Publicado
Extraction of molybdenite concentrates by leaching
Extracción de concentrados de molibdenita por lixiviación
DOI:
https://doi.org/10.15446/dyna.v91n234.115358Palabras clave:
molibdenita, lixiviación, cloruro férrico (es)molybdenite, leaching, ferric chloride (en)
Descargas
El concentrado de molibdenita (MoS2) es obtenido actualmente como un subproducto en el procesamiento de minerales provenientes de un pórfido de cobre, siendo la molibdenita considerada un componente menor. El procedimiento para la extracción comercial de molibdeno desde un sulfuro, la molibdenita, implica tostar el concentrado, purificar el calcinado resultante, ya sea por destilación de tiroxido de molibdeno (MoO3 )o por una ruta hidrometalúrgica, y finalmente reducir el trióxido con hidrógeno para obtener el metal
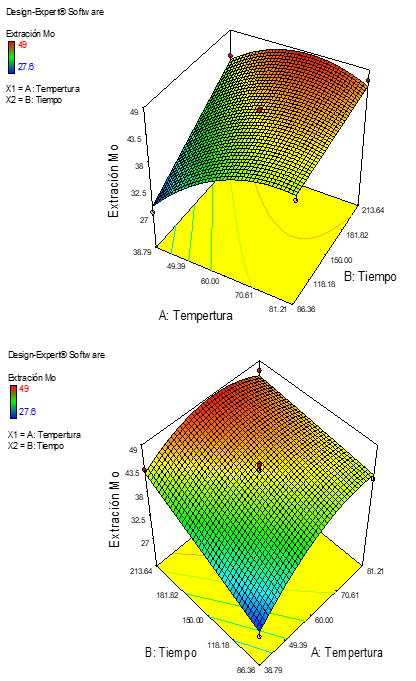
El objetivo del presente trabajo es estudiar la producción de molibdeno desde concentrados de molibdenita usando una solución acuosa de ácido clorhídrico (HCl) como lixiviante, cloruro de sodio (NaCl) como catalizador, hidróxido de sodio (OHNa) como regulador de pH, y por último cloruro férrico (FeCl3) como un oxidante. Los resultados muestran que trabajando en un pH más grande que 8 temperatura de 50ºC, con una concentración de ácido clorhídrico de 5% y una relación solida / liquido de 10:1, velocidad de agitación de 200- 300 rpm y la adición de un 2% de cloruro férrico, La lixiviación de Mo fue del 70% en condiciones experimentales en un tiempo de 180 minutos, con una remoción completa del hierro.
Molybdenum concentrate, as Molybdenite (MoS2), is nowadays obtained as a byproduct of the processing of porphyry copper ores, being molybdenite considered a minor component. The procedure for the commercial extraction of molybdenum from such a sulfide ore, involves the operations of roasting the concentrate, purifying the resulting calcination, either by distillation of molybdenum trioxide (MoO3) or by a hydrometallurgical pathway, and finally reducing the trioxide of molybdenum with hydrogen to obtain the metal.
The objective of the present work is to study the production of molybdenum from molybdenite concentrates using aqueous solution of hydrochloric acid (HCl) as a lixiviant, sodium chloride (NaCl) as a catalyst, sodium hydroxide (NaOH) as a pH regulator, and lastly ferric chloride (FeCl3) as an oxidant. The results show that working pH greater than 8, temperature of 50 °C, hydrochloric acid concentration of 5%, solid/liquid ratio of 10:1, stirring rate of 200-300 rpm and the addition of 2% ferric chloride. Mo leaching was 70% under experimental conditions at a time of 180 minutes, with a complete removal of iron.
Referencias
[1] Salehi, H., Tavakoli, H., Aboutaleb, M.R., and Samim, H.R., Recovery of molybdenum and rhenium in scrub liquors of fumes and dusts from roasting molybdenite concentrates, Hydrometallurgy, 185, pp. 142-148, 2019. ISSN 0304-386X, DOI: https://doi.org/10.1016/j.hydromet.2019.02.004
[2] Kumar, M., Mankhand, T.R., Murthy, D.S.R., Mukhopadhyay, R., and Prasad, P.M., Refining of a low-grade molybdenite concentrate, Hydrometallurgy, 86(1–2), pp. 56-62, 2007. ISSN 0304-386X, DOI: https://doi.org/10.1016/j.hydromet.2006.11.003
[3] Lasheen, T.A., El-Ahmady, M.E., Hassib, H.B., and Helal, A.S. Molybdenum metallurgy review: hydrometallurgical routes to recovery of molybdenum from ores and mineral raw materials. Mineral Processing and Extractive Metallurgy Review, 36(3), pp. 145–173, 2014. DOI: https://doi.org/10.1080/08827508.2013.868347
[4] Bazán, V., Brandaleze, E. y Colque, E., Cinética de tostación de concentrados de baja ley de molibdenita. DYNA.80(181), pp. 146-152, 2013.
[5] Marin, T., Utigard, T., and Hernandez, C., Roasting kinetics of molybdenite concentrates. Canadian Metallurgical Quarterly, 48(1), pp. 73-80, 2009. DOI: https://doi.org/10.1179/000844309794239206
[6] Yang, J-G, Yang, J-Y, Tang, M-T, Tang, C-B, and Liu, W., The solvent extraction separation of bismuth and molybdenum from a low grade bismuth glance flotation concentrate, Hydrometallurgy, 96(4), pp. 342-348, 2009. ISSN 0304-386X, DOI: https://doi.org/10.1016/j.hydromet.2008.12.006
[7] Sharia, M.H., Setoodeh, N., and Atash, R., Optimizing conditions for hydrometallurgical production of purified molybdenum trioxide from roasted molybdenite of sarcheshmeh, Minerals Engineering, 14(7), pp. 815-820, 2001. ISSN 0892-6875, DOI: https://doi.org/10.1016/S0892-6875(99)00000-X
[8] Cao, Z-F., Zhong, H., Qiu, Z-H., Liu, G-Y., and Zhang, W-X., A novel technology for molybdenum extraction from molybdenite concentrate. Hydrometallurgy, 99(1–2), pp. 2-6, 2009. ISSN 0304-386X, DOI: https://doi.org/10.1016/j.hydromet.2009.05.001
[9] Behmadi, R., Mirzaei, M., Afshar, M.R., and Najafi, H. Investigation of chalcopyrite removal from low-grade molybdenite using response surface methodology and its effect on molybdenum trioxide morphology by roasting. The Royal Society of Chemistry, 13, pp. 14899-14913, 2023. DOI: https://doi.org/10.1039/D3RA02384B
[10] Khalil, M.M.H., Al-Wakeel, K.Z., Abd El Rehim, S.S., and Abd El Monem, H., Adsorption of Fe(III) from Aqueous Medium onto Glycine-Modified chitosan resin: equilibrium and kinetic studies. Journal of Dispersion Science and Technology, 35(12), pp. 1691–1698. 2014. DOI: https://doi.org/10.1080/01932691.2013.859624
[11] Kuma, J.A., Amarnath, D.J., Kumar, P.S., Kaushik, C.S., Varghese, M.E., and Saravanan, A., Mass transfer and thermodynamic analysis on the removal of naphthalene from aqueous solution using oleic acid modified palm shell activated carbon, Desal. Wat. Treat., 106, pp. 238–250, 2018. DOI: https://doi.org/10.5004/dwt.2018.22066
[12] Elwakeel, K.Z., Aly, M.H., El-Howety, M.A. et al., Synthesis of chitosan activated carbon beads with abundant amino groups for capture of Cu(II) and Cd(II) from Aqueous Solutions. J Polym Environ 26, pp. 3590–3602, 2018. DOI: https://doi.org/10.1007/s10924-018-1243-2
[13] Khalil, M.M.H., Al-Wakeel, K.Z., Abd El Rehim, S.S. and Abd El Monem, H., Efficient removal of ferric ions from aqueous medium by amine modified chitosan resins. Journal of Environmental Chemical Engineering, 1, pp. 566-573, 2013. DOI: https://doi.org/10.1016/j.jece.2013.06.022.
[14] Baba, A.A., Ayinla, K.I., Adekola, F.A., Ghosh, M.K., Ayanda O.S., Bale, R.B., Sheik, A.R., and Pradhan, S.R., A review on novel techniques for chalcopyrite ore processing. Int. J. Min. Eng. Miner. Process. 1(1), pp. 1-16, 2012. DOI: https://doi.org/10.5923/j.mining.20120101.01
[15] Bazan, V., Fernandez, P., Medina, M., y Lara, R., Lixiviación de concentrados molibdeno. La Revista Latinoamericana de Metalurgia y Materiales, RLMM, S8(1), pp. 1-18, 2019.
[16] Nicol, M., Miki, H., and Velásquez-Yévenes, L., The dissolution of chalcopyrite in chloride solutions: Part 3. Mechanisms, Hydrometallurgy, 103(1–4), pp. 86-95, 2010. ISSN 0304-386X, DOI: https://doi.org/10.1016/j.hydromet.2010.03.003
[17] Li, Y., Wang, F., Yang, B. et al., Experimental investigation of molybdenum disulfide purification through vacuum distillation. J. Sustain. Metall. 6, pp. 419–427, 2020. DOI: https://doi.org/10.1007/s40831-020-00284-5
[18] Cao, Z-F., Zhong, H., Qiu, Z-H., Liu, G-Y., and Zhang, W-X., A novel technology for molybdenum extraction from molybdenite concentrate, Hydrometallurgy, 99(1–2), pp. 2-6, 2009. DOI: https://doi.org/10.1016/j.hydromet.2009.05.001
[19] Shalchian Hossein, Birloaga Ionela, Bagheri Motahareh Moghaddam, Nasiri Hadi, Vegliò Francesco, A hydrometallurgical process flowsheet for recovering MoO3 from Molybdenite, Hydrometallurgy, 228, art. 106355, 2024. ISSN 0304-386X, DOI: https://doi.org/10.1016/j.hydromet.2024.106355
[20] Hesami, R., Ahmadi, A., Hosseini, R.M., Manafi, Z., Electroleaching kinetics of molybdenite concentrate of Sarcheshmeh copper complex in chloride media, Minerals Engineering, 186, art. 107721, 2022. ISSN 0892-6875. DOI: https://doi.org/10.1016/j.mineng.2022.107721
[21] Padilla, R., Letelier, H., and Ruiz, M.C., Kinetics of copper dissolution in the purification of molybdenite concentrates by sulfidation and leaching, Hydrometallurgy, 137, pp. 78-83, 2013. ISSN0304-386X, DOI: https://doi.org/10.1016/j.hydromet.2013.05.012
[22] Nguyen, H.N.H., Nguyen, T.T.H., and Lee, M.S., Leaching of molybdenite by hydrochloric acid solution containing sodium chlorate. Resources Recycling, 31(5), pp. 26-33, 2022. DOI: https://doi.org/10.7844/kirr.2022.31.5.26
[23] Ojo, J., Ipinmoroti, K., and Adeeyinwo, C., Solvent extraction of molybdenum (VI) from diluted and concentrated hydrochloric acid. Global Journal of Pure and Applied Sciences. 14(3), pp: 289-294, 2008. DOI: https://doi.org/10.4314/gjpas.v14i3.16810
[24] Tumen-Ulzii, N., Batnasan, A., and Gunchin, B., Selective dissolution of copper and iron from molybdenite concentrate using acidic sodium nitrate solution, Minerals Engineering, 185, art. 107715. 2022. DOI: https://doi.org/10.1016/j.mineng.2022.107715
[25] Li, L.F., Cao, Z.F., Zhong, H. et al., The selective leaching and separation of molybdenum from complex molybdenite concentrate containing copper. Mining, Metallurgy & Exploration 30, pp. 233–237, 2013. DOI: https://doi.org/10.1007/BF03402467
[26] Xu, Y., Liu, X., Zhao, Z., Chen, X., Li, J., He, L., and Sun, F., Kinetics and mechanism of selective leaching of bismuth from molybdenite and bismuthinite mixed ore, Hydrometallurgy, 224, art. 106258, 2024. ISSN 0304-386X, DOI: https://doi.org/10.1016/j.hydromet.2023.106258
[27] Lessard, J.D., and Shekhte, L.N., Thermodynamic modeling of atmospheric hydrometallurgical removal of chalcopyrite from molybdenite concentrates, Hydrometallurgy, 150, pp. 9-13, 2014. ISSN 0304-386X, DOI: https://doi.org/10.1016/j.hydromet.2014.08.013
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. B. K. Kenzhaliev, S. Zh. Aibagarov, N. Azatbekuly, A. A. Ultarakova. (2026). DEVELOPMENT OF AN INFORMATION SYSTEM FOR MODELING AND ANALYZING HYDROMETALLURGICAL PROCESSING OF MOLYBDENITE CONCENTRATES. Bulletin of Shakarim University. Technical Sciences, 1(4(20)), p.179. https://doi.org/10.53360/2788-7995-2025-4(20)-21.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2024 DYNA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
El autor o autores de un artículo aceptado para publicación en cualquiera de las revistas editadas por la facultad de Minas cederán la totalidad de los derechos patrimoniales a la Universidad Nacional de Colombia de manera gratuita, dentro de los cuáles se incluyen: el derecho a editar, publicar, reproducir y distribuir tanto en medios impresos como digitales, además de incluir en artículo en índices internacionales y/o bases de datos, de igual manera, se faculta a la editorial para utilizar las imágenes, tablas y/o cualquier material gráfico presentado en el artículo para el diseño de carátulas o posters de la misma revista.
















