Use of white rot fungi in the degradation of an azo dye from the textile industry
Utilización de hongos de la podredumbre blanca en la degradación de un colorante tipo azo de la industria textile
DOI:
https://doi.org/10.15446/dyna.v83n198.52923Palabras clave:
Solid-state fermentation, white-rot fungi, basic red 46, banana peel. (en)Fermentación en estado sólido, hongos de podredumbre blanca, rojo básico 46, cascara de banano. (es)
Descargas
DOI: https://doi.org/10.15446/dyna.v83n198.52923
Use of white rot fungi in the degradation of an azo dye from the textile industry
Utilización de hongos de la podredumbre blanca en la degradación de un colorante tipo azo de la industria textil
Ana Zuleta-Correa a,b, Andrés Merino-Restrepo b, Sara Jiménez-Correa b, Angelina Hormaza-Anaguano b & Santiago Alonso Cardona-Gallo c
a Department of
Biological and Agricultural Engineering, North Carolina State University, Raleigh, USA. azuleta@ncsu.edu
b Facultad de Ciencias,
Universidad Nacional de Colombia, Medellín, Colombia. ramerinor@unal.edu.co, sjimenezc@unal.edu.co, ahormaza@unal.edu.co
c Facultad de Minas, Universidad Nacional de Colombia, Medellín, Colombia. scardona@unal.edu.co
Received: September 4th, 2015. Received in revised form: January 29th, 2016. Accepted: May 11th, 2016.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract
Textile industry effluents-a complex mix of chemicals,
among which colorants are of particular concern-impose great environmental
challenges. In this study, a full 23 factorial design was used for
determining the best conditions for the degradation of textile dye Basic Red 46
under solid state fermentation (SSF). Three white rot fungi Trametes versicolor, Pleurotus ostreatus, and Pleurotus pulmonarius were used in the fermentation process. A
maximum degradation percentage of 63.0% was achieved at 17 days of incubation
with T. versicolor under a moisture
content of 90%, carbon to nitrogen ratio of 12: 1, and at 20°C. P. ostreatus and P. pulmonarius reached a
maximum degradation percentage of 69.3% and 63.1%, respectively, after 25 days
of fermentation. The scale-up of the fermentation process using T. versicolor
led to a degradation percentage of 45.7% after 30 days of incubation.
Additionally, the enzyme activity of laccase, manganese peroxidase and lignin
peroxidase was measured. The results indicate that SSF offers a satisfactory
degradation, whose efficiency depends on the optimization of process
conditions.
Keywords: Solid-state fermentation; white-rot fungi; basic red 46; banana peel.
Resumen
Los efluentes de la industria
textil imponen grandes retos ambientales. Estos son una mezcla compleja de
productos químicos, donde colorantes son de particular interés. En este
estudio, se implementó un diseño factorial completo 23 para
establecer las condiciones más apropiadas para la degradación del rojo básico
46 bajo fermentación en estado sólido, FES. Tres hongos de podredumbre blanca Trametes versicolor, Pleurotus ostreatus y
Pleurotus pulmonarius fueron utilizados en el proceso de fermentación. Se alcanzó una degradación máxima de
63.0% a los 17 días de incubación con T.
versicolor con una humedad del 90%, proporción carbono:nitrógeno de 12:1 y
a 20°C. P. ostreatus y P. pulmonarius alcanzaron porcentajes máximos de degradación del 69.3% y 63.1% respectivamente
luego de 25 días de fermentación. El escalado del proceso con T. versicolor condujo a una degradación
del 45.7% a los 30 días de incubación. Adicionalmente, se cuantificó la
actividad de las enzimas lacasa, manganeso peroxidasa y lignina peroxidasa. Los
resultados señalan que la FES ofrece una degradación satisfactoria, cuya eficiencia
depende de la optimización de las condiciones del proceso.
Palabras clave: Fermentación en estado sólido; hongos de podredumbre blanca; rojo básico 46; cascara de banano.
1. Introduction
The textile and clothing industry is one of the main pillars of the Colombian economy. It generates more than 800,000 jobs directly and indirectly and it accounts for about 12.1% of the national industrial production, 6.0% of total exports and 13.3% of non-traditional products sales [1,2]. Colombian textiles are recognized by high quality, color, and design. Such characteristics have allowed its success in highly competitive markets like the United States, the European Union, and the Andean Community, among others [1].
Textile effluents are usually made up of acids, bases, salts, oils, fats, surfactants and various types of dyes. Concentration of such pollutants is highly variable since it depends on the stage of the process and type of fabric. The variability of effluent composition and extreme temperatures and pH, make textile industry effluents difficult to treat [3]. The dying stage, in particular, has the greatest negative environmental impact. It uses large amounts of water; it is energy intensive, and releases highly toxic dyes into surface water [4].
It is estimated that 10 to 14% of the colorants used in the dyeing and finishing processes are discarded in effluents [5]. Unfortunately, their presence, even in minimal concentrations of 1.0 ppm, is sufficient to cause the aesthetic deterioration of the environment [6]. The most dramatic impact of their presence is the negative effect on photosynthetic processes. They reflect solar radiation reducing the self-regeneration of water resources. They also increase the biological oxygen demand (BOD) and chemical oxygen demand (COD) [1, 7] that could lead to anoxic conditions affecting the whole aquatic ecosystem [8].
Act number 3930 of 2010 in Colombian law regulates water quality standards and parameters for industrial and domestic effluents. The absence, in both the current and preceding act (Act number 1594 of 1984), of the amount of dyes permitted in industrial effluents, has led to the appearance of colored bodies of water emerging around the country. This is as a recurring and disturbing phenomenon in large cities such as Medellín.
Physicochemical treatment technologies such as activated carbon adsorption, flocculation, chemical oxidation, ozonation and filtration, among others, exhibit satisfactory decoloration efficiency. However, their use is restricted due to high costs, incomplete removal of pollutant, and sludge and the generation of toxic byproducts. Therefore, it is important to develop alternative treatment methodologies [9-11]. Between these methods, adsorption with agricultural wastes has emerged as a promising strategy; nevertheless, its study has been primarily limited to metals [12,13].
Biological strategies have been implemented as a result of the limitations associated with the traditional methods and the need to solve the persisting environmental problem. These methodologies use the microorganism's metabolic potential for transforming the pollutants into smaller molecules or for affecting the functional groups involved in their toxicity [14]. In the present study, a novel "mixed" or "combined" strategy is employed for treating a simplified simulated textile effluent. It combines a physiochemical and a biological process. The first step consists of removing dissolved dye from solution without breaking the molecule. In the following step, solid state fermentation (SSF) is used to mineralize or partially degrade the previously adsorbed pollutant.
SSF is characterized by the growth of microorganisms on solid substrates in the absence of free water (low water activity). In this case, the residue-dye complex was used as a source of support and nutrition for the microorganism [15-19]. Previous studies demonstrated the efficiency of the methodology by reaching degradation percentages higher than 90% under the best conditions during the fermentation process [20]. Contaminant degradation is attributed to the action of various enzymes produced by the microorganism.
White rot fungi (WRF) are proven the most efficient microorganisms in the treatment of xenobiotic molecules such as synthetic dyes [21]. Their metabolic capacity to mineralize complex polymers, even like lignin, is attributed to the secretion of non-specific and non-stereoselective enzymes. Laccases (EC 1.10.3.2), manganese peroxidases (EC 1.11.1.13) and lignin peroxidases (EC 1.11.1.14) stand out among the biological catalyzers [22] and they have been able to degrade recalcitrant compounds under conditions similar to the natural habitat of WRF, which are recreated under SSF conditions [23].
Banana peel (BP) was selected as an adsorbent and subsequent substrate in SSF because it allowed us to simulate the natural environment of WRF. Additionally, the lignin, cellulose and hemicellulose content of BP [24] seem to be appropriate for enhancing the production of ligninolytic enzymes [25-27]. Moreover, BP also represents an alternative, highly available, low-cost agricultural residue. On the other hand, basic red 46 (BR46) was selected because it is an azo dye widely used in the textile industry and it is characterized as a highly recalcitrant xenobiotic [28].
In the present study, the most appropriate conditions for the biodegradation of the synthetic dye BR46 under SSF were evaluated. Residues from the banana industry were implemented as substrates. P. ostreatus, P. pulmonarius, and T. versicolor were used to inoculate the fermentation.
2. Materials and methods
2.1. Microorganisms and culture conditions
Three WRF were evaluated, P. ostreatus, P. pulmonarius and T. versicolor. Microorganisms were obtained from Plant Tissue Laboratory at the University of Antioquia, Medellín, Colombia. They were preserved in Petri dishes with PDA agar at 4 °C and subcultured every two months. Before each fermentation, 1.2 cm disks containing the microorganism were subcultured and incubated at 28 °C for a period of 5 to 7 days. For degradation of RB46, small discs (diameter 1.2 cm) were taken from the edge of fungal growth zone for ensuring the exponential growth.
2.2. Adsorbent pretreatment and adsorption of BR46
Banana Peel was acquired in one of the dining halls at the Universidad Nacional de Colombia -Medellín Campus. It was washed, dried at 100 °C, and ground in an Ika mill. Then, it was sieved and particles ranging in size from 300 to 500 µm were chosen for performing the SSF experiments. To neutralize organic acids, BP was washed again with a KOH solution (83.17 mM) for 60 minutes [27,29]. The BR46 dye CI 110 825, was purchased from a local company.
The adsorption was performed in 500 mL beakers at room temperature and 150 rpm for 3 h in a Heidolph Unimax 1010 shaker; 500 mg of BP were added to 250 mL of BR46 solution at 800 ppm. A preliminary analysis of the influence of pH on dye removal showed that a pH greater than 7.0 favors adsorption. Therefore, pH=7.0 was selected for avoiding the costs of adding an alkali. The concentration of the dissolved dye was determined using an UV-Vis Lambda 35 spectrophotometer, Perkin Elmer. Absorbance before and after the adsorption were determined at the wavelength of maximum absorption (lmax = 531 nm). The amount of dye impregnated in the substrate or removal percentage, %R, was calculated using Equation 1, where c0 and cf correspond to the initial and final concentrations of BR46, respectively.

2.3. Determination of the best conditions of degradation in 50 mL erlenmeyer flasks
For each microorganism, we evaluated the effect of temperature (20 and 28 °C), ATRO moisture (90 and 142), and carbon nitrogen ratio C:N (12:1 and 20:1) in the degradation of BR46 dye adsorbed on BP. A randomization scheme was implemented in a randomized full block factorial design with four replicates resulting in a total of eight treatments, whose characteristics are detailed in Table 1.
Fifty mL Erlenmeyer flasks were used with 140 mg of colored solid substrate (BP-BR46) that were inoculated with three disks (1.2 cm in diameter) of the respective fungus grown on PDA agar.
The ATRO moisture (water percentage in the dry matter of the substrate) was reached using a nutrient solution at the beginning of the fermentation and afterwards regulated by adding sterile distilled water. The solution contained NH4Cl 0.35 g/L, (NH4)2SO4 1.40 g/L, KH2PO4 2.00 g/L, CaCl2 0.30 g/L, MgSO4 0.30 g/L, FeSO4 5.00 mg/L, MnSO4 1.60 g/L, ZnSO4 1.40 mg/L, CuSO4 44.70 mg/L and peptone 1.00 g/L. Moisture was determined by gravimetry on a moisture balance. The C: N ratio was adjusted taking into account the results of the bromatological analysis of the BP, adding the required amount of glucose in the nutrient solution. Compositional analysis was performed at the Laboratory of Chemical and Bromatological Analysis at the Universidad Nacional de Colombia - Medellín Campus.
2.4. Optimization of the BR46 desorption
BR46 dye desorption was initially evaluated using different mixtures of polar and nonpolar solutions, finding that the combination of acetone-hydrochloric acid gave the best results. This mixture was optimized through a Box-Behnken design, in four blocks, where the concentration of acetone (84, 86 and 88% v/v), contact time (13, 16 and 19 h), and solid support dosage varied (80, 140 and 200 mg), reaching a maximum desorption of 99.5% with an acetone concentration of 86% v / v in 0.2 M HCl, a contact time of 16 hours and a dosage of 140 mg [30].
2.5. Degradation kinetics curves
BR46 dye degradation in the SSF process was quantified comparing the amount of desorbed dye in the control sample (substrate without the action of the microorganism) with the amount of desorbed dye in each test sample. The degradation was monitored every five days and from the tenth day, every three days for a total period of 30 days. Equation 2 was used to establish the degradation percentage of the samples at a specific time.
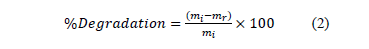
Where:
mi: mass of the desorbed dye in the control sample [mg]
mr: residual mass of desorbed dye in the study sample [mg]
Since the desorption achieved was close to 100%, no correction factor for the terms involved in equation 2 was implemented.
2.6. Evaluation of the degradation process in 100 mL erlenmeyer flasks
This preliminary scale-up of the SSF process was conducted with the fungal species that presented the best adaptation and growth on the solid substrate in the experiments carried out in 50 mL Erlenmeyer flasks, and under the conditions that allowed obtaining the highest degradation percentage.
Thus, 2,500 mg of BP were used. They were contained in a 100 mL Erlenmeyer flask, and were inoculated with five agar disks, 1.2 cm in diameter. The assembly included a total of 20 Erlenmeyer flasks. Ten experimental units were selected for determining the degradation percentage at 15 and 30 days of fermentation. Since the growth of the fungus is not homogeneous on the colored solid substrate, three samples of 140 mg were taken randomly from each experimental unit. They were used to perform the desorption process in triplicate and for the respective analysis.
2.7. Evaluation of the enzyme activity
In order to determine the ligninolytic activity, an enzymatic extraction was conducted by using previously reported protocols [14,25]. The experimental setup was similar to that described for the previous test of the scale-up in 100 mL Erlenmeyer flasks in terms of dosage and fermentation times.
For the extraction, 20 mL of acetate buffer (50 mM, pH = 5.5) were added to each experimental unit. These were stirred at 160 rpm, ensuring the agglomerate disintegration of the fermented substrate, in an ice bath for one hour and centrifuged at 6,000 rpm for five minutes. The supernatant was used to determine the nonspecific enzymatic activity of the selected fungal species.
Laccase (Lac) activity was established using 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) 2 mM as substrate and sodium citrate buffer (0.1 M, pH = 3.0), by measuring the increase in absorbance at 420 nm (e420 = 36.00 mM-1cm-1) [31,32]. Manganese peroxidase (MnP) activity was determined using 0.5 mM MnSO4 and 0.1 mM H2O2 in sodium malonate buffer (50 mM, pH = 4.5), measuring the increase in absorbance at 270 nm (e270 = 11.59 mM-1cm-1) [31,32]. Lignin peroxidase (LiP) activity was determined using 2.0 mM veratryl alcohol as substrate, and 0.5 mM H2O2 in sodium tartrate buffer (50 mM, pH = 2.5), by measuring the increase in absorbance at 310 nm (e310 = 9.30 mM-1cm-1) [31,33]. The enzyme activity is reported as enzyme units (U) per gram of solid substrate, gss, defined as the amount of enzyme required to produce 1.0 µmol product/min [32,33] or, in parallel, the amount necessary to oxidize a 1.0 µmol substrate/minutes [31].
2.8. Statistical analysis
All experimental trials were assessed with 95% confidence. To compare the results an analysis of variance was conducted (ANOVA), followed by the Scheffe's test. To determine the validity of the variance analysis, Shapiro-Wilk and Levene tests were performed with a confidence level of 99%, verifying the criteria of normality and homoscedasticity, respectively. To ensure independence, the sample selection and treatments were applied randomly. All results are reported as the average of measurements plus or minus (±) the standard deviation.
3. Results and discussion
3.1. Adsorbent pretreatment and BR46 adsorption
A sufficient amount of BP was prepared to carry out the BR46 adsorption process. Thus, with the conditions previously described concerning BP dosage, initial amount of dye, pH value, temperature and contact time, it was proceeded to quantify the concentration of the remnant BR46 in the solution. A solution of 40 ppm was obtained at the end of the process, which is equivalent to 95% removal. This high percentage of removal suggests that BP represents a suitable adsorbent material for its incorporation as a solid support in SSF processes aimed at treating colored effluents.
3.2. Determination of the best conditions of degradation in 50 mL erlenmeyer flasks
The results of BR46 degradation due to the action of the fungal species P. ostreatus, P. pulmonarius and T. versicolor are presented in Table 2. The eight treatments evaluated are shown in descending order of the mean percentage of degradation.
Treatments classified with the same letter for each microorganism exhibit no significant statistical difference. These results are derived from an ANOVA analysis with a confidence level of 95%.
The results suggest that the dye degradation process with P. ostreatus and P. pulmonarius worked best at the lowest temperature (treatments 1-4). In particular, a maximum degradation of 72.87% with P. ostreatus under treatment 1 was reached, which corresponds to a temperature of 20 °C, moisture content of 90% and C:N ratio of 12:1. That is, it includes the lowest levels for each factor, resulting in a decrease in the amount of glucose (lower C:N ratio) and in the volume of water (low moisture). For the above reasons, the conditions of this treatment were selected for constructing the kinetic curves.
It is noteworthy that under the same conditions, the highest percentage of RB46 degradation with P. pulmonarius was 63.38%, which is satisfactory but about 10 percentage units lower compared to P. ostreatus. These results are probably due to the difference in the intrinsic metabolism of the mentioned microorganisms, which belong to different species. The effect of a temperature increase (treatments 5-8), is verified with a significant decrease in the percentage of BR46 degradation; in the case of P. ostreatus, down to 1.78%. One possible explanation for this behavior is attributed to a faster drying process that the solid medium has at this temperature compared to 20 °C, which prevents the proper growth of the microorganism. At 28 °C, the inoculum dries and contracts preventing the proper colonization of the entire substrate. It is emphasized that for treatment 8, the synergy between high levels of the C:N ratio and moisture, mitigates the impact of temperature favoring the availability of nutrients, allowing appropriate development of the microorganism, and resulting in 51.5% degradation. Meanwhile, P. pulmonarius shows a greater sensitivity to the temperature rise, leading to a lack of degradation (treatment 5, corresponding to the conditions of lower moisture and less addition of carbon source).
The degradation process of the BR46 dye using T. versicolor was not affected under the conditions of temperature, moisture or C:N ratio evaluated, since a significant statistical difference between the pairs of treatment methods was not found. These results contrast with those found with P. pulmonarius and P. ostreatus, and a possible explanation for such a trend is taxonomy. This microorganism belongs to another genus within WRF, and therefore it may adapt differently to diverse nutritional conditions, directly influencing its development [34]. In particular, for this species the additional supply of carbon source (C:N 20:1 in treatments 3, 4, 7 and 8), did not favor the degradation; it is clear that the presence of carbon can support the primary metabolism; however, ligninolytic enzymes associated with dye degradation are part of the secondary metabolism [21].
A maximum degradation of 64.1% was achieved with treatment 8 using T. versicolor, which corresponds to a temperature of 28 ° C, an ATRO moisture of 142% and a C:N ratio of 20:1. Since no significant difference occurs with treatment 1 and 8 for T. versicolor and the highest degradation with P. ostreatus and P. pulmonarius, were reached under treatment 1 conditions, these parameters were taken to evaluate the degradation kinetics curves for the three strains.
3.3. Degradation kinetics in 50 mL erlenmeyer flasks
BR46 dye degradation by P. ostreatus, P. pulmonarius and T. versicolor, under the best growing conditions, determined from the previous experimental designs, is shown in Fig. 1. These conditions were as follows: temperature of 20 °C, C:N ratio of 12:1, and 90% moisture. The amount of solid substrate used in each assay was 140 mg.
It is noted that in a period of 408 hours (approximately 17 days) T. versicolor reached the maximum degradation percentage corresponding to 63.0±3.1%. In turn, P. ostreatus and P. pulmonarius reached their maximum values of degradation in 600 hours (approximately 25 days) with percentages of 69.3±1.6% and 63.1±3.7%, respectively. Likewise, it is emphasized that the error bars indicate quite high deviations at the beginning of the process, which decrease as time advances (Fig. 1). This occurs because in the early days of the fermentation, the microorganisms have not colonized the entire substrate and the growth on the residue is not homogeneous. As time progresses, the fungus grows throughout the substrate, acting on the BP-BR46 residue-dye complex. Furthermore, while the experimental units were treated with adequate homogeneity, the inoculum of each fermentation could have come from a different Petri dish, which may contribute to the increased in difference between the assays due to the fact that the microorganisms may have exhibited different adaptation times to the solid medium. Similar degradation results were reported by Robinson et al. [16], who evaluated a mixture of five reactive dyes adsorbed on barley husk. In their study, the WRF Bjerkandera adusta was used and a maximum degradation percentage of 53.1% after 21 fermentation days was reached. This process was performed using 5.0 g of the residue-dye mixture as substrate, with culture conditions similar to those used in the present study, an incubation temperature of 25 ° C, a moisture of 85% and a medium supplemented with glucose and micronutrients. It is noteworthy that the C:N ratio is not detailed.
The results of the kinetics allow to establish that T. versicolor offered the best performance in BR46 degradation. It reached a satisfactory degradation of 63.0%, in 17 days, whereas P. pulmonarius and P. ostreatus only achieved similar percentages in 25 days.
3.4. Evaluation of the degradation process in 100 mL Erlenmeyer flasks.
T. versicolor was selected to evaluate the process on a larger scale. The selection was based on its performance in kinetic studies. The fermentation was carried out at the same temperature, moisture, and C:N ratio conditions of previous experiments. However, the amount of solid substrate was increased to 2,500 mg. The degradation percentage at 15 and 30 days of fermentation are presented in Table 3.
When results of scale-up experiments are compared with the previous tests, a considerable decrease in the maximum degradation is observed. After 15 days of fermentation, the degradation percentage was 37 percentage points below the expected value (63%). However, after 30 days of fermentation, the degradation percentage corresponds to 72% of the maximum expected value. It may be noted that standard deviation is high even though each value corresponds to the weighted average of ten experimental units with three measurements for each sample. This uncertainty can be due to the heterogeneous fungal growth on the substrate: it was inoculated in the periphery of the Erlenmeyer flask, and then the substrate located in the center will be colonized at later stages. Besides, random samples were taken from each experimental unit and they could correspond to variable degradation regions.
The preliminary results suggest that the scaling-up of this process is feasible since the mass of the BP-BR46 complex increased over 18 times, but a satisfactory degradation percentage was achieved in a period that did not double the time reported for the fermentation process at a lower scale. Results also indicate that the amount of solid support to be treated has a direct impact on the rate of degradation and the time required to carry out the fermentation process. Therefore, modifications need to be implemented to promote further fungal growth and their enzyme activity. Some alternatives include the use of different inoculation techniques that enable a more homogeneous growth and a higher rate of colonization of substrate. It has also been reported that various metal inductors and co-substrates act as enhancing agents for enzyme activity and they lead to a substantial increase in the degradation of recalcitrant compounds. In particular, Baldrian and Gabriel, indicate that copper and cadmium addition led to eight and twelve fold increase of laccase activity compared to a medium without these metallic inductors [35].
3.5. Determination of enzyme activity
The ligninolytic activity of T. versicolor was quantified after 15 and 30 days of the fermentative process (Table 3).
After 15 days of fermentation, enzyme activity for Lac, MnP and LiP were 15.62 ± 2.53 U/gss, 6.42 ± 2.89 U/gss and 1.83 ± 1.23 U/gss, respectively. The value obtained for laccase activity in this investigation is lower when compared to the results reported by Iandolo et al. [36]. They obtained an activity of 35 U/gss with T. versicolor at 16 days of fermentation using a mixture of tomato waste and sorghum stalks. However, it should be noted that the production of enzymes under SSF conditions without further catalytic applications was evaluated in this study. In a period of 30 days, an increase in LiP (3.1 ± 1.8 U/s) was recorded while Lac and MnP activity decreased significantly, registering values of 5.4 ± 2.2 U/gss and 1.0 ± 0.4 U/gss respectively. It is noteworthy that a greater degradation efficiency is achieved at 30 days compared to the results for 15 days, but the degradation percentage is a cumulative variable, while the enzyme activity can change from time to time.
The production of ligninolytic enzymes by different WRF depends mainly on the fungal species, the substrate composition and culture conditions [36,37]. The results obtained in this study indicate that enzyme activity varies over time, a trend also evidenced by Winquist et al., with enzyme production of T. versicolor on oat hulls [38]; Reddy et al. with two Pleurotus strains using banana waste [39]; Robinson et al. with grim B. adusta on barley husk [16]; Sun et al. with Trametes sp. AH28-2 using four agricultural byproducts [40]; and Tychanowics et al., with P. pulmonarius on corn residues [33], among others.
It is also noteworthy that Robinson et al. [16] evaluated the enzyme activity during the degradation of adsorbed dyes and they found that it changes over time. In the early days of fermentation, low rates of degradation were observed, which coincided with the absence or minimum enzyme activity. As the fermentation process progressed, a higher content of ligninolytic enzymes was detected, even before the degradation of the dye could be perceived. This behavior allows to suggest that the enzymes are associated initially to the lignocellulose cleavage and subsequently to the dye degradation. Enzyme content increased to a maximum value, which occurs at a different time for each enzyme, after such a maximum value is reached, the activity starts to decrease. These authors reached maximum activities of LiP, MnP and laccase of 17.0 U/gss, 15.8 U/gss and 2.0 U/gss at nine, fifteen and twelve days, respectively, obtaining a maximum degradation percentage of 53.0% after 21 days.
The results of this study point out that BR46 degradation, impregnated on an agricultural waste, with WRF, represents a highly efficient and satisfactory alternative process for its bioremediation. In particular, further studies should continue to explore appropriate conditions to achieve an efficient degradation of this azo dye with the fungal species T. versicolor at a larger scale.
4. Conclusions
Degradation percentages higher than 60% were achieved at 20 °C, a moisture content of 90% and a C:N ratio of 12:1 using T. versicolor, which proved to be the most efficient fungus between the tested species for the degradation of the azo dye BR46. Seventeen days were needed to reach the maximum level of degradation with T versicolor, while P. ostreatus and P. pulmonarius required 25 days.
T. versicolor is an outstanding candidate for large-scale fermentation due to its versatility against changes in the evaluated variables, which allow it to achieve efficient BR46 degradation. It is therefore a suitable species to develop an efficient and low-cost strategy with a positive environmental impact for the bioremediation of xenobiotic compounds such as synthetic dyes.
The enzymatic potential of WRF depends on their different ligninolytic enzymes, primarily laccases. Their unspecific action and great oxidative ability allow them to degrade a wide variety of organic compounds, including polymers such as lignin, chlorinated phenols, dioxins, chloroanilines and dyes. Thus, their application in several biotechnology fields and in the bioremediation of pollutants will undoubtedly be important in the future.
Acknowledgements
The authors would like to thank the Universidad Nacional de Colombia -Medellín Campus for its support through the infrastructure of the Laboratory of Experimental Chemistry and the funding of project number 25769, "Programa Nacional de Semilleros de Investigación, Creación e Innovación de la Universidad Nacional de Colombia 2014".
References
[1] Cámara de Comercio de Medellín para Antioquia. Inexmoda: Un caso exitoso de liderazgo. Documentos Comunidad Clúster. CCMA: Medellín, 2007, 210 P.
[2] Cámara de Comercio de Medellín para Antioquia. Clúster: Una estrategia para Crear Ventaja Competitiva. Documentos Comunidad Clúster. CCMA: Medellín, 2006, 110 P.
[3] Ministerio del Medio Ambiente and FUNDES, Guía de Buenas Prácticas para el Sector Textiles. 2008, 53 P.
[4] Usepa. EPA Office of Compliance Sector Notebook Project: Profile of the Textile Industry. [Online]. 1997. [date of reference February 23rd of 2015]. Available at: http://www.clu-in.org/download/toolkit/textilsn.pdf.
[5] Vaidya, A.A. and Datye, K.V., Environmental-pollution during chemical-processing of synthetic-fibers. Colourage, 29(1), pp. 3-10, 1982.
[6] Banat, I.M., Nigam, P., Singh, D. and Marchant, R., Microbial decolorization of textile-dye- containing effluents: A Review. Bioresource Technology, 58(1996), pp. 217-227, 1997.
[7] Yesilada, O., Cing, S. and Asma, D., Decolourisation of the textile dye astrazon red FBL by funalia trogii pellets. Bioresource Technology, 81(2), pp. 155-157, 2002. DOI: 10.1016/S0960-8524(01)00117-1
[8] Walsh, G.E., Bahner, L.H. and Horning, W.B., Toxicity of textile mill effluents to freshwater and estuarine algae, crustaceans and fishes. Environmental Pollution Series A, Ecological and Biological, 21(3), pp. 169-179, 1980. DOI: 10.1016/0143-1471(80)90161-0
[9] Robinson, T., McMullan, G., Marchant, R. and Nigam, P., Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresource Technology, 77(3), pp. 247-255, 2001. DOI: 10.1016/S0960-8524(00)00080-8
[10] Rafatullah, M., Sulaiman, O., Hashim, R. and Ahmad, A., Adsorption of methylene blue on low-cost adsorbents: A review. Journal of Hazardous Materials, 177(1-3), pp. 70-80, 2010. DOI: 10.1016/j.jhazmat.2009.12.047
[11] Gupta, V.K. and Suhas., Application of low-cost adsorbents for dye removal - A review. Journal of Environmental Management, 90(8), pp. 2313-2342, 2009. DOI: 10.1016/j.jenvman.2008.11.017
[12] Vargas-Nieto, C., Garriazo, J.G. and Castillo, E., Estudio de materiales adsorbentes de bajo costo para remover Cr (VI) de efluentes acuosos. Ingeniería e Investigacón, 31(1), pp.154-162, 2011
[13] Higuera-Cobos, O.F., Florez-García, L.C. and Arroyave-Londoño, J.F., Estudio de la biosorción de cromo con hoja de café. Ingeniería e Investigacón, 29(2), pp.59-64. 2009.
[14] Rauf, M.A. and Salman-Ashraf, S., Survey of recent trends in biochemically assisted degradation of dyes. Chemical Engineering Journal, 209, pp. 520-530, 2012. DOI: 10.1016/j.cej.2012.08.015
[15] Krishna, C., Solid-state fermentation systems-an overview. Critical Reviews in Biotechnology, 25(1-2), pp. 1-30, 2005. DOI: 10.1080/07388550590925383
[16] Robinson, T. and Nigam, P.S., Remediation of textile dye waste water using a white-rot fungus Bjerkandera adusta through solid-state fermentation (SSF). Applied Biochemistry and Biotechnology, 151(2-3), pp. 618-628, 2008. DOI: 10.1007/s12010-008-8272-6
[17] Papadopoulou, K., Kalagona, I.M., Philippoussis, A. and Rigas, F., Optimization of fungal decolorization of azo and anthraquinone dyes via Box-Behnken design. International Biodeterioration and Biodegradation, 77, pp.31-38, 2013. DOI: 10.1016/j.ibiod.2012.10.008.
[18] Tisma, M., Komar, M., Rajic, M., Pavlovic, H. and Zelic, B., Decolorization of dyes by Aspergillus ochraceus cultivated under solid state fermentation on sugar beet waste. Chemical Engineering Transactions, 27, pp.145-150, 2012.
[19] Buitrago H.G., Ospina, M.P. y Rengifo, L.J.R., Construcción de un fermentador para operación en estado sólido y diseño de sistemas de control. Ingeniería e Investigacón, 18, pp.45-53, 1989.
[20] Jaramillo, A., Jimenez, S., Merino, A. y Hormaza, A., Obtención de un inóculo fúngico para la degradación de un colorante azo por fermentación en estado sólido. Rev.Udcaactual.Divulg.Cient, 17(2), pp. 577-585, 2014.
[21] Wesenberg, D., Kyriakides, I. and Agathos, S.N., White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnology Advances, 22(1-2), pp. 161-187, 2003. DOI: 10.1016/j.biotechadv.2003.08.011
[22] Levin, L., Papinutti, L. and Forchiassin, F., Evaluation of Argentinean white rot fungi for their ability to produce lignin-modifying enzymes and decolorize industrial dyes. Bioresource Technology, 94(2), pp. 169176, 2004. DOI: 10.1016/j.biortech.2003.12.002
[23] Kadam, A.A., Telke, A.A., Jagtap, S.S. and Govindwar, S.P., Decolorization of adsorbed textile dyes by developed consortium of Pseudomonas sp. SUK1 and Aspergillus ochraceus NCIM-1146 under solid state fermentation. Journal of Hazardous Materials, 189(1-2), pp. 486-494, 2011. DOI: 10.1016/j.jhazmat.2011.02.066
[24] Shah, M.P., Reddy, G.V., Banerjee, R., Ravindra-Babu, P. and Kothari, I.L., Microbial degradation of banana waste under solid state bioprocessing using two lignocellulolytic fungi (Phylosticta spp. MPS-001 and Aspergillus spp. MPS-002). Process Biochemistry, 40(1), pp. 445-451, 2005. DOI: 10.1016/j.procbio.2004.01.020
[25] Nigam, P., Armour, G., Banat, I.M., Singh, D. and Marchant, R., Physical removal of textile dyes from effluents and solid-state fermentation of dye-adsorbed agri-cultural residues. Bioresour. Technol., 72, pp. 219-226, 2000.
[26] Boer, C.G., Obici, L., De Souza, C.G.M. and Peralta, R.M., Decolorization of synthetic dyes by solid state cultures of Lentinula (Lentinus) edodes producing manganese peroxidase as the main ligninolytic enzyme. Bioresource Technology, 94(2), pp. 107-112, 2004. DOI: 10.1016/j.biortech.2003.12.015
[27] Osma, J.F., Toca-Herrera, J.L. and Rodríguez-Couto, S., Banana skin: A novel waste for laccase production by Trametes pubescens under solid-state conditions. Application to synthetic dye decolouration. Dyes and Pigments, 75(1), pp. 32-37, 2007. DOI: 10.1016/j.dyepig.2006.05.021
[28] Deniz, F. and Karaman, S., Removal of basic red 46 dye from aqueous solution by pine tree leaves. Chemical Engineering Journal, 170(1), pp. 67-74, 2011. DOI: 10.1016/j.cej.2011.03.029
[29] Stredansky, M. and Conti, E., Xanthan production by solid state fermentation. Process Biochemistry, 34, pp. 581-587, 1999.
[30] Jimenez, S., Merino, A., Zuleta, A. y Hormaza, A., Optimización de la desorción como metodología de cuantificación de la biodegradación del colorante rojo básico 46 por Pleurotus pulmonarius bajo fermentación en estado sólido, XIII Congreso Argentino de Microbiología, de Microbiología, 2013, 255 P.
[31] Faraco, V., Pezzella, C., Miele, A., Giardina, P. and Sannia, G., Bio-remediation of colored industrial wastewaters by the white-rot fungi Phanerochaete chrysosporium and Pleurotus ostreatus and their enzymes. Biodegradation, 20(2), pp. 209-220, 2009. DOI: 10.1007/s10532-008-9214-2
[32] Murugesan, K., Nam, I.H., Kim, Y.M. and Chang, Y.S., Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture. Enzyme and Microbial Technology, 40(7), pp. 1662-1672, 2007. DOI: 10.1016/j.enzmictec.2006.08.028
[33] Tychanowicz, G.K., Zilly, A., De Souza, C.G.M. and Peralta, R.M., Decolourisation of industrial dyes by solid-state cultures of Pleurotus pulmonarius. Process Biochemistry, 39(7), pp. 855-859, 2004. DOI: 10.1016/S0032-9592(03)00194-8
[34] Kaushik, P. and Malik, A., Fungal dye decolourization: Recent advances and future potential. Environment International, 35(1), pp. 127-141, 2009. DOI: 10.1016/j.envint.2008.05.010
[35] Baldrian, P. and Gabriel, J., Copper and cadmium increase laccase activity in Pleurotus ostreatus. FEMS Microbiology Letters, 206(1), pp. 69-74, 2002. DOI: 10.1016/S0378-1097(01)00519-5
[36] Iandolo, D., Piscitelli, A., Sannia, G. and Faraco, V., Enzyme production by solid substrate fermentation of Pleurotus ostreatus and Trametes versicolor on tomato pomace. Applied Biochemistry and Biotechnology, 163(1), pp. 40-51, 2011. DOI: 10.1007/s12010-010-9014-0
[37] Stajić, M., Persky, L., Friesem, D., Hadar, Y., Wasser, S.P., Nevo, E., and Vukojević, J., Effect of different carbon and nitrogen sources on laccase and peroxidases production by selected Pleurotus species. Enzyme and Microbial Technology, 38(1-2), pp. 65-73, 2006. DOI: 10.1016/j.enzmictec.2005.03.026
[38] Winquist, E., Moilanen, U., Mettälä, A., Leisola, M. and Hatakka, A., Production of lignin modifying enzymes on industrial waste material by solid-state cultivation of fungi. Biochemical Engineering Journal, 42(2), pp. 128-132, 2008. DOI: 10.1016/j.bej.2008.06.006
[39] Reddy, G.V., Ravindra-Babu, P., Komaraiah, P., Roy, K.R.R.M., and Kothari, I.L., Utilization of banana waste for the production of lignolytic and cellulolytic enzymes by solid substrate fermentation using two Pleurotus species (P. ostreatus and P. sajor-caju). Process Biochemistry, 38(10), pp. 1457-1462, 2003. DOI: 10.1016/S0032-9592(03)00025-6
[40] Sun, Q.Y., Hong, Y.Z., Xiao, Y.Z., Fang, W. and Fang, J., Decolorization of textile reactive dyes by the crude laccase produced from solid-state fermentation of agro-byproducts. World Journal of Microbiology and Biotechnology, 25(7), pp. 1153-1160, 2009. DOI: 10.1007/s11274-009-9994-5
A. Zuleta-Correa, completed her BSc. Eng in Biological Engineering at Universidad Nacional de Colombia, Medellín Campus in 2009; and her MSc degree in Environment and Development at Universidad Nacional de Colombia, Medellín Campus in 2013. She was a very active member of the Research Group "Synthesis, Reactivity and Transformation of Organic Compounds" (SIRYTCOR) and her researching experience in the environmental topic allowed her to direct several projects. Currently, she is a PhD student in Biological and Agricultural Engineering at North Carolina State University. ORCID: 0000-0001-8674-6396
A. Merino-Restrepo, completed his BSc. Eng in Biological Engineering at Universidad Nacional de Colombia, Medellín Campus in 2016. He is a MSc Biotechnology student and a member of the Research Group "Synthesis, Reactivity and Transformation of Organic Compounds" (SIRYTCOR). His research experience in the environmental topic, specifically in the biological degradation processes through Solid State Fermentation, has strengthened due to his important participation in Master's projects and his expertise in high-end equipment. He has taken part in several research projects. ORCID: 0000-0001-6818-3251
S. Jiménez-Correa, completed her BSc. Eng in Biological Engineering at Universidad Nacional de Colombia, Medellín Campus in 2016. She is a MSc Biotechnology student and a member of the "Synthesis, Reactivity and Transformation of Organic Compounds" research group. Her researching experience in the environmental field, specifically in biological degradation processes by Solid State Fermentation, has improved thanks to her participation in Master's projects and her expertise in high-tech equipment. She has attended several worldwide congresses. ORCID: 0000-0001-7103-7474
A. Hormaza-Anaguano, completed her BSc. in Chemistry at Universidad de Nariño in 1994; her MSc in Chemical Science at Universidad del Valle in 1997; and her PhD in Natural Sciences at the Johannes Gutenberg-University Mainz, Germany, in 2003. She began working at Universidad Nacional de Colombia Medellín Campus in 1997 as an Assistant Professor and she is currently a full-time Associate Professor in the School of Chemistry, School of Sciences, Universidad Nacional de Colombia, Medellín Campus. Since 2003 she has been the Director of the Research Group "Synthesis, Reactivity and Transformation of Organic Compounds", SIRYTCOR, whose research lines are focused on the treatment of industrial effluents, exploration and evaluation of alternative adsorbents, adsorption and desorption, and biological processes by solid state fermentation. ORCID: 0000-0001-5825-3885
S. Cardona-Gallo, completed his BSc. Eng. in Sanitary Engineering in 1997; his MSc. degree Environmental Engineering at Universidad Nacional Autónoma of Mexico in 2000; his PhD. in Environmental Engineering at Universidad Nacional Autónoma of Mexico in 2004; and his Post. PhD in Rice University in 2012. He has been a professor at the Department of Geosciences and the Environemnt, School of Mines, Universidad Nacional de Colombia, Medellín Campus, Colombia, since 2005. His research interests include water quality, soil quality, remediation, bioremediation, hazardous waste, and process design. ORCID: 0000-0002-1875-7330
Referencias
Cámara de Comercio de Medellín para Antioquia. Inexmoda: Un caso exitoso de liderazgo. Documentos Comunidad Clúster. CCMA: Medellín, 2007, 210 P.
Cámara de Comercio de Medellín para Antioquia. Clúster: Una estrategia para Crear Ventaja Competitiva. Documentos Comunidad Clúster. CCMA: Medellín, 2006, 110 P.
Ministerio del Medio Ambiente and FUNDES, Guía de Buenas Prácticas para el Sector Textiles. 2008, 53 P.
Usepa. EPA Office of Compliance Sector Notebook Project: Profile of the Textile Industry. [Online]. 1997. [date of reference February 23rd of 2015]. Available at: http://www.clu-in.org/download/toolkit/textilsn.pdf.
Vaidya, A.A. and Datye, K.V., Environmental-pollution during chemical-processing of synthetic-fibers. Colourage, 29(1), pp. 3-10, 1982.
Banat, I.M., Nigam, P., Singh, D. and Marchant, R., Microbial decolorization of textile-dye- containing effluents: A Review. Bioresource Technology, 58(1996), pp. 217-227, 1997.
Yesilada, O., Cing, S. and Asma, D., Decolourisation of the textile dye astrazon red FBL by funalia trogii pellets. Bioresource Technology, 81(2), pp. 155-157, 2002. DOI: 10.1016/S0960-8524(01)00117-1
Walsh, G.E., Bahner, L.H. and Horning, W.B., Toxicity of textile mill effluents to freshwater and estuarine algae, crustaceans and fishes. Environmental Pollution Series A, Ecological and Biological, 21(3), pp. 169-179, 1980. DOI: 10.1016/0143-1471(80)90161-0
Robinson, T., McMullan, G., Marchant, R. and Nigam, P., Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresource Technology, 77(3), pp. 247-255, 2001. DOI: 10.1016/S0960-8524(00)00080-8
Rafatullah, M., Sulaiman, O., Hashim, R. and Ahmad, A., Adsorption of methylene blue on low-cost adsorbents: A review. Journal of
Hazardous Materials, 177(1-3), pp. 70-80, 2010. DOI: 10.1016/j.jhazmat.2009.12.047
Gupta, V.K. and Suhas., Application of low-cost adsorbents for dye removal - A review. Journal of Environmental Management, 90(8), pp. 2313-2342, 2009. DOI: 10.1016/j.jenvman.2008.11.017
Vargas-Nieto, C., Garriazo, J.G. and Castillo, E., Estudio de materiales adsorbentes de bajo costo para remover Cr (VI) de efluentes acuosos. Ingeniería e Investigacón, 31(1), pp.154-162, 2011
Higuera-Cobos, O.F., Florez-García, L.C. and Arroyave-Londoño, J.F., Estudio de la biosorción de cromo con hoja de café. Ingeniería e Investigacón, 29(2), pp.59-64. 2009.
Rauf, M.A. and Salman-Ashraf, S., Survey of recent trends in biochemically assisted degradation of dyes. Chemical Engineering Journal, 209, pp. 520-530, 2012. DOI: 10.1016/j.cej.2012.08.015
Krishna, C., Solid-state fermentation systems-an overview. Critical Reviews in Biotechnology, 25(1-2), pp. 1-30, 2005. DOI: 10.1080/07388550590925383
Robinson, T. and Nigam, P.S., Remediation of textile dye waste water using a white-rot fungus Bjerkandera adusta through solid-state fermentation (SSF). Applied Biochemistry and Biotechnology, 151(2-3), pp. 618-628, 2008. DOI: 10.1007/s12010-008-8272-6
Papadopoulou, K., Kalagona, I.M., Philippoussis, A. and Rigas, F., Optimization of fungal decolorization of azo and anthraquinone dyes via Box-Behnken design. International Biodeterioration and Biodegradation, 77, pp.31-38, 2013. DOI: 10.1016/j.ibiod.2012.10.008.
Tisma, M., Komar, M., Rajic, M., Pavlovic, H. and Zelic, B., Decolorization of dyes by Aspergillus ochraceus cultivated under solid state fermentation on sugar beet waste. Chemical Engineering Transactions, 27, pp.145-150, 2012.
Buitrago H.G., Ospina, M.P. y Rengifo, L.J.R., Construcción de un fermentador para operación en estado sólido y diseño de sistemas de control. Ingeniería e Investigacón, 18, pp.45-53, 1989.
Jaramillo, A., Jimenez, S., Merino, A. y Hormaza, A., Obtención de un inóculo fúngico para la degradación de un colorante azo por fermentación en estado sólido. Rev.Udcaactual.Divulg.Cient, 17(2), pp. 577-585, 2014.
Wesenberg, D., Kyriakides, I. and Agathos, S.N., White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnology Advances, 22(1-2), pp. 161-187, 2003. DOI: 10.1016/j.biotechadv.2003.08.011
Levin, L., Papinutti, L. and Forchiassin, F., Evaluation of Argentinean white rot fungi for their ability to produce lignin-modifying enzymes and decolorize industrial dyes. Bioresource Technology, 94(2), pp. 169176, 2004. DOI: 10.1016/j.biortech.2003.12.002
Kadam, A.A., Telke, A.A., Jagtap, S.S. and Govindwar, S.P., Decolorization of adsorbed textile dyes by developed consortium of Pseudomonas sp. SUK1 and Aspergillus ochraceus NCIM-1146 under solid state fermentation. Journal of Hazardous Materials, 189(1-2), pp. 486-494, 2011. DOI: 10.1016/j.jhazmat.2011.02.066
Shah, M.P., Reddy, G.V., Banerjee, R., Ravindra-Babu, P. and Kothari, I.L., Microbial degradation of banana waste under solid state bioprocessing using two lignocellulolytic fungi (Phylosticta spp. MPS-001 and Aspergillus spp. MPS-002). Process Biochemistry, 40(1), pp. 445-451, 2005. DOI: 10.1016/j.procbio.2004.01.020
Nigam, P., Armour, G., Banat, I.M., Singh, D. and Marchant, R., Physical removal of textile dyes from effluents and solid-state fermentation of dye-adsorbed agri-cultural residues. Bioresour. Technol., 72, pp. 219-226, 2000.
Boer, C.G., Obici, L., De Souza, C.G.M. and Peralta, R.M., Decolorization of synthetic dyes by solid state cultures of Lentinula (Lentinus) edodes producing manganese peroxidase as the main ligninolytic enzyme. Bioresource Technology, 94(2), pp. 107-112, 2004. DOI: 10.1016/j.biortech.2003.12.015
Osma, J.F., Toca-Herrera, J.L. and Rodríguez-Couto, S., Banana skin: A novel waste for laccase production by Trametes pubescens under solid-state conditions. Application to synthetic dye decolouration. Dyes and Pigments, 75(1), pp. 32-37, 2007. DOI: 10.1016/j.dyepig.2006.05.021
Deniz, F. and Karaman, S., Removal of basic red 46 dye from aqueous solution by pine tree leaves. Chemical Engineering Journal, 170(1), pp. 67-74, 2011. DOI: 10.1016/j.cej.2011.03.029
Zuleta-Correa et al / DYNA 83 (198), pp. 128-135, Septiembre, 2016.
Stredansky, M. and Conti, E., Xanthan production by solid state fermentation. Process Biochemistry, 34, pp. 581-587, 1999.
Jimenez, S., Merino, A., Zuleta, A. y Hormaza, A., Optimización de la desorción como metodología de cuantificación de la biodegradación del colorante rojo básico 46 por Pleurotus pulmonarius bajo fermentación en estado sólido, XIII Congreso Argentino de Microbiología, de Microbiología, 2013, 255 P.
Faraco, V., Pezzella, C., Miele, A., Giardina, P. and Sannia, G., Bio-remediation of colored industrial wastewaters by the white-rot fungi Phanerochaete chrysosporium and Pleurotus ostreatus and their enzymes. Biodegradation, 20(2), pp. 209-220, 2009. DOI: 10.1007/s10532-008-9214-2
Murugesan, K., Nam, I.H., Kim, Y.M. and Chang, Y.S., Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture. Enzyme and Microbial Technology, 40(7), pp. 1662-1672, 2007. DOI: 10.1016/j.enzmictec.2006.08.028
Tychanowicz, G.K., Zilly, A., De Souza, C.G.M. and Peralta, R.M., Decolourisation of industrial dyes by solid-state cultures of Pleurotus pulmonarius. Process Biochemistry, 39(7), pp. 855-859, 2004. DOI: 10.1016/S0032-9592(03)00194-8
Kaushik, P. and Malik, A., Fungal dye decolourization: Recent advances and future potential. Environment International, 35(1), pp. 127-141, 2009. DOI: 10.1016/j.envint.2008.05.010
Baldrian, P. and Gabriel, J., Copper and cadmium increase laccase activity in Pleurotus ostreatus. FEMS Microbiology Letters, 206(1), pp. 69-74, 2002. DOI: 10.1016/S0378-1097(01)00519-5
Iandolo, D., Piscitelli, A., Sannia, G. and Faraco, V., Enzyme production by solid substrate fermentation of Pleurotus ostreatus and Trametes versicolor on tomato pomace. Applied Biochemistry and Biotechnology, 163(1), pp. 40-51, 2011. DOI: 10.1007/s12010-010-9014-0
Stajić, M., Persky, L., Friesem, D., Hadar, Y., Wasser, S.P., Nevo, E., and Vukojević, J., Effect of different carbon and nitrogen sources on laccase and peroxidases production by selected Pleurotus species. Enzyme and Microbial Technology, 38(1-2), pp. 65-73, 2006. DOI: 10.1016/j.enzmictec.2005.03.026
Winquist, E., Moilanen, U., Mettälä, A., Leisola, M. and Hatakka, A., Production of lignin modifying enzymes on industrial waste material by solid-state cultivation of fungi. Biochemical Engineering Journal, 42(2), pp. 128-132, 2008. DOI: 10.1016/j.bej.2008.06.006
Reddy, G.V., Ravindra-Babu, P., Komaraiah, P., Roy, K.R.R.M., and Kothari, I.L., Utilization of banana waste for the production of lignolytic and cellulolytic enzymes by solid substrate fermentation using two Pleurotus species (P. ostreatus and P. sajor-caju). Process Biochemistry, 38(10), pp. 1457-1462, 2003. DOI: 10.1016/S0032-9592(03)00025-6
Sun, Q.Y., Hong, Y.Z., Xiao, Y.Z., Fang, W. and Fang, J., Decolorization of textile reactive dyes by the crude laccase produced from solid-state fermentation of agro-byproducts. World Journal of Microbiology and Biotechnology, 25(7), pp. 1153-1160, 2009. DOI: 10.1007/s11274-009-9994-5
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Devikaben Bharatbhai Vishani, Anupama Shrivastav. (2022). Development in Wastewater Treatment Research and Processes. , p.419. https://doi.org/10.1016/B978-0-323-85657-7.00020-1.
2. Isma Fayyaz, Salina Saddick, Raja Tahir Mahmood, Muhammad Javaid Asad, Muhammad Altaf Hussain, Jiandong Hu, Muhammad Awais, M. Ijaz Khan, Shaxnoza Saydaxmetova. (2025). Biodegradation of Azo and disperse dyes by Trametes versicolor: Process optimization and MnP enzyme dynamics. Results in Engineering, 25, p.103980. https://doi.org/10.1016/j.rineng.2025.103980.
3. Andrés Merino, Gemma Eibes, Angelina Hormaza. (2019). Effect of copper and different carbon and nitrogen sources on the decolorization of an industrial dye mixture under solid-state fermentation. Journal of Cleaner Production, 237, p.117713. https://doi.org/10.1016/j.jclepro.2019.117713.
4. Indunil S. Herath, Dhanushka Udayanga, D.J. Jayasanka, Choolaka Hewawasam. (2024). Textile dye decolorization by white rot fungi – A review. Bioresource Technology Reports, 25, p.101687. https://doi.org/10.1016/j.biteb.2023.101687.
5. B. S. Shanthi Kumari, Kanderi Dileep Kumar, K. Sai Geetha, G. Narasimha, B. Rajasekhar Reddy. (2021). Bioenergy Research: Basic and Advanced Concepts. Clean Energy Production Technologies. , p.93. https://doi.org/10.1007/978-981-33-4611-6_4.
6. Afnan Ahmadi Zahuri, Wan Hanna Melini Wan Mohtar, Zarimah Mohd Hanafiah, Muhamad Fazly Abdul Patah, Pau-Loke Show, Yusufjon Gafforov, Wan Abd Al Qadr Imad Wan-Mohtar. (2024). Mycoremediation of Industrial Textile Wastewater Using Ganoderma lucidum Pellets and Activated Dolomite in Batch Bioreactor. Molecular Biotechnology, https://doi.org/10.1007/s12033-023-01035-z.
7. Ismail Fitri Mohd Hafidz, Muhamad Syaffuan Ramli, Nur Raihan Abdullah, Wan Abd Al Qadr Imad Wan-Mohtar, Nur Hafizah Azizan, Faez Sharif. (2022). Decolorization potential of bacteria isolated from Sungai Lembing hot springs and Ganoderma lucidum on methyl red dye. INTERNATIONAL CONFERENCE ON BIOENGINEERING AND TECHNOLOGY (IConBET2021). INTERNATIONAL CONFERENCE ON BIOENGINEERING AND TECHNOLOGY (IConBET2021). 2454, p.050027. https://doi.org/10.1063/5.0078615.
8. Ankita Srivastava, Radha M Rani, Dipesh S Patle, Sushil Kumar. (2022). Emerging bioremediation technologies for the treatment of textile wastewater containing synthetic dyes: a comprehensive review. Journal of Chemical Technology & Biotechnology, 97(1), p.26. https://doi.org/10.1002/jctb.6891.
9. Anu Kalia, Swarnjeet Singh. (2020). Myco-decontamination of azo dyes: nano-augmentation technologies. 3 Biotech, 10(9) https://doi.org/10.1007/s13205-020-02378-z.
10. Eko L. Fitriana, Erika B. Laconi, Anuraga Jayanegara, Dewi A. Astuti. (2023). The determination of residual fiber composition from agricultural by-products after being treated with solid-state fermentation and black soldier fly larvae rearing. THE FIRST INTERNATIONAL CONFERENCE ON NEUROSCIENCE AND LEARNING TECHNOLOGY (ICONSATIN 2021). THE FIRST INTERNATIONAL CONFERENCE ON NEUROSCIENCE AND LEARNING TECHNOLOGY (ICONSATIN 2021). 2679, p.020024. https://doi.org/10.1063/5.0118349.
11. Juan Carlos Cueva-Orjuela, Angelina Del Socorro Hormaza-Anaguano, Andrés Merino-Restrepo. (2017). Sugarcane bagasse and its potential use for the textile effluent treatment. DYNA, 84(203), p.291. https://doi.org/10.15446/dyna.v84n203.61723.
12. Evans C. Egwim, Oluwafemi A. Oyewole, Japhet G. Yakubu. (2023). Bioremediation for Environmental Pollutants. , p.181. https://doi.org/10.2174/9789815123494123010009.
13. Ines Mnif, Raouia Fendri, Dhouha Ghribi. (2022). Decolorization of methyl red using Bacillus thuringiensis RI16 strain: Enhanced bacterial treatment by SPB1 biosurfactant addition. Water Practice and Technology, 17(12), p.2570. https://doi.org/10.2166/wpt.2022.143.
14. Sara Jiménez, Carolina Velásquez, Felipe Mejía, Mario Arias, Angelina Hormaza. (2019). Comparative studies of pure cultures and a consortium of white-rot fungi to degrade a binary mixture of dyes by solid-state fermentation and performance at different scales. International Biodeterioration & Biodegradation, 145, p.104772. https://doi.org/10.1016/j.ibiod.2019.104772.
15. Andrés Merino-Restrepo, Felipe Mejía-Otálvaro, Carolina Velásquez-Quintero, Angelina Hormaza-Anaguano. (2020). Evaluation of several white-rot fungi for the decolorization of a binary mixture of anionic dyes and characterization of the residual biomass as potential organic soil amendment. Journal of Environmental Management, 254, p.109805. https://doi.org/10.1016/j.jenvman.2019.109805.
16. Thupakula Venkata Madhukar Sreekanth, Patnamsetty Chidanandha Nagajyothi, Gutturu Rajasekhara Reddy, Jaesool Shim, Kisoo Yoo. (2019). Urea assisted ceria nanocubes for efficient removal of malachite green organic dye from aqueous system. Scientific Reports, 9(1) https://doi.org/10.1038/s41598-019-50984-6.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2016 DYNA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
El autor o autores de un artículo aceptado para publicación en cualquiera de las revistas editadas por la facultad de Minas cederán la totalidad de los derechos patrimoniales a la Universidad Nacional de Colombia de manera gratuita, dentro de los cuáles se incluyen: el derecho a editar, publicar, reproducir y distribuir tanto en medios impresos como digitales, además de incluir en artículo en índices internacionales y/o bases de datos, de igual manera, se faculta a la editorial para utilizar las imágenes, tablas y/o cualquier material gráfico presentado en el artículo para el diseño de carátulas o posters de la misma revista.




















