EVALUATION OF FLY ASHES FOR THE REMOVAL OF CU, NI AND CD FROM ACIDIC WATERS
EVALUATION OF FLY ASHES FOR THE REMOVAL OF CU, NI AND CD FROM ACIDIC WATERS
EVALUACIÓN DE LAS CENIZAS VOLANTES PARA LA ELIMINACIÓN DEL CU, NI Y CD DE AGUAS ÁCIDAS
BEGOÑA
FERNÁNDEZ PÉREZ
Escuela
de Minas de la Universidad
de Oviedo, España, fernandezbegona@uniovi.es
JULIA
AYALA ESPINA
Escuela
de Minas de la Universidad
de Oviedo, España, jayala@uniovi.es
Received for review June 16 th, 2009, accepted December 20th, 2009, final version December 23th, 2009
ABSTRACT: The presence of sulphides in many mine wastes and the formation of acid mine drainages (AMD) has been widely recognized as one of the great environmental problems nowadays. Waters from many of the abandoned mines, with thousands of cubic meters of residue scattered in dumps and ponds, are affected by this type of pollution characterized by its acidity, high contents of sulphates and heavy metals such as Fe, Mn, Al, Cu, Ni, Cd.
This study was designed to study the effect of use flying ash coming from Power Stations as a neutralizer for acidic waters resulting from this type of abandoned facilities. In this study, and due to the heterogeneity of the contaminants present, we have studied the removal of Ni, Cu and Cd. Different parameters were studied: metal concentration and pH of the solution to be treated, time reaction and pulp density. Fly ash can be used as a neutralization/fixation agent. Fly ash will add alkalinity and increase the pH on contacting AMD. This will result in precipitation of metal hydroxides.
KEY WORDS: AMD, Fly Ash, Ni, Cu, Cd.
RESUMEN: La presencia de sulfuros en la mayoría de los residuos mineros y la subsiguiente formación de los drenajes ácidos de mina (AMD) ha sido ampliamente reconocida como uno de los grandes problemas medioambientales actuales. Las aguas procedentes de las minas abandonadas, con miles de metros cúbicos de residuos dispersos en escombreras y balsas mineras, se ven afectadas por este tipo de contaminación caracterizada por su acidez alto contenido en sulfatos y metales pesados como el Fe, Mn, Al, Cu, Ni, y Cd.
Este estudio fue diseñado para evaluar el efecto del uso de cenizas volantes procedentes de centrales eléctricas como un neutralizador de las aguas ácidas resultantes de este tipo de instalaciones abandonadas. En este trabajo, y debido a la heterogeneidad de los contaminantes presentes en dicho residuo hemos estudiado la eliminación del Ni, Cu y Cd. Para ello se estudiaron diferentes parámetros: la concentración de metal y el pH de la solución a tratar, el tiempo de reacción y la densidad de pulpa. Las cenizas volantes se pueden utilizar como agentes de neutralización o fijación. Su utilización en contacto con AMD permite aumentar la alcalinidad y el pH. Esto dará lugar a la precipitación de los hidróxidos metálicos correspondientes.
PA LABRAS CLAVE: AMD, Cenizas Volantes, Ni, Cu, Cd.
1. INTRODUCTION
The acid mine drainage (AMD) is a worldwide major cause of degradation of water resources.
Characterized by low pH and high concentrations of heavy metals and other toxic elements, AMD can severely contaminate surface and groundwater, as well as soils. [1]. In nature, sulphides remain in the subsurface in absence of oxygen and only small outcrops of these deposits came up to the surface. The drainage is formed when sulphide minerals in rocks, primarily pyrite (FeS2), are exposed to oxidizing conditions, forming sulphuric acid and dissolved iron. Upon exposure to water and oxygen, pyritic minerals oxidize to form acidic, iron and sulphate-rich drainage. Contamination associated with AMD depends on type and amount of sulphide mineral oxidized, as well as type of gangue minerals present in the rock. The leaching reaction produce the hydrolysis of other minerals that are dissolved Al, Ca, Mg, Mn, Na, Si, etc [2]
Also referred to as Acid Rock Drainage (ARD), AMD emanating from mine waste rock, tailings, and mine structures, such as pits and underground workings, is primarily a function of the mineralogy of local rock material and the availability of water and oxygen. The need to prevent their formation has led to the development of numerous investigations on the mechanisms of oxidation and its prevention. [3-4] However, despite the many studies that exist, because mineralogy and other factors affecting AMD formation so they are highly variable from site-to-site, predicting the potential and treatment for AMD can be exceedingly challenging and costly.
The present work, given the wide variations in composition with the acid water for the wide range of geochemical and biogeochemical processes that cause, has focused on studying the removal of Cd, Cu and Ni. [5]. Synthetic water, obtained in laboratory, have been used for analysis and have been neutralized through the use of fly ash from two Power Station [6-7]
2. MATERIALS AND METHODS
2.1 Characterization of fly ashes
Tests were carried out with two fly ashes from different Power Starions: CVN y CVS. In both cases the surface area (by BET method-N2), the chemical composition (by atomic adsorption spectrophotometry with a Pye Unican analyzer) and the mineralogical composition (by X-ray diffraction with a Philips PW 1830) was determined. It also calculated the loss of ignition (LOI), bulk density and average particle size and morphology by electron microscopy (SEM). (Table 1, Figures 1 and 2).
Table 1. Chemical
analysis and properties of fly ashes


Figure 1. X-Ray Diffractogram
of CVN and CVS fly ash
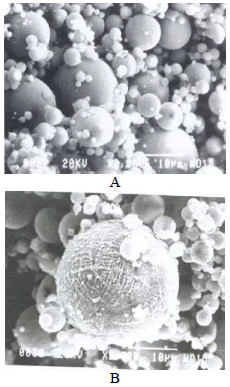
Figure 2. A. SEM-without react B. SEM-treated
The chemical analysis carried out on the two fly ashes shows that corresponds to an ash residue rich in Si (more than 50 wt% SiO2), iron and aluminium and low in calcium (less than 5 wt% CaO) (Table 1) and that these therefore may be classified according to ASTM C618, such as a low calcium fly ashes, (class F), since they contain less than 10% CaO and more than 70% is composed of SiO2 , Al2O3 and Fe2O3.
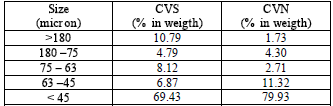
The mineral composition of the ash presents mostly vitreous phase and mullite and quartz as crystalline phase Fig.1. Fig. 2 shows that the fly ash particles have a spherical morphology with a 70% ,by weight, of medium grain size lower to 45μm, Table 2.
Table 2. CVS and CVN fly ash
granulometry
2.2 Acid water
characterization
All concentrated solutions
of heavy metals were prepared using analytical grade reagents and distilled and
deionised water. The pH was measured using a pH-meter Crisom 2000 and Sulphuric
acid (Probus,
Badalona, Spain ) was used for adjust.
Tests were carried out with concentrated solutions of nickel, copper and cadmium. In Ni case three solutions were prepared: one of 0.001M, a second one of 0.004M and last of 0.01M, since in most cases, industrial discharges do not exceed these concentrations. In Cu case, the concentrations used were 0.002, 0.004, and 0.005 M and for Cd, 0.001, 0.003 and 0.005 M. The final pH of the solution was measured for all of them.
2.3 Method
The adsorption
method consisted of mixing, in polyethylene bottles, different quantities of
fly ash with water from concentrated acid solutions of Cu, Ni and Cd. During
the experiments varied both the source of the ash used, the pulp density,
stirring time, pH and initial concentration of metal ions in solutions used. At
the end of the process, fly ash was filtered in a 45 microns filter and the
residual liquor was analysed by atomic adsorption.
3. RESULTS AND DISCUSSION
3.1 Study of Nickel elimination
To study the elimination of
nickel ions in acidic solutions different solutions were prepared from
different concentrates: 0.001, 0.004 and 0.01M, at pH 5. Figure 3 shows
the results of tests carried out with an agitation time ranged from 5, 15, 30
to 60 minutes, with samples of 25 ml each of acidic solutions 100 above
mentioned and with a pulp density of
40 g / l.

Figure 3. % Extraction of Ni vs time for CVS and CVN fly
ashes and different initial metal concentration. Pulp density 40 g/l
Figure 3 shows eliminations close to 100% in the conditions mentioned for the solution 0.001 M on CVS ash during the first 5 minutes of stirring. CVN ash needed 30 minutes to achieve 95% removal and 60 minutes to reach 100%. After 5 minutes the pH is 5, arriving at pH 8 after 60 minutes. Final pH =8.4.
CVS ash presents similar behaviour for the 0.004M solution, grazing 95% of elimination in 5 minutes of treatment, and 100% at 30 minutes. However, as seen in the previous case, CVN ashes have a worse behaviour. In this case they do not exceed 20% elimination after 60 minutes of agitation.
For CVS ashes experiments were performed with more concentrated solutions, 0.1M. The results returned to be very favourable reaching 100% elimination after 30 minutes of agitation.
Subsequently, the influence of pulp density on the percentage of extraction was studied. For CVS ash, since good results were obtained with small times of stirring, experiments were realized with low pulp densities and 15 minutes of stirring time Figure 4. Good results were obtained.

Figure
4. %
Extraction of Ni vs different pulp density for CVS ash, 15 minutes of stirring
time and initial solutions 0.003 and
0.004 M
For CVN ashes, taking into account the low percentage of adsorption obtained to high molar ratio, pulp density in the experiments was increased reaching densities of 250 grams / litre, achieving under these conditions results of about 90% elimination for 0.003 molar solution. Figure 5.

Figure 5. % Extraction of Ni vs different pulp density for CVN
ash, 15 minutes of stirring time and initial solutions of 0.003 and 0.004 M
Finally, tests were carried out to evaluate the impact on the final adsorption result, of the initial pH of the solution. Although CVS ashes were not influenced themselves it can be seen a worse result in the case of CVN ashes, with a higher acidity of the initial solution. Figure 6.
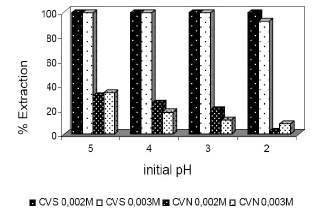
Figure 6. % Extraction of Ni vs different initial pH solution: 15
minutes of agitation time,40 g/l of CVS and CVN ash, and
initial solution 0.003 and 0.002 M
3.2 Copper elimination
In copper case, dissolutions 0.002M, 0.004M and
0.005M were prepared. Figure 7 shows the results obtained for tests realized with CVS and CVA ash, with acidic solutions with
a pH of 5, pulp density of
40 g
/ l and stirring time from 5 to 60 minutes. As in the case of nickel, the final
pH of the samples was measured.
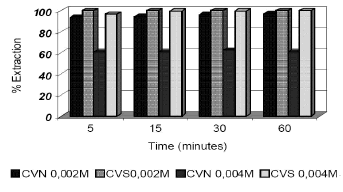
Figure 7. %
Extraction of Cu vs time for CVS and CVN fly ash and different initial metal
concentration. Pulp density 40 g/l
In the figure is observed how, as it happened in the case of nickel, CVS ashes allow to obtain the best results in the first 5 minutes of reaction, whereas for the CVN ashes, molar concentrations of 0.004 down the percentage of extraction to 60% even after 1 hour of stirring.
In view of the above mentioned results for tests realized with different pulp densities, the stirring time was fixed in 15 minutes (Figure 8.A) and 60 minutes (Figure 8.B) to assure a total reaction. Figures A and B show the good results for both ashes, reaching the total elimination of Cu with a high pulp density, both with 15 minutes of agitation and with 60 minutes.

Figure 8. A. % Extraction of Cu
for different pulp density, 15 minutes of agitation time and 0.004M. B. %
Extraction of Cu for different pulp density from CVN, 60 minutes of agitation
time and 0.005M
Finally, the study of the influence of the initial pH of the solution shows that at pH values of 3 or lower the results are considerably worse. Figure 9. In both cases, CVS ash shows a high metal removal capacity with low pulp density, due to its high specific surface, while CVN ash needs more density to achieve the complete elimination of the metal even with 60 minutes of reaction
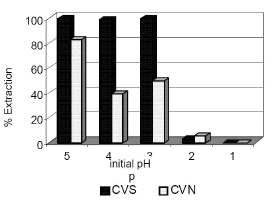
Figure 9. % Extraction of Cu
vs different initial pH solution: 30
minutes of agitation time, 40 g/l of CVS and CVN ash,
and initial solution 0.004 M
3.3 Cadmium Elimination
Dissolutions 0.001M, 0.003M
and 0.005M were prepared. Figure 10 shows the results obtained in the
same conditions as in the previous cases: CVS and CVN
ashes, pH 5, pulp density of
40
g / l and a stirring time from 5 to 60 minutes with a 0.003 M solution of Cd. CVS
ashes got the best results as opposed to the CVN ashes that are kept in
percentages of elimination not superior than 50% in all tests.

Figure
10. % Extraction of Cd vs time for CVS and CVN fly ash and solution 0.004 M.
Pulp density 40 g/l
In Figures 11.A and 11.B is observed how at low concentrations 100% of elimination is achieved with low pulp densities. As the molar value of the solution increases, the results are worse, being kept only for very high pulp densities, as expected, since the fly ash coming to lose its capacity of adsorption by saturation.

Figure 11. A. % Extraction of Cd
for different pulp density with CVN fly ash, 60 minutes of stirring time and
different molarity B. % Extraction of Cd
for different pulp density with CVS fly ash, 60 minutes of stirring time and
different molarity
Regarding the influence of the pH, for the case of Cd, seems have a minor influence on the results obtained. Figure 12.

Figure 12. % Extraction of Cd
vs different initial pH solution: 30 minutes of stirring time, 40 g/l of CVS and CVN ash and
0.004 M
The results obtained in the experiments carried out have become clear the influence that can have on the process of adsorption and neutralization on fly ash, parameters as the contact time, pH, initial metal concentration in solution and the physicochemical characteristics of the ashes. [8-9]
The removal of heavy metals is mainly due to a neutralization of the dissolution by increasing the pH, making the precipitation of the metal ions in solution as hydroxides. Therefore, the most important parameter in the captation of metal will be the initial pH of wastewater. Fly ashes have alkaline and alkaline earth metals on their surfaces that are dissolved to put in touch with the residual dissolution, increasing its pH. The metal is deposited in these conditions as a hydroxide on the surface of the ashes. The smaller the particle size and major its specific surface, the contact between solid and liquid will be better, thus increasing the retention of the metal. But for values of pH very acids, the dissolution of the above mentioned alkaline and alkaline earth metals are not sufficient to achieve the precipitation of metal hydroxides.
4. CONCLUSIONS
The study for the removal of Cu, Ni and Cd in acid mine waters realized in the laboratory reveals the following conclusions:
Both ashes from the power plant that burns coal as the one that burns anthracite are suitable for the removal of heavy metals such as nickel, copper and cadmium from contaminated waste water. The retention capacity is not the same for both ashes. It depends on both the physical and chemical properties of the ashes. In this case, the ash from coal has a higher retention capacity and for a short period of time. Thus, for the same initial concentration of nickel, it would be necessary a little more than 5 times the amount of anthracite ash than the coal ash to achieve the same results.
The kinetics of the reaction is faster for the ash from coal than for the anthracite. For concentrations of Ni among 0.001 and 0.01 M a removed between 90 and 100% of the metal is reached during the first five minutes. In the case of the ashes of anthracite (CVN) shows that more time is needed to achieve the complete elimination of nickel. The same can be say for the other two metals. With the increasing of concentrations of heavy metals in waste solutions a major time of contact between the ash and the dissolution it is necessary for the total elimination of the metal. This fact is more pronounced for the ashes from anthracite for having a lower capacity of captation.
Finally we can conclude that the captation of nickel, copper and cadmium on fly ash is an effective process that can become an effective alternative for sealing and closure of residual acidic leaching accumulations.
REFERENCES
[1] SINGER, P.C. & STUMM, W. Acid Drainage: The Rate Determining Step, Science, 167, 1121-1123, 1970.
[2] YOUNGER, P.L. The longevity of mine water pollution: a basis for decision-making. Science of the Total Environment, 194/195, 457-466, 1997.
[3] LOVERA ET AL. "Tecnologías limpias", Rev. Inst. investig. Fac. minas metal cienc. geogr, N° 03. 1999.
[4] KLEINMANN, R.L.P. Biogeochemistry of acid mine drainage and a method to control acid formation, Min. Eng. 33, 300-305, 1981.
[5] YADAVA K.P., TYAG B.S. I, PANDAY K.K. AND SINGH V.N. Fly ash for the treatment of Cd(II) rich effluents, Environ. Technol. Lett. 8, 225234, 1987.
[6] AYALA, J., BLANCO F., GARCIA P., RODRIQUEZ P. AND SANCHO J. Asturian fly ash as a heavy metals removal material, Fuel 77, 1147 1154, 1988.
[7] AYALA, J. & FERNÁNDEZ, B. Adsorption of copper cyanide species from tailings pond on flying ash, IMWA, 649-654, 2005.
[8] R. PÉREZ-LÓPEZ, J. M. NIETO, G. RUIZ DE ALMODÓVAR. Utilization of fly ash to improve the quality of the acid mine drainage generated by oxidation of a sulphide-rich mining waste: Column experiments. Chemosphere 67, 16371646, 2007.
[9] RICOU P., LECUYER I AND LE CLOIREC P. Influence of pH on removal of heavy metallic cations by fly ash in aqueous solution, Environmental Technology 19,10051016,1998.
[10] POTGIETER-VERMAA SS, POTGIETER JH, MONAMA P, VANGRIEKEN R. Comparasion of limestone , dolomite and fly ash as pre-treatment agents for acid mine drainage. Miner Eng.; 19:454-62, 2006
[11] GITARI MW, PETRIK LF, ETCHEBERS O,KEY DL IWUOHA E, OKUJENI C. Treatment of acid mine drainage with fly ash : removal of major contaminants and trace elements. J. Environ.Sci Health-Part A; 41 (8): 1729-47, 2006
[12] GITARI MW, PETRIK LF, ETCHEBERS O,KEY DL IWUOHA E, OKUJENI C, Passive neutralization of acid mine drainage by fly ash and its derivatives: a colum leaching study.Fuel 87 , 1637-1650, 2008.
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2010 DYNA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
El autor o autores de un artículo aceptado para publicación en cualquiera de las revistas editadas por la facultad de Minas cederán la totalidad de los derechos patrimoniales a la Universidad Nacional de Colombia de manera gratuita, dentro de los cuáles se incluyen: el derecho a editar, publicar, reproducir y distribuir tanto en medios impresos como digitales, además de incluir en artículo en índices internacionales y/o bases de datos, de igual manera, se faculta a la editorial para utilizar las imágenes, tablas y/o cualquier material gráfico presentado en el artículo para el diseño de carátulas o posters de la misma revista.

















