Publicado
Synthesis and evaluation of the antibacterial activity of Cu(II) and Ni(II) complexes with mixed ligands based on glycine and dicarboxylic acids
Síntesis y evaluación de la actividad antibacteriana de complejos de Cu(II) y Ni(II) con ligandos mixtos basados en glicina y ácidos dicarboxílicos
DOI:
https://doi.org/10.15446/dyna.v91n234.112116Palabras clave:
complexes, amino acids, glycine, itaconic acid, oxalic acid (en)complejos, aminoácidos, glicina, ácido itacónico, ácido oxálico (es)
Descargas
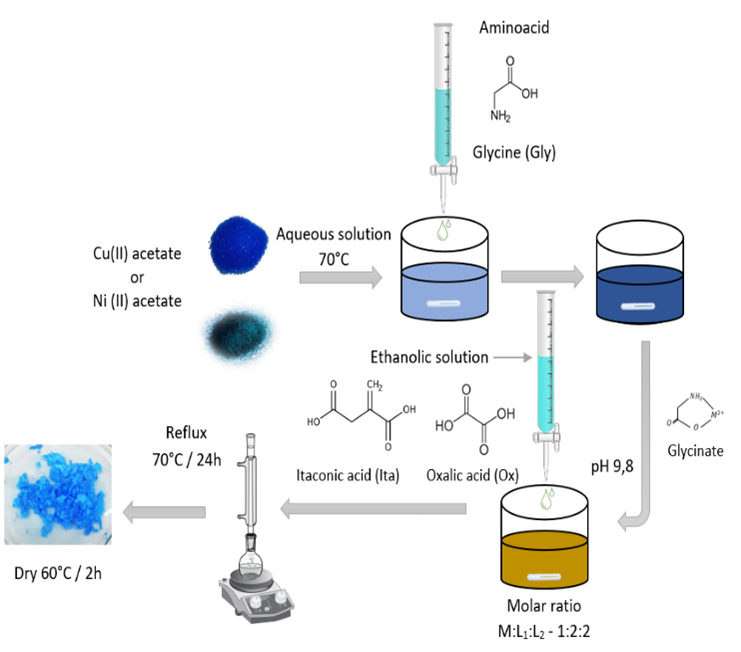
A large number of metal complexes have the ability to inhibit bacterial growth. Cu(II) and Ni(II) complexes based on glycine (Gly), itaconic acid (Ita), and oxalic acid (Ox) were synthesized by conventional methods and evaluated for their antibacterial activity. The metal complexes were characterized by TGA, FTIR, UV-vis spectroscopy, and XRD. The metal: ligands (M:L1:L2) stoichiometry of these complexes is 1:2:2, and coordination around Cu(II) and Ni(II) seems to be octahedral, with the ligands bound through the N atom of the amino group and O atoms of the bridging carboxylate group. These compounds are crystalline and stable at temperatures between 250 to 300°C. The metal complexes were screened for their antibacterial activity against the bacterial species Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, Salmonella, and Escherichia coli. These compounds were shown to have antibacterial activity mainly against gram-positive strains, with a minimum inhibitory concentration of 20 ppm.
Un gran número de complejos metálicos tienen la capacidad de inhibir el crecimiento bacteriano. Se sintetizaron complejos de Cu(II) y Ni(II) basados en glicina (Gly), ácido itacónico (Ita) y ácido oxálico (Ox) mediante métodos convencionales y se evaluó su actividad antibacteriana. Los complejos metálicos se caracterizaron por TGA, FTIR, espectroscopia UV-vis y XRD. La estequiometría metal:ligandos (M:L1:L2) de estos complejos es 1:2:2, y la coordinación alrededor de Cu(II) y Ni(II) parece ser octaédrica, con los ligandos unidos a través del átomo de N del grupo amino y los átomos de O del grupo carboxilato puente. Estos compuestos son cristalinos y estables a temperaturas entre 250 y 300°C. Los complejos metálicos se probaron para su actividad antibacteriana contra las especies bacterianas Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, Salmonella y Escherichia coli. Estos compuestos demostraron tener actividad antibacteriana principalmente contra cepas gram-positivas, con una concentración mínima inhibitoria de 20 ppm.
Referencias
[1] Subramaniyam, A., Ravi, P.V. and Pichumani, M. Structure co-ordination of solitary amino acids as ligands in metal-organic frameworks (MOFs): A comprehensive review. J Mol Struct, 1251 (3) 131931, 2022. https://doi.org/10.1016/j.molstruc.2021.131931
[2] An, J., Geib, S.J. and Rosi, N.L. High and Selective CO2 Uptake in a Cobalt Adeninate Metal−Organic Framework Exhibiting Pyrimidine- and Amino-Decorated Pores. J Am Chem Soc, 132 (1), pp. 38–39, 2010. https://doi.org/10.1021/ja909169x
[3] Anderson, S.L., Boyd, P.G., Gładysiak, A., Nguyen, T.N., Palgrave, R.G., Kubicki, D., Emsley, L., Bradshaw, D., Rosseinsky, M.J., Smit,B. and Stylianou, K.C. Nucleobase pairing and photodimerization in a biologically derived metal-organic framework nanoreactor. Nat Commun, 10, (1), 2019. https://doi.org/10.1038/s41467-019-09486-2
[4] Saikumari, N. Synthesis and characterization of amino acid Schiff base and its copper (II) complex and its antimicrobial studies. Materials Today: Proceedings, 47, pp. 1777–1781. 2021. https://doi.org/10.1016/j.matpr.2021.02.607
[5] Siddiki, N.A., Islam, S., Begum, S. and Salam, M.A. Synthesis, spectral characterization, thermal behavior and biological activities study of ternary metal complexes of alanine and 1,8-diaminonapthalene with Co(III), Ni(II), Cu(II), Zn(II) and Cd(II). Materials Today: Proceedings, 46, pp. 6374–6381, 2019. https://doi.org/10.1016/j.matpr.2020.06.126
[6] An, J., Geib, S.J. and Rosi, N.L. Cation-Triggered Drug Release from a Porous Zinc-Adeninate Metal-Organic Framework. J Am Chem Soc, 131 (24), pp. 8376–8377, 2009. https://doi.org/10.1021/ja902972w
[7] İnci, D., Aydın, R. and Zorlu, Y. Biomacromolecular interactions and radical scavenging activities of one-dimensional (1D) copper(II) glycinate coordination polymer. Journal of the Iranian Chemical Society, 18 pp. 3017-3030, 2021. https://doi.org/10.1007/s13738-021-02249-1
[8] Aljahdali, M. Synthesis, characterization and equilibrium studies of some potential antimicrobial and antitumor complexes of Cu(II), Ni(II), Zn(II) and Cd(II) ions involving 2-aminomethylbenzimidazole and glycine. Spectrochim Acta A Mol Biomol Spectrosc, 112, pp. 364–376, 2013. https://doi.org/10.1016/j.saa.2013.03.057
[9] Li, B., Luo, Y., Zheng, Y., Liu, X., Tan, L. and Wu, S. Two-dimensional antibacterial materials. Prog Mater Sci, 130 (10), p. 100976 2022. https://doi.org/10.1016/j.pmatsci.2022.100976
[10] [Akbar. S.A. and Morsali, A. Linker functionalized metal-organic frameworks. Coord Chem Rev, 399, p. 213023, 2019. https://doi.org/10.1016/j.ccr.2019.213023
[11] MacLaren, J.K. and Janiak, C. Amino-acid based coordination polymers. Inorganica Chim Acta, 389, (7) pp. 183–190, 2012. https://doi.org/10.1016/j.ica.2012.03.010
[12] Weiss, I.M., Muth, C., Drumm, R. and Kirchner, H. Thermal decomposition of the aminoacids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginineand histidine. BMC Biophys, 2018. https://doi.org/10.1186/s13628-018-0042-4
[13] Yablokov, V.Y., Smel’tsova, I.L., Zelyaev, I.A. and Mitrofanova, S.V. Studies of the rates of thermal decomposition of glycine, alanine, and serine. Russ J Gen Chem, 79 (8), pp. 1704–1706, 2009. https://doi.org/10.1134/S1070363209080209
[14] Cao, P., Wu, X., Zhang, W., Zhao, L., Sun, W. and Tang, Z. Killing Oral Bacteria Using Metal-Organic Frameworks. Ind Eng Chem Res, 59 (4), pp. 1559–1567, 2020. https://doi.org/10.1021/acs.iecr.9b05659
[15] Alisir, S.H., Demir, S., Sariboga, B. and Buyukgungor, O. A disparate 3-D silver (I) coordination polymer of pyridine-3,5-dicarboxylate and pyrimidine with strong intermetallic interactions: X-ray crystallography, photoluminescence and antimicrobial activity. J Coord Chem, 68 (1), pp. 155–168, 2015. https://doi.org/10.1080/00958972.2014.978307
[16] Lucena, G.N., Alves, R.C., Abuçafy, M.P., Chiavacci, L.A., da Silva, I.C., Pavan, F.R. and Galvão, R.C. Zn-based porous coordination solid as diclofenac sodium carrier. J Solid State Chem, 260, pp. 67–72, 2018. https://doi.org/10.1016/j.jssc.2018.01.011
[17] Yuan, K., Ye, X., Liu, W., Liu, K., Wu, D., Zhao, W., Qian, Z., Li, S., Huang, C., Yu, Z. and Chen, Z. Preparation, characterization and antibacterial activity of a novel Zn(II) coordination polymer derived from carboxylic acid. J. Mol Struct, 1241, p. 130624, 2021. https://doi.org/10.1016/j.molstruc.2021.130624
[18] Akbarzadeh, F., Motaghi, M., Singh Chauhan, N.P. and Sargazi, G. A novel synthesis of new antibacterial nanostructures based on Zn-MOF compound: design, characterization and a high-performance application. Heliyon, 6 (1), pp. e03231, 2020. https://doi.org/10.1016/J.HELIYON.2020.E03231
[19] Wiegand, I., Hilpert, K. and Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc, 3 (2), pp. 163–175, 2008. https://doi.org/10.1038/nprot.2007.521
[20] Liu, A., Wang, C.C., Wang, C.Z., Fu, H.F., Peng, W., Cao, Y.L., Chu, H.Y. and Du, A.F. Selective adsorption activities toward organic dyes and antibacterial performance of silver-based coordination polymers. J Colloid Interface Sci, 512, pp. 730–739, 2018. https://doi.org/10.1016/j.jcis.2017.10.099
[21] Sevgi, F., Bagkesici, U., Kursunlu, A.N. and Guler, E. Fe (III), Co(II), Ni(II), Cu(II) and Zn(II) complexes of schiff bases based-on glycine and phenylalanine: Synthesis, magnetic/thermal properties and antimicrobial activity. J Mol Struct, 1154, pp. 256–260, 2018. https://doi.org/10.1016/j.molstruc.2017.10.052
[22] Chávez, N.K., Ramírez, R.I., Sheen, P., Zimic, M.J. and Valderrama, A.C. Síntesis, caracterización y evaluación de la actividad biológica de compuestos de coordinación de cobalto con pirazinamida. Rev Soc Quím Perú, 86 (3), pp. 315–328, 2020. https://doi.org/10.37761/rsqp.v86i3.303
[23] Deacon, G.B. and Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev., 33, pp. 227–250, 1980. https://doi.org/10.1016/S00108545(00)80455-5.
[24] El-Newehy, M.H., Osman, S.M., Refat, M.S., Al-Deyab, S.S and El-Faham, A. Microwave synthesis of copolymers based on itaconic acid moiety and their utility for scavenging of copper (II) and lead (II). Journal of Macromolecular Science, Part A: Pure and Applied Chemistry, 52 (7), pp. 561–576, 2015. https://doi.org/10.1080/10601325.2015.1039335
[25] Köse, D.A., Toprak, E., Kasąrci, A., Avci, E., Avci, G.A., Sąhin, O. and Buyükgüngör, O. Synthesis, Spectral, and Thermal Studies of Co(II), Ni(II), Cu(II), and Zn(II)-Glycinato Complexes and Investigation of Their Biological Properties: Crystal Structure of [Cu(μ-gly)2(H2O)]n. Synthesis and Reactivity in Inorganic, Metal-Organic and Nano-Metal Chemistry, 46 (7), pp. 1109–1118, 2016. https://doi.org/10.1080/15533174.2013.801855
[26] Nguyen, H.G.T., Tao, R. and Van Zee, R.D. Porosity, Powder X-Ray Diffraction Patterns, Skeletal Density, and Thermal Stability of NIST Zeolitic Reference Materials RM 8850, RM 8851, and RM 8852. J Res Natl Inst Stand Technol, 126, (126047), pp. 1–10, 2021. https://doi.org/10.6028/jres.126.047
[27] Newsam, J.M., Yang, C.Z., King, H.E., Jones, R.H. and Xie, D. Experiences in studying zeolites and related microporous materials by synchrotron x-ray diffraction. Journal of Physics and Chemistry of Solids, 52 (10), pp. 1281–1288, 1991. https://doi.org/10.1016/0022-3697(91)90204-D
[28] Eswaramoorthy, M., Neeraj, S. and Rao, C.N.R. Synthesis of hexagonal microporous silica and aluminophosphate by supramolecular templating of a short-chain amine. Microporous and Mesoporous Materials, 28 (1), pp. 205–210, 1999. https://doi.org/10.1016/S1387-1811(98)00309-6
[29] Dharmaraja, J., Esakkidurai, T., Subbaraj, P. and Shobana, S. Mixed ligand complex formation of 2-aminobenzamide with Cu(II) in the presence of some amino acids: Synthesis, structural, biological, pH-metric, spectrophotometric and thermodynamic studies. Spectrochim Acta A Mol Biomol Spectrosc, 114, pp. 607–621, 2013. https://doi.org/10.1016/j.saa.2013.05.043
[30] Wyszogrodzka, G., Marszałek, B., Gil, B. and Dorozyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov Today, 21 (6), pp. 1009–1018, 2016. https://doi.org/10.1016/j.drudis.2016.04.009
[31] Agboola, T.D. and Bisi-Johnson, M.A. Occurrence of Listeria monocytogenes in irrigation water and irrigated vegetables in selected areas of Osun State, Nigeria. Sci Afr, 19, p. e01503, 2023. https://doi.org/10.1016/j.sciaf.2022.e01503
[32] Rojas, S., Arenas, A. and Horcajada, P. Metal-organic frameworks: A novel platform for combined advanced therapies. Coordination Chemistry Reviews, 388, pp. 202–226, 2019. https://doi.org/10.1016/j.ccr.2019.02.032
[33] Fan, X., Gurtler, J.B. and Mattheis, J.P. Possible sources of Listeria monocytogenes contamination of fresh-cut apples and antimicrobial interventions during antibrowning treatments: A review,” J Food Prot, 86 (6) p. 100100, 2023. https://doi.org/10.1016/j.jfp.2023.100100
[34] Doyle, M.E., Archer, J., Kaspar, C.W. and Weiss, R. Human Illness Caused by E. coli O157: H7 from Food and Non-food Sources. FRI Briefings [Online]. 10, pp. 1–37, 2006, [Date of reference October 18 of 2023]. Available: https://fri.wisc.edu/files/Briefs_File/FRIBrief_EcoliO157H7humanillness.pdf
[35] Liu, C.B., Gong, Y.N., Chen, Y. and Wen, H.L. Self-assembly and structures of new transition metal complexes with phenyl substituted pyrazole carboxylic acid and N-donor co-ligands. Inorganica Chim Acta, 383, pp. 277–286, 2012. https://doi.org/10.1016/J.ICA.2011.11.015
[36] Jo, J.H., Kim, H.C., Huh, S., Kim, Y. and Lee, D.N. Antibacterial activities of Cu-MOFs containing glutarates and bipyridyl ligands. Dalton Transactions, 48 (23), pp. 8084–8093, 2019. https://doi.org/10.1039/c9dt00791a
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2024 DYNA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
El autor o autores de un artículo aceptado para publicación en cualquiera de las revistas editadas por la facultad de Minas cederán la totalidad de los derechos patrimoniales a la Universidad Nacional de Colombia de manera gratuita, dentro de los cuáles se incluyen: el derecho a editar, publicar, reproducir y distribuir tanto en medios impresos como digitales, además de incluir en artículo en índices internacionales y/o bases de datos, de igual manera, se faculta a la editorial para utilizar las imágenes, tablas y/o cualquier material gráfico presentado en el artículo para el diseño de carátulas o posters de la misma revista.
















