Sample to sample comparisons were made with the DÍA module. Images were manually edited to remove dust partióle signáis and protein spots outside the separation range. Two standard deviations of mean volume ratios (95th percentile confidence) were used as threshold to
|
determine confidence levéis for each sample. The mean valué for two standard deviations of volume ratios was 1.67. Statistical analysis and gel-to-gel comparisons were carried out with the BVA (Biological Variation Analysis) module, included with DeCyder 6.0.
Protein identification: Proteins displaying differential expression between control and test samples, as determined by the conditions imposed with Decyder, were removed from the gel using the Ettan Spot Picker (GE Healthcare). Proteins were in-gel digested with modified trypsin (Invitrogen). The resulting peptides1 molecular weights were determined by mass spectrometry at the University
|
||
|
|
|||
|
51
|
|||
|
|
|||
|
|
|||
|
Rev. Colomb. Biotecnol. Vol. VIII N° 1 Julio 2006 48-56
|
|||
|
|
|||
|
of North Carolina at Chapel Hill's mass spectrometry facility by peptide mass fingerprinting (Parker et ál., 2005). Briefly, MALDI-MS/MS data were acquired using an ABI Voyager 4700 MALDI-TOF/TOF mass spectrometer (Applied Biosystems, Inc. (ABI), Framingham, MA). MS and MS/MS spectra were acquired and the 8 most intense peaks with a signal-to-noise ratio greater than 25 were automatically selected for MS/MS analysis. The peptide mass fingerprinting and sequence tag data from the TOF/ TOF were evaluated with GPS Explorer scores (ABI). MS/MS spectra were submitted to the NCBI datábase for producing ion scores via the Mascot search engine (Parker et ál., 2005).
RESULTS
TGF-p regulates a large number of proteins in human glioma cell lines. First, the reproducibility of DIGE experiments using glioma cell lines was demonstrated by running individual gels. Figures 2A and 2B show two individual experiments. The experiments proved highly reproducible. For the first experimental setting, proteins isolated from cells treated with DMSO (control) were labelled with Cy2 (blue), proteins from cells treated with TGF-(3 were labelled with Cy3 (green) and proteins isolated from cells treated with SB-431542 were labelled with Cy5 (red) (Figure 2, panel C). The distribution of proteins affected by TGF-p and SB-431542 is shown in Figure 2C. Proteins whose expression was decreased by TGF-p appear as green spots and proteins decreased by SB-431542 are shown as red spots.
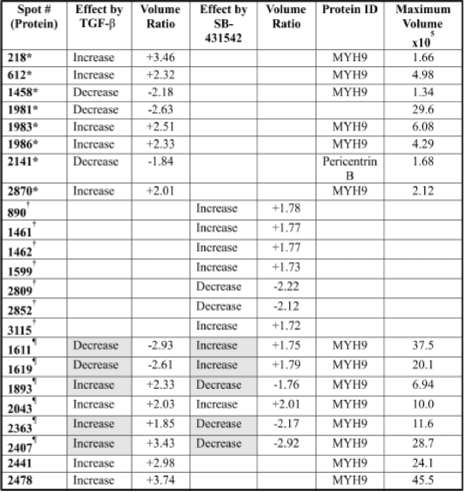
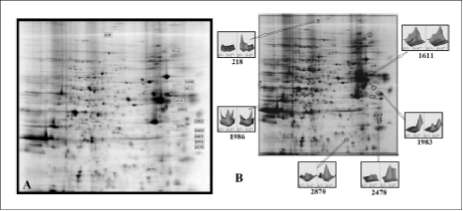
For the second experimental setting (Figure 2D), proteins affected by TGF-p and SB-431542 were labelled with Cy5 and fractionated by 2D-PAGE following the diagram displayed in figure 1. Contrasting with expressional changes of proteins shown in figure 2C, figure 2D indicates that cells treated with TGF-á+SB-431542 had less affected proteins, and that the effect was smaller in those already altered by the treatments. This result suggested that SB-431542 performed a contra effect on TGF-p. The detailed map of proteins affected by TGF-á and counter-affected by SB-431542 is shown in figure 3A and detailed in the table 1. The expression of the proteins indicated by numbers 218, 612, 1893, 1983, 1986, 2043, 2363, 2407, 2441, 2478 and 2870 increased in the presence of TGF-
|
P; whilst expression of protein spots having numbers 1458, 1611, 1981, 1619 and 2141 decreased with TGF-p (figure 3A and table 1).
When the cells were treated with a combination of TGF-p and SB-431542, five proteins affected by TGF-p alone were counter-affected by SB-431542 (spot numbers 1611, 1619, 1893, 2363 and 2407), suggesting that these proteins were involved in the regulation pathways depending on TGF-p. A total of
|
||
 |
|||
|
Figure 2. TGF-p regulates many proteins in human glioma cells: Proteins isolated from cells treated with DMSO (control) were labelled with Cy2 (blue), proteins from cells treated with TGF-p, labelled with Cy3 (green) and proteins from cells treated with SB-431542, labelled with Cy5 (red) (panel C). There was a high degree of reproducibility of 2D-DIGE for glioma analysis (panels A, B). Proteins affected by both TGF-p and SB431542 (panel C). Proteins whose expression was decreased by TGF-p are shown as green spots and proteins decreased by SB-431542 are shown as red spots (panel C).
|
|||
|
|
|||
|
52
|
|||
|
|
|||
|
|
|||
|
PROTEOMICS OF GLIOMA CELL UNES
|
|||
|
|
|||
|
Table 1. Perícentrín B and non muscle myosin are differentially regulated in gliomas: Out of 16 proteins displaying regulation by TGF-p, 5 showed counter-regulation by SB-431542. Fourteen proteins were identified using tándem mass spectrometry. All fragments except one were identified as being non muscle myosin (MYH9). The other protein was identified as being pericentrin B.
|
|||
|
|
|||
 |
|||
|
|
|||
|
*■
|
Affected only by SB-431542 Affected only by TGF-P Regulated by both treatments
|
||
|
|
|||
|
53
|
|||
|
|
|||
|
|
|||
|
Rev. Colomb. Biotecnol. Vol. VIII N° 1 Julio 2006 48-56
|
|||
|
|
|||
|
11 proteins whose expression increased with TGF-p and 5 proteins whose expressions decreased with TGF-p were found (table 1). These 16 proteins were the best targets for studying TGF-P's regulatory mechanism on glioma cell lines.
The effect of SB-432542 alone on protein expression was determined by comparing internal control (cells in DMSO, labelled with Cy2) and protein lysates from cells treated with SB-431542 (labelled with Cy3; figure 2D) and cells treated with SB-431542 and TGF-p (labelled with Cy5; figure 2D1). Six proteins (spot numbers 890, 900, 980, 1082, 1186 and 1675; not shown) showed increased expression from Cy2 to Cy3 and from Cy2 to Cy5. Six other proteins (1374, 1381, 2593, 2635, 2809 and 2852; not shown) displayed decreased expression. The changes in protein level were below the mínimum 20% change in expression used for cut-off in expression analysis. Cy3-labelled and Cy5-labelled protein expression were very similar, which was to be expected as the effects of TGF-p are regulated by SB-431542.
Pericentrin B and non muscle myosin are differentially regulated in glioma cells. Out of
the 16 proteins displaying regulation by TGF-p, five showed opposite regulation by SB-431542 (table 1). Fourteen proteins from this subset were identified using tándem mass spectrometry. All the
|
fragments, but one, were identified as being non-muscle myosin (MYH9). The other protein was identified as pericentrin B (table, spot number 2141 in figure 3A).
DISCUSSION
The present work describes a very powerful method for identifying molecular biomarkers (proteins) using proteomics. These techniques were used to search for proteins differentially regulated in human glioma cell lines. This research was performed using cell lines isolated from human brain tumours which were properly cultured in experimental conditions aimed at identifying biomarkers involved in tumour progression. The main techniques described here are differential in gel expression (DIGE) analysis and mass spectrometry.
To obtain suitable systems for DIGE proteomics, proteins must be isolated and prepared so that protein lysates can be labelled and quantified, without interference from other molecules such as carbohydrates, lipids or nucleic acids and dust partióles. Proteins are labelled with fluorophores allowing for visualisation and quantitation when the fluorophores are excited by the proper wavelength. This technology's main advantages include eliminating experimental variations resulting from individual analysis of múltiple samples, fluorophore
|
||
|
|
|||
 |
|||
|
|
|||
|
Figure 3. Map displaying proteins decreased and increased by both treatments, with explanations and identifications shown in table 1. figure 3B shows the 3D map of some of the differentially expressed proteins.
|
|||
|
|
|||
|
54
|
|||
|
|
|||
|
|
|||
|
PROTEOMICS OF GLIOMA CELL UNES
|
|||
|
|
|||
|
labels1 sensitivity (which increase protein detection levéis by up to 5 femtogram scale), experiments' reproducibility and flexibility for identifying proteins by spectroscopic methods, by mass spectrometry or by immunodetection.
Two proteins differentially regulated in glioma cells were found using DIGE proteomics: pericentrin B and non muscle myosin heavy chain. Pericentrin B, also known as kendrin, is a 350 kDa protein encoded by a gene located at the 21q22.3-qter locus. Kendrin is the human protein homologue of the yeast Spc110p protein (Flory et ál., 2000). Spc110p provides the link between (3-tubulin and the centrosome core, resulting in centrosomal microtubule nucleation (Flory et ál., 2000). The N-terminal fragment of pericentrin B is highly homologous with pericentrin, a centrosome component known to interact with tubulin. Pericentrin B localises to the centrosome during the cell cycle (Flory et ál., 2000). Pericentrin B is expressed in the centrosomes and is an essential pericentriolar material (Li et ál., 2001). Previous results have shown that the gene encoding pericentrin B (AI194767) decreases by 3.2-fold during tumour formation in embryonic fibroblasts derived from rasv12/EIA mice (Vasseur et ál., 2005).
MYH9 is a protein also known as non muscle myosin heavy chain 9. The encoding gene is located in the 22q12 locus. This protein has 1,960 amino acids (-227 kDa). The globular región possesses the binding sites for actin and the light chain. MYH9 has been implicated in several diseases including autosomal dominant giant platelet disorder, the May-Hegglin anomaly (MHA), the Fechtner syndrome (FTNS), the Sebastian syndrome (SBS) and the Epstein syndrome.
The specific role of pericentrin B and non muscle myosin in brain tumour development requiresfurther investigaron. Changes in thesetwo proteins1 expression levéis is presented here as the possible result of glioma development, as these changes were induced by TGF-p and counter-affected with the TGF-0 inhibitor, SB431542. This type of research is possible because of DIGE's differential ability. These experiments would require more extensive experimentation if any other currently available technique were to be used.
|
As indicated by MYH9 distribution in the gel, it is clear that the protein is fragmented. A similar result was seen with pericentrin B since the molecular weight for the holo-protein (350 kDa) is about ten times larger than the fragment identified here by mass spectrometry. It is proposed that such fragments result from activating specific proteases associated with the mechanism responsible for tumour progression. Using the peptide sequences and the molecular weights derived from tándem mass spectrometry it is postulated here that pericentrin B is cleaved by a metalloprotease and non muscle myosin is cleaved by caspase 9. These results were obtained by using peptide cutter (http:// us.expasy.org/tools/peptidecutter/). Further studies are required to valídate the activation of these proteases in glioma cell line regulation. It is expected that further research would allow the de-termination of all the proteases involved in brain tumour activation.
ACKNOWLEDGMENTS
We would like to thank Mauricio Ramírez and Lissete Betancur for critically reviewing the manuscript. This research was partially supported with start-up funds from Duke University's Neurobiology Department.
BIBLIOGRAPHY
Alban, A.; David, S.O.; Bjorkesten, L;Andersson, O; Sloge, E.; Lewis, S.; Currie, I. 2003. A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled interna! standard. Proteomics. 3: 36-44.
Blobe, G.C.; Schiemann, W.P.; Lodish, H.F. 2000. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 342. 1350-1358.
Bottner, M.; Krieglstein, K.; Unsicker, K. 2000. Thetransforming growth factor-betas: structure, signaling, and roles in nervous system development and functions. J. Neurochem. 75: 2227-2240.
Chang, H.L.; Gillett, N.; Figari, I.; López, A.R.; Palladino, M.A.; Derynck, R. 1993. Increased transforming growth factor beta expression inhibits cell proliferation in vitro, yet increases tumorigenicity and tumor growth of Meth a sarcoma cells. Cancer Res. 53: 4391-4398.
|
||
|
|
|||
|
55
|
|||
|
|
|||
|
|
|||
|
Rev. Colomb. Biotecnol. Vol. VIII N° 1 Julio 2006 48-56
|
|||
|
|
|||
|
Dang, H.; Geiser, A.G.; Letterio, J.J.; Nakabayashi, T.; Kong, L; Femandes, G; Talal, N. 1995. SLE-like autoantibodies and Sjogren's syndrome-like lymphoproliferation in TGF-beta knockout mice. J. Immunol. 155: 3205-3212.
Diebold, R.J.; Eis, M.J.; Yin, M.; Ormsby, I.; Boivin, G.P.; Darrow, B.J.; Saffitz, J.E.; Doetschman, T. 1995. Early-onset multifocal inflammation in the transforming growth factor beta 1 -null mouse is lymphocyte mediated. Proc. Natl. Acad. Sci. U S A. 92: 12215-12219.
Flory, M.R.; Moser, M.J.; Monnat, R.J. Jr; Davis, T.N. 2000. Identification of a human centrosomal calmodulin-binding protein that shares homology with pericentrin. Proc. Natl. Acad. Sci. U S A. 97: 5919-5923.
Friedman, D.B.; Hill, S.; Keller, J.W.; Merchant, N.B.; Levy, S.E.; Coffey, R.J.; Caprioli, R.M. 2004. Proteome analysis of human colon cáncer by two-dimensional difference gel electrophoresis and mass spectrometry. Proteomics. 4: 793-811.
Geiser, A.G.; Letterio, J.J.; Kulkarni, A.B.; Karlsson, S.; Roberts, A.B.; Sporn, M.B. 1993. Transforming growth factor beta 1 (TGF-beta 1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-beta 1 null mouse phenotype. Proc. Natl. Acad, Sci. U S A. 90: 9944-9948.
Hjelmeland, M.D.; Hjelmeland, A.B.; Sathomsumetee, S.; Reese, E.D.; Herbstreith, M.H.; Laping, N.J.; Friedman, H.S.; Bigner, D.D.; Wang, X.F.; Rich, J.N. 2004. SB-431542, a small molecule transforming growth factor-beta-receptor antagonist, inhibits human glioma cell line proliferation and motility. Mol. Cáncer Ther. 3: 737-745.
Jemal, A.; Murray, T; Samuels, A.; Ghafoor, A.; Ward, E.; Thun, M.J. 2003. Cáncer statistics, 2003. CA. Cancer J. Clin. 53: 5-26.
Jennings, M.T.; Maciunas, R.J.; Carver, R.; Bascom, C.C.; Juneau, P.; Misulis, K.; Moses, H.L. 1991. TGF beta 1 and TGF beta 2 are potential growth regulators for low-grade and malignant gliomas in vitro: evidence in support of an autocrine hypothesis. Int. J. Cáncer. 49: 129-139.
Kehrl, J.H.; Roberts, A.B.; Wakefield, L.M.; Jakowlew, S.; Sporn, M.B.; Fauci. A.S. 1986a. Transforming growth factor beta is an important
|
immunomodulatory protein for human B lymphocytes. J. Immunol. 137: 3855-3860.
Kehrl, J.H.; Wakefield, L.M.; Roberts, A.B.; Jakowlew, S.; Álvarez-Mon, M.; Derynck, R.; Sporn, M.B.; Fauci, A.S. 1986b. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 163: 1037-1050.
Kingsley, D.M. 1994. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 8: 133-146.
Kjellman, C; Olofsson, S.P.; Hansson, O.; Von Schantz.T; Lindvall, M.; Nilsson, I.; Salford, L.G.; Sjogren, H.O.; Widegren, B. 2000. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int. J. Cáncer. 89: 251-258.
Li, Q.; Hansen, D.; Killilea, A.; Joshi, H.C.; Palazzo, R.E.; Balczon, R. 2001. Kendrin/pericentrin-B, a centrosome protein with homology to pericentrin that complexes with PCM-1. J. CelI Sci. 114: 797-809.
Munoz-Sanjuan, I.; Brivanlou, A.H. 2002. Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 3: 271-280.
Parker, CE.; Warren, M.R.; Loiselle, D.R.; Dicheva, N.N.; Scarlett, C.O.; Borchers, C.H. 2005. Identification of components of protein complexes. Methods Mol. Biol. 301: 117-151.
Rich, J.N. 2003. The role of transforming growth factor-beta in primary brain tumours. Front Biosci. 8: e245-260.
Rich, J.N.; Shi, Q.; Hjelmeland, M.; Cummings, T.J.; Kuan, C.T.; Bigner, D.D.; Counter, C.M.; Wang, X.F. 2003. Bone-related genes expressed in advanced malignancies induce invasión and metástasis in a genetically defined human cáncer model. J. Biol. Chem. 278: 15951-15957.
Vasseur, S.; Malicet, C.; Calvo, E.L.; Dagorn, J.C.; Iovanna, J.L. 2005. Gene expression profiling of tumours derived from rasV12/E1A-transformed mouse embryonic fibroblasts to identify genes required for tumour development. Mol. Cancer. 4: 4.
Zhao, B.; Schwartz, J.P. 1998. Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. J. Neurosci Res. 52: 7-16.
|
||
|
|
|||
|
56
|
|||
|
|
|||
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2006 Revista Colombiana de Biotecnología

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
Esta es una revista de acceso abierto distribuida bajo los términos de la Licencia Creative Commons Atribución 4.0 Internacional (CC BY). Se permite el uso, distribución o reproducción en otros medios, siempre que se citen el autor(es) original y la revista, de conformidad con la práctica académica aceptada. El uso, distribución o reproducción está permitido desde que cumpla con estos términos.
Todo artículo sometido a la Revista debe estar acompañado de la carta de originalidad. DESCARGAR AQUI (español) (inglés).


















