Publicado
Sol-gel synthesis of zinc oxide nanoparticle at three different temperatures and its characterization via XRD, IR and EPR
DOI:
https://doi.org/10.15446/dyna.v83n195.50833Palabras clave:
X-Ray diffraction, IR spectroscopy, sol-gel method, electron paramagnetic resonance, zinc oxide. (es)Descargas
DOI: https://doi.org/10.15446/dyna.v83n195.50833
Sol-gel synthesis of zinc oxide nanoparticle at three different temperatures and its characterization via XRD, IR and EPR
Síntesis sol-gel de nanopartículas de óxido de zinc a tres termperaturas diferentes y su caracterización vía XRD, IR y EPR
Manuel Acosta-Humánez a, Luis Montes-Vides b & Ovidio Almanza-Montero c*
a Departamento de Física. Universidad Nacional de Colombia. Bogotá.
Colombia. mafacostahu@unal.edu.co
b Departamento de Geociencias. Universidad Nacional de Colombia.
Bogotá. Colombia. lamontesv@unal.edu.co
c Departamento de Física. Universidad Nacional de Colombia. Bogotá.
Colombia. oaalmanzam@unal.edu.co
Received: May 25th, 2015. Received in revised form: September 11th, 2015. Accepted: September 21th, 2015.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract
In this work, nanoparticles of zinc
oxide were synthesized; they were formed using the sol-gel method (citrate
route) at calcination temperatures (Tc) of 500, 550 and 600 °C. For all samples
studied, IR spectroscopy showed the presence of the bands associated with water
molecules present in the zinc oxide and carbon dioxide adsorbed on its surface.
The formation of zinc oxide phase was confirmed by XRD, which showed that from
500 ºC it had this type of Wurtzite structure. However, samples calcinated at
600 ºC have higher crystallinity. Crystallite size was calculated using the Scherrer
equation. The Rietveld method was used
to obtain lattice parameters a and c for Wurtzite cell types as well as cell
volume and the ratio c/a of each sample. These parameters do not show
significant changes when they are compared with values obtained from samples
with different calcination temperatures. Electron paramagnetic resonance showed
the presence of defects in the zinc oxide. Three signals with g values of 1.96,
2.00 and 2.04 were associated with oxygen and zinc vacancies. Defects in the
structure disappear when the calcination temperature is increased. The sample
that was highlighted with the highest concentration of vacancies has a mean
crystallite size greater than 30 nm, and this may also be responsible for this
feature.
Keywords: X-Ray diffraction, IR spectroscopy, sol-gel method, electron paramagnetic resonance, zinc oxide.
Resumen
Se
sinterizaron nanopartículas de óxido de zinc (ZnO) mediante el método sol-gel
(ruta citrato) a las temperaturas de calcinación (Tc) de 500, 550 y 600 °C.Se
mostró por espectroscopía IR la presencia de las bandas asociadas a moléculas
de agua presentes en el óxido de zinc así como de dióxido de carbono adsorbido
en su superficie, para todas las muestras estudiadas. La tenencia de una
estructura tipo wurtzita, propia del ZnO, incluso para las muestras calcinadas
a 500 ºC, fue confirmada por XRD, Las muestras preparadas a Tc de 600 ºC tienen
mayor cristalinidad que las muestras calcinadas a 500 ºC. El tamaño de cristal
fue evaluado por la ecuación de Scherrer. El método Rietveld fue usado para
obtener los parámetros de red a y c para la celda tipo wurtzita, así como el
volumen de celda y la relación c/a de cada muestra. Estos parámetros no
muestran desviaciones significativas cuando ellos son comparados entre valores
obtenidos para muestras con distintas temperaturas de calcinación. Por
resonancia paramagnética electrónica se mostró la presencia de defectos en el
óxido de zinc. Tres señales, con valores de g = 1.96, 2.00 y 2.04 fueron
asociados a vacancia de oxígenos y vacancias de Zinc respectivamente. Los
defectos en la estructura desaparecen a medida que se incrementa la temperatura
de calcinación. La muestra con la mayor concentración de vacancias tiene un
tamaño medio de cristalito mayor de 30 nm y esto último puede ser el
responsable de esta característica.
Palabras clave: Difracción de rayos X, espectroscopía IR, método sol-gel, resonancia paramagnética electrónica, ZnO.
1. Introduction
Zinc oxide (ZnO) is a type II-VI semiconductor with a direct band gap of 3.37 eV and stable Wurtzite type structure with lattice parameters a = 3.25 Å and c = 5.21 Å [1]. It is an important semiconductor material due to its applications, which include transparent conductive oxides (TCO) [2,3], ultraviolet (UV) blockers, and photocatalysts, among others. As photocatalysts, the reduction of organic pollutant compounds and the remediation of organic contaminants, mainly azo type (compounds that interact with visible light), can be mentioned as being the most important in terms of usefulness. The presence of free radicals or vacancies in them are responsible for these applications and some authors have established that the amount of them is dependent on the crystal size, given that the ratio surface / volume increases as the size of the material decreases to a nanometer range [4,5]. ZnO is a relatively open structure with a hexagonal-close-packed lattice where Zn atoms occupy half of the tetrahedral sites, while all octahedral sites are empty [6]. The open structure also influences the nature of defects and the diffusion mechanism. The identity, quantity, and stability of these radicals or vacancies are features to consider when this material is required in a particular application. Applications for these types of materials depend on their electrical and magnetic properties and these depend on the method by which they were prepared [1-5].
Many methods for the production of ZnO nanostructures have been described in the literature such as laser ablation [7], hydrothermal methods [8], electrochemical depositions [9], chemical vapor deposition [10], thermal decomposition [11], the combustion method [12] and the co-precipitation method [13,14], resulting in zinc oxide nanoparticles with a nanometric size. The sol-gel method allows the mixture of the initial reagent on an atomic level, which, while there is control of chemical composition and there are quite homogeneous materials in its composition, reduces the possibility of having impurities that are difficult to detect and has good reproducibility. Materials of high surface area can be prepared at a low cost and the experimental procedure can be easily undertaken [15,16]. In this work, pure zinc oxide was synthesized using the sol-gel method (citrate route) at various calcination temperatures. Structural properties and the mean crystal size were determined by XRD, and its purity was assessed by infrared spectroscopy. The presence of free radicals, vacancies or defects was observed by electron paramagnetic resonance, which could be associated to potential uses of these materials as reductors of organic pollutant compounds.
2. Materials and methods
Nanoparticles of Zinc oxide were prepared by the sol-gel (citrate route) method using Zinc Nitrate, Zn(NO3)26H2O (Panreac) as the starting materials. Citric acid C6H8O7H2O (Panreac) was used as a complexing agent. A stoichiometric weight of metallic cation was homogeneously mixed in deionized water using magnetic stirring at 70 °C. Then, Citric acid was also dissolved in deionized water for 30 minutes. The Citrate/Nitrate ratio used was 1. The solution of Zinc Nitrate in the solution of Citric acid was added slowly. The reaction mixture was heated at 70 °C with continuous stirring until gel formation. The product obtained was dried at 130 °C for 12 hours and pulverized for 30 minutes using an agate mortar. The precursor materials were calcined at the calcination temperature (Tc) of 500, 550 and 600 °C for 12 hours [4]. These samples were denominated in ZnOTc form. The reaction of zinc oxide formation can be written as described in equation (1):
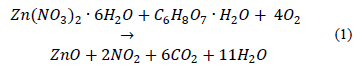
Infrared spectra were carried out in a Fourier transform spectrophotometer Shimadzu IRAffinity-1 in the range of wavenumber 4000-600 cm-1. X-ray diffraction measurements were performed on a diffractometer Panalytical Pro MPD using polycrystalline silicon as a calibration standard in 2q range of 10-90, and using CuKa1 radiation and a step size (2q) of 0.0260. Sweep time was 18.6860 s. The EPR spectra were carried out on a Bruker X-band spectrometer ESP-300 at different temperatures. A frequency of 9.45 GHz and a modulation amplitude of 10.496 G were also used in this procedure.
3. Results and discussion
The purity and molecular structure of the samples were determined by infrared spectroscopy. Fig. 1 shows the FTIR spectra of the nanocrystalline powders of zinc oxide. The two peaks at ~3428 and 1590 cm-1 are attributed to the stretching vibrations of the -OH group and the deformation of H-O-H bond, which are associated with small amounts of water existing in the zinc oxide [1]. The band corresponding to ~ 2400 cm-1 is associated with CO2 molecules that are adsorbed on the surface of ZnO [17,18]. The bands that are near to 1000 cm-1 are associated with metal-Oxygen tension and bending, in this case, Zn-O. An increase of the temperature in this band is associated with high Zn-O bonding due to thermal evolution of samples [18]. Two small bands are observed at 2920 and 2850 cm-1, possibly due to -CH2 groups belonging to the metal-organic chain formed during synthesis [18]; these are small residues and the bands are not intense. All these results were independent of calcination temperature (Tc).
Fig. 2 shows diffractograms for ZnO samples as well as the precursor material studied at different calcinations temperatures. At this point, the evolution of the precursor material is displayed, which is an organometallic compound (zinc citrate), and its evolution towards zinc oxide can be appreciated when calcination temperature is higher than 500 ºC. For all samples studied, all diffraction peaks were associated with Zinc oxide according to the PDF-Card 36-1451 (2004 PDF2 database) and the structure was a wurtzite type. There were no observed peaks corresponding to some of the reagent precursors or other secondary phases, and all samples have good crystallinity, which increases at the same time as the calcination temperature Tc. The Wurtzite structure for ZnO, is therefore observed from Tc = 500 ºC and this calcination temperature could be enough to obtain samples that are going to be used as a photocatalyst, that in turn decreasing production costs.
It is worthwhile mentioning that the diffraction peak associated with the plane (101) is the most intense one, and this may be due to the synthesis method used since it has been reported that for thin films the most intense level corresponds to the (002) reflection [19-21]. The preferred orientation of growth, determined using a texture coefficient TC (hkl) was calculated using equation (2) [19] and showed that for all samples calcinated, the preferred growth plane was (100) except ZnO calcinated at 600 °C, which grew preferentially in the plane direction (101).

In this equation, I (hkl) is the measured relative intensity of the plane (hkl), I0 (hkl) is the relative intensity of the standard plane (hkl) taken from the JCPDS database, N is the number of reflections in the diffractogram, 7 being the most intense (Fig. 2), and n is the number of used diffraction peaks, which in our case was 3 associated with the planes (100), (002) and (101). For a sample that has randomly oriented crystallites, the value of TC(hkl) is equal to 1, whereas if this value increases, it means that the greater abundance of crystals is oriented in one particular direction (hkl) [20] . TC(hkl) data for the three main diffraction peaks (100), (002) and (101) are shown in Table 1.
Mean crystallite size was estimated from the Scherrer equation, with a gaussian fit,
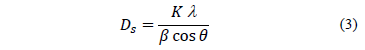
where b is integral breadth (in radians), l is the wavelength of X rays (CuKa radiation, 0.1540598 nm), K is Scherrer's constant that depends on the directions of the crystal lattice and crystal morphology [21]; its value is 0.94 and q is Bragg's angle. Integral breadths of diffraction peaks for all samples were calculated using the following equation:
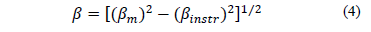
Where binstr is the integral breadth for a calibration standard, and bm the integral breadth for diffraction peaks of synthesized samples. The results for Dsare shown in Table 2, and Ds values are nanometric. The lowest Ds is obtained when calcination temperature is 550 °C; ZnO calcinated at 500 °C has an average crystallite size above 30 nm. Differences in crystal sizes (Ds) are determined by the Scherrer equation, and this is true for all samples as they have different integral breadth values as consequence of the fact they were calcinated at different temperature Tc.
The lattice parameters a and c as well as cell volume (V) were calculated by the Rietveld method, using the Fullprof program [22]. Table 2 shows the calculated values. a and c do not change significantly with calcination temperature. The ratio c/a shows the deviation that crystalline structure of the samples presented with respect to the theoretical network hcp (hexagonal compact packing, the value of which is 1.633). The deviations regarding the theoretical value may be products of microstrains and the concentration of defects in the crystal.
Fig. 3 shows the EPR spectra for the Zinc oxide samples that are calcinated at three different temperatures. At Tc = 500 ° C three signals are observed having g values of 1.96, 2.00 and 2.04. Some authors have associated these lines to defects in Zinc oxide [4]. The signal localized at the g = 1.96 line was attributed to Oxygen vacancies [4,23,24]. The other two lines are associated with Zinc vacancies [25]. Stehr et al. attributed these lines to shallow donors, which show a slight anisotropy because of the Wurtzite type crystal structure of zinc oxide [26]. The sample calcinated at 550 °C showed a small concentration of defects associated with Oxygen vacancies, while the sample calcinated at 600 ° C showed no vacancies, at least in the order of determination of these defects by EPR. It means that pure ZnO calcinated at 550 ºC could be used as a photocatalyst if the oxidation/reduction activity were caused by the presence of defects or vacancies. The sample is highlighted with the highest concentration of vacancies that has a mean crystallite size greater than 30 nm, and this may also be responsible for this feature.
4. Conclusion
Zinc oxide nanoparticles were synthesized at calcination temperatures of 500, 550 and 600 °C. For all samples, the formation of wurtzite-type structures of zinc oxide was observed. The crystal size indicates that samples contain nanosized crystals, and that there are also small microstrains associated with small variations in the hexagonal lattice of zinc oxide. Lattice parameters showed no significant variations in the unit cell due to variation of the calcination temperature. The presence of water and Carbon dioxide molecules on the surface of zinc oxide was evidenced by infrared spectroscopy. Zinc vacancies and oxygen were shown by electron paramagnetic resonance, a process in which their disappearance is shown as long as the calcination temperature increases. The presence of vacancies in samples calcined at Tc = 500 °C, which reacts with a mean crystallite size above 30 nm should be the best catalyst performance or oxidizing/reducing agent.
Acknowledgment
We would like to give thanks to the DIB of the National University of Colombia for their financial support through project 15848. Additionally, we thank professor Mario Barrera for facilitating our use of the equipment and laboratories for the material synthesis.
References
[1] Jagannatha-Reddy, A., Kokila, M.K, Nagabhushana, H., Chakradhar, R.P.S, Shivakumara, C., Rao J.L. and Nagabhushana, B.M., Structural, optical and EPR studies on ZnO:Cu nanopowders prepared via low temperature solution combustion synthesis. J. Alloys. Compd. 509(17), pp. 5349-5355, 2001. DOI: 10.1016/j.jallcom.2011.02.043
[2] Hisono, H., Recent progress in transparent oxide semiconductors: Materials and device application. Thin Solid Films. 515(5), pp. 6000-6014, 2007. DOI: 10.1016/j.tsf.2006.12.125
[3] Becheri, A., Durr, M., Lo Nostro, P. and Baglioni, P., Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV absorbers. J. Nanopart. Res. 10, pp. 679-689, 2008. DOI: 10.1007/s11051-007-9318-3
[4] Acosta-Humánez, F., Cogollo-Pitalúa, R. and Almanza, O., Electron paramagnetic resonance in Zn1-xCoxO. J. Magn. Magn. Mater. 329, pp. 39-42, 2013. DOI: 10.1016/j.jmmm.2012.10.026
[5] Wu, C., Shen, L., Shang, Y.C. and Huang, Q., Solvothermal synthesis of Cr-doped ZnO nanowires with visible light-driven photocatalytic activity. Mat. Lett. 65(12), pp. 1794-1796, 2011. DOI: 10.1016/j.matlet.2011.03.070
[6] Cao, B., Gong, H., Zeng, H. and Cai, W.P. Photoluminiscence / Fluorescence spectroscopic technique for nanomaterials characterization, in Nanomaterials: Processing and characterization with lasers, Weinheim, Wiley, 2012, pp. 597-616.
[7] Scarisoreanu, N., Metai, D.G., Dinescu, G, Epurescu, G, Ghica, C, Nistor, L.C. and Dinescu, M., Properties of ZnO thin films prepared by radio-frequency plasma beam assisted laser ablation. Appl. Surf. Sci. 247, pp.518-525, 2005. DOI: 10.1016/j.apsusc.2005.01.140
[8] Ni, Y.H., Wei, X.W., Hong, J.M. and Ye, Y., Hydrothermal synthesis and optical properties of ZnO nanorods. Mater. Sci. Eng., B, 121, pp. 42-47, 2005. DOI: 10.1016/j.mseb.2005.02.065
[9] Chang, S., Yoon, S.O., Park, H.J. and Sakai, A., Luminescence properties of Zn nanowires prepared by electrochemical etching. Mater. Lett. 53, pp. 432-436, 2002. DOI: 10.1016/S0167-577X(01)00521-3
[10] Wu, J.J. and Liu, S.C., Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv. Mater. 14, pp. 215-218, 2002. DOI: 10.1002/1521-4095(20020205)14:3<215::AID-ADMA215>3.0.CO;2-J
[11] Wang, R.C. and Tsai, C.C., Efficient synthesis of ZnO nanoparticles, nanowalls, and nanowires by thermal decomposition of zinc acetate at a low temperature. Appl. Phys. A. 94, pp. 241-245, 2009. DOI 10.1007/s00339-008-4755-0
[12] Lamas, D.G., Lascalea, G.E. and Walsoc, N.E., Synthesis and characterization of nanocrystalline powders for partially stabilized zirconia ceramics. J. Eur. Ceram. Soc. 18, pp. 1217-1221, 1998. DOI: 10.1016/S0955-2219(98)00045-4
[13] Kumar, S.S., Venkateswarlu, P., Rao, V.R. and Rao, V.R., Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett [Online]. 30(3), pp. 2-6, 2013. [Date of reference 11th October of 2015]. Available at: http://www.inl-journal.com/content/pdf/2228-5326-3-30.pdf
[14] Ruiz, C.V. and Rodríguez-Páez, J.E., Aluminatos de sodio obtenidos del sistema Al(NO3)3×9H2O a través del método de precipitación controlada. Ingeniería e Investigación [Online]. 30(2), pp. 16-24, 2010. [Date of reference 13th October of 2015]. Available at: http://www.revistas.unal.edu.co/index.php/ingeinv/article/view/15726/34068
[15] Jokela, S.J. and McCluskey, M.D., Structure and stability of O-H donors in ZnO from high-pressure and infrared spectroscopy. Phys. Rev. B. 72(11), pp. 113201(1-4), 2005. DOI: 10.1103/PhysRevB.72.113201
[16] Montenegro-Hernández, A. y Rodríguez-Páez, J.E., Síntesis óxido de estaño altamente reactivo utilizando como precursor etilhexanoato de estaño. Ingeniería e Investigación [Online]. 29(1), pp. 47-52, 2009 [Date of reference 13th October of 2015] Available at: http://www.revistas.unal.edu.co/index.php/ingeinv/article/view/15142/34413.
[17] Nejati, K., Rezvani, Z. and Pakizevand, R., Synthesis of ZnO nanoparticles and investigation of the ionic template effect on their size and shape. Int. Nano Lett. [Online]. 1, pp. 75-81, 2011 [date of reference December 15th of 2014]. Available at: http://inljournal.com/?_action=article&vol=1&issue=4&_is=Volume+1%2C+Number+2+%28July+2011%29
[18] Nakamoto, K., Infrared and raman spectra of inorganic and coordination compounds, Applications in coordination, organometallic, and bioinorganic chemistry. Wiley, New York, 2009.
[19] Caglar, Y., Sol-gel derived nanostructure undoped and cobalt doped ZnO: Structural, optical and electrical studies J. Alloys Compd. 560, pp. 181-188, 2013. DOI: 10.1016/j.jallcom.2013.01.080
[20] Xu, C., Cao, L., Su, G., Liu, W., Qu, X. and Yu, Y., Preparation, characterization and photocatalytic activity of Co-doped ZnO powders. J. Alloys Compd. 497(1-2), pp. 373-376, 2010. DOI: 10.1016/j.jallcom.2010.03.076
[21] Yang, J.H, Zhao, L.Y., Ding, X., Yang, L.L., Zhang, Y.J., Wang, Y. X. and Liu, H.L., Magnetic properties of Co-doped ZnO prepared by sol-gel method. Materials Science and Engineering B., 162(3), pp. 143-146, 2009. DOI: 10.1016/j.mseb.2009.03.020
[22] Rodríguez-Carvajal, J., Fullprof suite software: Crystallographic tools for rietveld, profile matching and integrated intensity refinements for X-ray and/or neutron data. Version 5.30. Laboratorie Leon, Brillounin, Gifsuryvette, France, 2012.
[23] Vlasenko, L.S., Magnetic resonance studies of intrinsic defects in ZnO: Oxygen vacancy. Appl Magn Reson. 39(1), pp. 103-111, 2010. DOI: 10.1007/s00723-010-0140-1
[24] Janotti, A. and Van de Walle, C.G., Native point defects in ZnO. Phys. Rev. B. 76(16), pp. 165202(22), 2007. DOI: 10.1103/PhysRevB.76.165202
[25] Vlasenko, L.S. and Watkins, G.D., Optical detection of electron paramagnetic resonance for intrinsic defects produced in ZnO by 2.5-MeV electron irradiation in situ at 4.2 K. Phys. Rev. B. 72(3), pp. 035203(12), 2005. DOI: 10.1103/PhysRevB.72.035203
[26] Stehr, J.E., Meyer, B.K. and Hofmann, D.M., Magnetic resonance of impurities, intrinsic defects and dopants in ZnO. Appl Magn Reson. 39(1), pp. 137-150, 2010. DOI: 10.1007/s00723-010-0142-z
M. Acosta-Humánez, received his BSc. in Chemistry in 2009 from the Universidad de Córdoba, Montería, Colombia. In 2015 he received and MSc. Eng. Degree in Materials and Processes from the Universidad Nacional de Colombia, Bogotá, Colombia. His research interests include: Chemical synthesis process of inorganic materials (oxides), characterization techniques (XRD, EPR, IR, UV-vis) as well as electrochemical methods for materials synthesis and characterization. ORCID: 0000-0003-0610-4831.
L. Montes-Vides, received his BSc. in Physicist - graduated from the Universidad Nacional de Colombia, MSc. in Engineering in 1987, from the Universidad Nacional de Colombia, and Dr. in Science - Geophysics in 1998. from the Universidade Federal do Pará, Brazil. He has worked at the Universidad Nacional de Colombia in Bogotá since 1990 as a lecturer on the following programs: undergraduate program in Geology, graduate programs in MSc. Geophysics and Doctorate program in Geosciences, all from the Faculty of Sciences. His research areas focus on Seismic prospecting, seismic inversion, seismic modeling and applied computing mathematics. He is a reviewer of the following scientific journals: ESRJ, Revista da SBGf, J.Appl. Geophysics and CT&F. ORCID: 0000-0002-7470-9202
O. Almanza-Montero, received his PhD. in Physics in 2000, from the Universidad de Valladolid, Spain, and his MSc. in Physics from the Universidad Nacional de Colombia. He is currently a professor in this University and has worked there since 1996. His research is focused on Materials science such as semiconductor and antioxidant activity of Colombian fruits. He is author of more than 50 papers published in different journals. He regularly uses the following characterization techniques: (XRD, EPR, IR, UV-vis). ORCID: 0000-0002-5141-6079
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Bouchra Ba Mohammed, Younes Dehmani, Hassane Lgaz, Taibi Lamhasni, Younes Brahmi, Han-seung Lee, Awad A. Alrashdi, Abouarnadasse Sadik, Khalid Yamni. (2024). Unveiling the role of transition metal catalysts in low-temperature, liquid-phase isopropanol oxidation. Inorganic Chemistry Communications, 159, p.111716. https://doi.org/10.1016/j.inoche.2023.111716.
2. Anusuya Mariappan, Leelavathi Harikrishnan, Jayanthi Eswaran, Nagaveni Arumugham, Yogeswari Balasubramaniam, Santhanaraj Daniel, Rajakumar Kanthapazham. (2024). Green Synthesis of Metal-Doped ZnO Nanoparticles Using Bauhinia racemosa Lam. Extract and Evaluation of Their Photocatalysis and Biomedical Applications. ACS Applied Bio Materials, 7(4), p.2519. https://doi.org/10.1021/acsabm.4c00132.
3. Gee Een Lau, Che Azurahanim Che Abdullah, Wan Amir Nizam Wan Ahmad, Suvik Assaw, Alvin Lim Teik Zheng. (2020). Eco-Friendly Photocatalysts for Degradation of Dyes. Catalysts, 10(10), p.1129. https://doi.org/10.3390/catal10101129.
4. Xuan-Sang Nguyen, Minh-Quan Nguyen, Xuan-Thang Trinh, Alexandra Camelia Joita, Sergiu Vasile Nistor. (2020). Correlation of native point defects and photocatalytic activity of annealed ZnO nanoparticle studied by electron spin resonance and photoluminescence emission. Semiconductor Science and Technology, 35(9), p.095035. https://doi.org/10.1088/1361-6641/aba168.
5. Saima Naz, Ahmad Manan Mustafa Chatha, Guillermo Téllez-Isaías, Shakeeb Ullah, Qudrat Ullah, Muhammad Zahoor Khan, Muhammad Kamal Shah, Ghulam Abbas, Azka Kiran, Rubina Mushtaq, Baseer Ahmad, Zulhisyam Abdul Kari. (2023). A Comprehensive Review on Metallic Trace Elements Toxicity in Fishes and Potential Remedial Measures. Water, 15(16), p.3017. https://doi.org/10.3390/w15163017.
6. Muhammad Tayyab Noman, Michal Petru, Jiří Militký, Musaddaq Azeem, Muhammad Azeem Ashraf. (2019). One-Pot Sonochemical Synthesis of ZnO Nanoparticles for Photocatalytic Applications, Modelling and Optimization. Materials, 13(1), p.14. https://doi.org/10.3390/ma13010014.
7. Renu Yadav, Tejpal S. Chundawat, Praveen K. Surolia, Dipti Vaya. (2022). Photocatalytic degradation of textile dyes using β-CD-CuO/ZnO nanocomposite. Journal of Physics and Chemistry of Solids, 165, p.110691. https://doi.org/10.1016/j.jpcs.2022.110691.
8. Huma Tariq, Fahad Azad, Sami-Ullah Rather. (2022). Functional Properties of Donor (Al) and Acceptor (Cu) Codoped High Dielectric Constant ZnO Nanoparticles. Journal of Nanomaterials, 2022(1) https://doi.org/10.1155/2022/3855582.
9. Solomon Kebede Urge, Solomon Tiruneh Dibaba, Abebe Belay Gemta, Vidya Nand Singh. (2023). Green Synthesis Method of ZnO Nanoparticles using Extracts of Zingiber officinale and Garlic Bulb (Allium sativum) and Their Synergetic Effect for Antibacterial Activities. Journal of Nanomaterials, 2023, p.1. https://doi.org/10.1155/2023/7036247.
10. A.A. Blinova, A.V. Blinov, O.A. Baklanova, M.A. Yasnaya, I.S. Baklanov, A.A. Siddiqui, A.B. Golik, A.I. Okolelova, A.A. Gvozdenko, A.N. Simonov, S.N. Povetkin. (2021). Study of Wound-Healing Ointment Composition based on Highly Dispersed Zinc Oxide Modified with Nanoscale Silver. International Journal Of Pharmaceutical And Phytopharmacological Research, 11(1), p.134. https://doi.org/10.51847/Cu6KX0JDE3.
11. Renu Yadav, Tejpal S Chundawat, Pooja Rawat, Gyaneshwar K Rao, Dipti Vaya. (2021). Photocatalytic degradation of malachite green dye by ZnO and ZnO–β-cyclodextrin nanocomposite. Bulletin of Materials Science, 44(4) https://doi.org/10.1007/s12034-021-02533-z.
12. Jyoti, Satyendra Singh, Sumistha Das, Shaili Srivastava. (2023). Comparative study for removal of toxic hexavalent chromium by zinc oxide nanoparticles, chitosan, chitin and zinc-chitosan nano-biocomposite. Environmental Technology & Innovation, 32, p.103310. https://doi.org/10.1016/j.eti.2023.103310.
13. Seyyed Vahid Mousazad Goorabjavari, Fateme Golmohamadi, Saba Haririmonfared, Hosein Ahmadi, Soheil Golisani, Hadi Yari, Anwarul Hasan, Zehra Edis, Mahsa Ale-Ebrahim, Majid Sharifi, Behnam Rasti, Fahimeh Nemati, Mojtaba Falahati. (2021). Thermodynamic and anticancer properties of inorganic zinc oxide nanoparticles synthesized through co-precipitation method. Journal of Molecular Liquids, 330, p.115602. https://doi.org/10.1016/j.molliq.2021.115602.
14. Renu Yadav, Tejpal S. Chundawat, Monu Verma, Dipti Vaya. (2024). β-cyclodextrinbased magnetic ternary nanocomposite for efficient adsorption and photocatalytic degradation of Rhodamine Blue, Gunner 500, Cefixime, and p-Nitrophenol. Journal of Physics and Chemistry of Solids, 188, p.111930. https://doi.org/10.1016/j.jpcs.2024.111930.
15. Stefano Alberti, Irene Basciu, Marco Vocciante, Maurizio Ferretti. (2021). Experimental and Physico-Chemical Comparison of ZnO Nanoparticles’ Activity for Photocatalytic Applications in Wastewater Treatment. Catalysts, 11(6), p.678. https://doi.org/10.3390/catal11060678.
16. S. Farhat, M. Rekaby, R. Awad. (2018). Synthesis and Characterization of Er-Doped Nano ZnO Samples. Journal of Superconductivity and Novel Magnetism, 31(9), p.3051. https://doi.org/10.1007/s10948-017-4548-9.
17. S. Mustapha, M. M. Ndamitso, A. S. Abdulkareem, J. O. Tijani, D. T. Shuaib, A. O. Ajala, A. K. Mohammed. (2020). Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: a review. Applied Water Science, 10(1) https://doi.org/10.1007/s13201-019-1138-y.
18. Sameh H. Ismail, Ahmed Hamdy, Tamer Ahmed Ismail, Heba H. Mahboub, Walaa H. Mahmoud, Walid M. Daoush. (2021). Synthesis and Characterization of Antibacterial Carbopol/ZnO Hybrid Nanoparticles Gel. Crystals, 11(9), p.1092. https://doi.org/10.3390/cryst11091092.
19. Wubishet Kejela Tolossa, Paulos Taddesse Shibeshi. (2022). Structural, optical and enhanced antibacterial activities of ZnO and (Co, Fe) co-doped ZnO nanoparticles by sol-gel combustion method. Chemical Physics Letters, 795, p.139519. https://doi.org/10.1016/j.cplett.2022.139519.
20. Nguyen Xuan Sang, Nguyen Minh Quan, Nguyen Huu Tho, Nguyen Tri Tuan, Tran Thanh Tung. (2019). Mechanism of enhanced photocatalytic activity of Cr-doped ZnO nanoparticles revealed by photoluminescence emission and electron spin resonance. Semiconductor Science and Technology, 34(2), p.025013. https://doi.org/10.1088/1361-6641/aaf820.
21. Thanyalux Wanotayan, Joongjai Panpranot, Jiaqian Qin, Yuttanant Boonyongmaneerat. (2018). Microstructures and photocatalytic properties of ZnO films fabricated by Zn electrodeposition and heat treatment. Materials Science in Semiconductor Processing, 74, p.232. https://doi.org/10.1016/j.mssp.2017.10.025.
22. S Mustapha, M M Ndamitso, A S Abdulkareem, J O Tijani, D T Shuaib, A K Mohammed, A Sumaila. (2019). Comparative study of crystallite size using Williamson-Hall and Debye-Scherrer plots for ZnO nanoparticles. Advances in Natural Sciences: Nanoscience and Nanotechnology, 10(4), p.045013. https://doi.org/10.1088/2043-6254/ab52f7.
23. Samreen Zahra, Hamim Bukhari, Saboora Qaisar, Asma Sheikh, Athar Amin. (2022). Synthesis of nanosize zinc oxide through aqueous sol–gel route in polyol medium. BMC Chemistry, 16(1) https://doi.org/10.1186/s13065-022-00900-3.
24. Muhammad Tayyab Noman, Nesrine Amor, Michal Petru. (2022). Synthesis and applications of ZnO nanostructures (ZONSs): a review. Critical Reviews in Solid State and Materials Sciences, 47(2), p.99. https://doi.org/10.1080/10408436.2021.1886041.
25. Renu Yadav, Tejpal S. Chundawat, Praveen K. Surolia, Dipti Vaya. (2022). Photocatalytic Degradation of Ortho‐Nitrophenol using ZnO‐β‐CD Nanocomposite. ChemistrySelect, 7(23) https://doi.org/10.1002/slct.202200394.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2016 DYNA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
El autor o autores de un artículo aceptado para publicación en cualquiera de las revistas editadas por la facultad de Minas cederán la totalidad de los derechos patrimoniales a la Universidad Nacional de Colombia de manera gratuita, dentro de los cuáles se incluyen: el derecho a editar, publicar, reproducir y distribuir tanto en medios impresos como digitales, además de incluir en artículo en índices internacionales y/o bases de datos, de igual manera, se faculta a la editorial para utilizar las imágenes, tablas y/o cualquier material gráfico presentado en el artículo para el diseño de carátulas o posters de la misma revista.