Manufacturing and evaluation of Chitosan, PVA and Aloe Vera hydrogels for skin applications
Fabricación y evaluación de hidrogeles de Quitosano, PVA y Aloe Vera para aplicaciones cutáneas
DOI:
https://doi.org/10.15446/dyna.v84n203.62742Palabras clave:
Aloe Vera, Chitosan, Hydrogel, Mechanical Strength, Polyvinyl Alcohol (en)Aloe Vera, Quitosano, Hidrogel, Resistencia Mecánica, Alcohol PoliVinilico (es)
Descargas
The crosslinking degree between PVA/CH allowed obtaining a matrix with high absorption capacity and with gradual release of Aloe Vera, with mechanical properties and dimensional stability even after rehydration. Bactericide assays showed chitosan and Aloe Vera protective activity, which indicates this compound may be suitable for applications that help in wound healing.
El grado de entrecruzamiento entre el PVA y el Quitosano permitió obtener una matriz con alta capacidad de absorción y liberación controlada de Aloe Vera, con propiedades mecánicas y estabilidad dimensional aún después de rehidratadas. Las pruebas bactericidas mostraron actividad protectora del quitosano y del Aloe Vera, lo cual hace que este compuesto sea adecuado para aplicaciones que ayudan en la curación de heridas.
Recibido: 17 de febrero de 2017; Revisión recibida: 13 de septiembre de 2017; Aceptado: 3 de octubre de 2017
Abstract
The aim of this research was to develop membranes of polyvinyl alcohol (PVA) and chitosan (CH), capable of absorbing an Aloe Vera solution and gradually releasing to accelerate wound healing. The methodology involved varying the membrane’s composition using Chitosan and Polyvinyl Alcohol at 5 and 10% w/v, and implementing different PVA/CH relations of 30/70, 50/50 and 70/30 (v/v) and embed them in a 2% (v/v) Aloe Vera solution to create hydrogels. Once hydrogels were obtained, they were characterized by Infrared Spectroscopy and Scanning Electronic Microscopy, and evaluated by mechanical and absorption tests as well as bactericide activity assays.
The crosslinking degree between PVA/CH allowed obtaining a matrix with high absorption capacity and with gradual release of Aloe Vera, with mechanical properties and dimensional stability even after rehydration. Bactericide assays showed chitosan and Aloe Vera protective activity, which indicates this compound may be suitable for applications that help in wound healing.
Keywords:
Aloe Vera, Chitosan, Hydrogel, Mechanical Strength, Polyvinyl Alcohol..Resumen
El objetivo fue desarrollar membranas de Polivinil Alcohol (PVA) y Quitosano (Qo), capaces de absorber solución de Aloe Vera y luego liberarla gradualmente para acelerar la cicatrización de la herida. La metodología empleada consistió en variar la composición de las membranas usando quitosano y Polivinil Alcohol al 5 y 10% p/v, e implementar diferentes relaciones de PVA/Qo de 30/70, 50/50 y 70/30 (v/v), para posteriormente embeberlas en solución de Aloe Vera al 2% (v/v) y formar hidrogeles. Una vez obtenidos, éstos fueron caracterizados por Espectroscopía de Infrarrojo y Microscopía Electrónica de Barrido, y evaluadas por pruebas mecánicas, pruebas de absorción, y ensayos de actividad bactericida.
El grado de entrecruzamiento entre el PVA y el Quitosano permitió obtener una matriz con alta capacidad de absorción y liberación controlada de Aloe Vera, con propiedades mecánicas y estabilidad dimensional aún después de rehidratadas. Las pruebas bactericidas mostraron actividad protectora del quitosano y del Aloe Vera, lo cual hace que este compuesto sea adecuado para aplicaciones que ayudan en la curación de heridas.
Palabras clave:
Aloe Vera, Quitosano, Hidrogel, Resistencia Mecánica, Alcohol PoliVinilico.1. Introduction
Chitosan is a completely deacetylated (-(l-4)-2-amino-2-desoxi-D-glucopiranose, obtained by deacetylation of chitin through treatment in alkaline conditions. Is a non-toxic, biocompatible, and biodegradable polymer, which has attracted interest to a wide range of biomedical and pharmaceutical applications, cosmetics, and tissue engineering [1]. The use of biodegradable polymeric systems as implantable drug delivery systems is desirable, since the devices are degraded and eliminated from the body.
Biodegradable hydrogels would be ideal for the improvement of the existing dose form and the development of a new and better drug delivery system.
The incorporation of drugs in polymeric membranes forming hydrogels for the treatment and prevention of skin infections has been a common clinical practice due to the fact that hydrogels are a cross-linked network obtained from a macro- molecular hydrophilic polymer. It is stable upon swelling in water and capable of absorbing a large amount of water, which has caused great interest for several applications as drug delivery or wound protectors.
Thanks to its biocompatibility, hydrogels may be put in direct contact with wounds as they help hydrating the dead tissue, providing not-reactive materials that do not cause irritation and cooling the surface of the wound, help reducing pain and increasing patient’s acceptance [1-3].
Hydrogels based on chitosan have shown very good properties when combined with drugs, as they are biocompatible and present swelling properties in aqueous mediums, among other features of interest. In addition to natural polymers, synthetic polymers also come to play an important role in the preparation of hydrogels helping to improve mechanical properties. Polyvinyl alcohol (PVA), polyacrylamide (PA) or polymethyl methacrylate (PMMA) polymer, strengthen the network by way of a crosslinking agent to confer to these improvements in mechanical properties, depending on the links are achieved and radical form entering the network [4-6].
The main objective of this research was to evaluate the effects of crosslinking on the mechanical properties of Aloe Vera embedded membranes so they can be used as hydrogels in medical applications by strengthening the network of chitosan adding polyvinyl alcohol to 5 and 10% as crosslinking agent [7-9].
2. Experimental
2.1. Preparation of CH/PVA membranes
A 1% (w/v) chitosan (CH) solution (Sigma Aldrich percentage deacetylation >75%) was solubilized in 1% (v/v) acetic acid (Merck) by magnetic stirring during 3 hours at room temperature until completing homogenization. Parallelly, 5% and 10% of Polyvinyl Alcohol (PVA) (Sigma Aldrich, 98% hydrolyzed) solutions were prepared varying the concentration for 5 and 10% (w/v), dissolving PVA in distilled water under constant stirring for 2 hours at 70°C.
To prepare membranes, solutions of Polyvinyl Alcohol and Chitosan were mixed for each concentration of PVA with magnetic stirrer during 30 min, to which relations 30/70, 50/50 and 70/30 v/v PVA/CH were varied; then the mixtures were molded to 60x15mm forms and isolated in the aeration chamber at room temperature for 24 hours to remove bubbles formed during the process. Upon completion, the mixtures were taken to the oven for 8 days at 40°C. Finally, dried membranes are removed from Petri dishes and labeled. All membranes were made in triplicate for each ratio of PVA/CH used.
2.2. Characterization of PVA/CH membranes
Obtained membranes were characterized by Fourier Transform Infra-Red (FTIR) on a Perkin Elmer Spectrum One model with a DTGS detector, with a wavelength range between 4000 and 400 cm-1 to observe the composition of present functional groups and to identify the corresponding bands of PVA/CH composite both membranes and hydrogels.
Morphological characterization of the membrane structure was carried out with Scanning Electron Microscopy (SEM) using a JEOL JSM 6490 LV microscope. Samples were fixed in a graphite tape, underwent a thin gold coating using vapor deposition techniques on a Denton Vacuum Desk IV computer and were analyzed under high vacuum mode. The surface was then scanned using 2000X and 5000X magnification in order to evaluate surface morphology and porosity of hydrogels before and after being embedded in a 2% Aloe Vera solution.
2.3. Mechanical tests
Specimens were prepared in tape form with dimensions of 114mm x 29mm of each PVA/CH (30/70, 50/50 and 70/30) sample with 5% and 10% PVA (as specified by the ASTM D882-09 [10] to polymers with thickness below 1mm), and brought to the Universal Testing Machine (DIGIMESS) using a 500 N load distribution and a 5 mm/min tension strain rate, to assess the mechanical properties of the crosslinked membranes. The ultimate strain and stress (from the point of failure), the modulus (from the slope of the linear region), and the Yield Strength were calculated from pattern and specimens prepared with various PVA percentages.
2.4. Aloe Vera absorption test
For this assay, a membrane with 22mm x 21mm dimensions was extracted, and it in turn was divided into three 22mm x 7mm fractions in order to carry out each test in triplicate. Each of the fractions was soaked in a 2% Aloe Vera solution (Cristaloe() for 48 hours. Weight change measurements were performed in an electronic balance during two hours in order to achieve stability.
2.5. Antibacterial activity test
Antibacterial activity of PVA/CH hydrogels was carried out in the presence of two bacterial species: Staphylococcus Aureus (S. Aureus) and Escherichia Coli (E.Coli), placing hydrogels on 5mm diameter discs which were previously sterilized with ethylene oxide. Discs were embedded in a 2% Aloe Vera solution, seeded in agars inoculated with the bacterial strains in nutritive agar, and underwent incubation for 24 hours at 37°C. Hydrogel antibacterial inhibition was determined by measuring the diameter of each clear zone around the gel in millimeters, using a ruler. All of the operation procedures were carried out under aseptic conditions.
3. Results and Discussion
3.1. Appearance of PVA/CH membranes
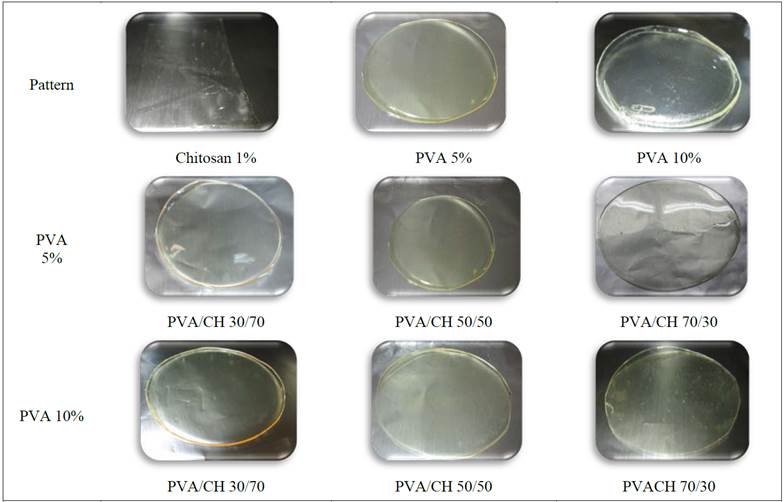
Fig. 1 shows the appearance of PVA/CH membranes for several concentrations of PVA. As shown in Fig. 1, obtained membranes are transparent, homogeneous and inflexible, have good consistency and their thickness is fairly uniform. After visual and tactile inspection, it is possible to determine that membranes with the lower PVA proportion are less rigid than the membranes with a higher PVA proportion. The conditions reported in the obtained membranes are similar to those reported by Zhao, [11] and León [12].
Figure 1: Photographs of PVA/CH membranes and pattern
3.2. Characterization of PVA/CH membranes and pattern
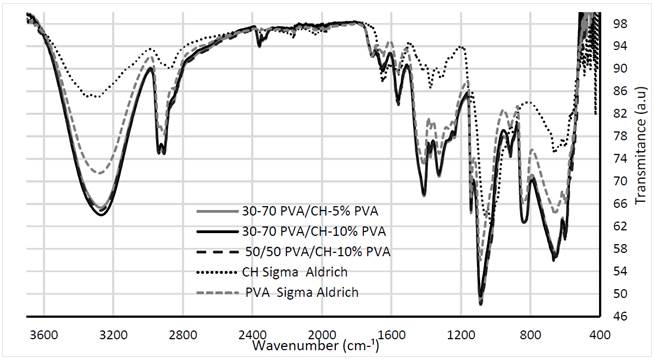
Infrared spectra of the pattern and PVA/CH dry membranes (before imbibition in a 2% Aloe Vera solution) can be seen in Fig. 2.
Figure 2: FTIR spectra of the standard membrane and crosslinking membranes
Membranes made with pattern materials present typical bands and the pure PVA has a band between 3600 and 3100 cm-1 associated with the stretching of OH- groups due to the strong hydrogen bonds, whereas the band located between 2950 and 2850 cm-1 refers to alkyl group (CH). In the chitosan spectrum, the band between 3600 and 3200 cm-1 is attributed to the extent of OH- and NH, and the band at 1655 cm-1 corresponds to stretching in the amide I (C=O) NH2 for 1580 cm-1, and the band 1313 cm-1 corresponds to the amide III. All these correspond to what has been reported in the literature for both polymers [13].
PVA/CH membranes present increase in the bands between 3200 and 3600 cm-1 of the OH and NH2 groups (with increasing percentage of chitosan and PVA) and bands between 2930 and 2850 cm-1 can be observed. However, in terms of chitosan amine NH2, the displacement of the absorption band at 1580 cm-1 indicates that the amine interacts with the OH- groups of PVA
Interaction among amine groups (~ 800 cm-1) and chitosan anomeric groups is evident, There are also bonds between double chains belonging to both molecules, which is evidenced by marked changes in transmittance, which shows a marked cross-linking of the molecules in these areas.
Pattern and PVA/CH hydrogels FTIR after imbibition in a 2% Aloe Vera solution can be seen in Fig. 3.
Figure 3: FTIR spectra of the standards and crosslinking samples embedded at 2% Aloe Vera
Fig. 3 shows how the band of OH- of PVA in 3300 cm-1 lose definition for overlap with the C-H bonds of chitosan; a decrease in transmittance showing the formation of molecular bonds (crosslinking) is also evidenced. Thus, the PVA/CH 30/70 -10% PVA sample is above the PVA/CH 30/70 -5% PVA sample and in turn, the sample lies above the PVA/CH 50/50 -10% PVA. This could be attributed to the amount of water in the Aloe Vera solution, which reacted with the PVA and CH and incorporated itself into the interstitial spaces created by the polymeric network.
An increase is observed in the transmittance of the bonds between 2800 and 3000 cm-1, denoting the formation of new peaks with greater transmittance, showing that these links were lost in the neo molecule formed of PVA/CH-Aloe Vera in lower proportion to PVA/CH 30/70 -10% PVA and increased in PVA/CH 50/50 -10% PVA and PVA/CH 30/70 -5% PVA samples.
Similarly, the formation of a carboxylic bond in the region of 1600 cm-1 is possibly due to the fact that Aloe Vera’s aromatic groups reacted with PVA and Chitosan, decreasing transmittance in this area, showing a molecular bond, higher for PVA/CH 50/50 -10% PVA hydrogel, followed by PVA/CH 30/70 -5% PVA and finally PVA/CH 30/70 -10% PVA.
Changes were seen in PVA C-O-C bonds in 1000-1300 cm-1 and chitosan amide bond III (1250-1360 cm-1). It can be seen that PVA/CH 30/70 -10% PVA hydrogel retains the defined shape of these peaks similarly to PVA, although transmittance increases, but in smaller amounts for the other two, showing a molecular link between the C-O-C and the chitosan amide bond.
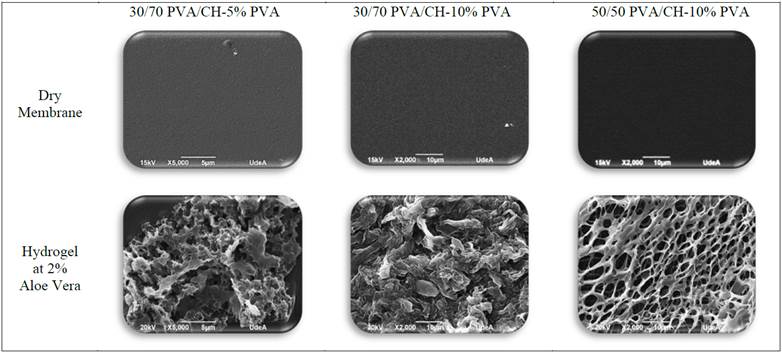
Fig. 4 shows images obtained with Scanning Electronic Microscopy of membranes prepared varying PVA/CH with different percentages of PVA.
Figure 4: SEM images of PVA/CH membranes before and after imbibed at Aloe Vera
Before imbibing them in a 2% Aloe Vera solution (above), the texture of PVA/CH membranes is highly uniform and homogeneous, very similar in appearance, a fact that demonstrates the high reproducibility of the process. Apparent roughness or porous structures cannot be noticed, in accordance with the structure of the samples reported by Nagahama [14].
Fig. 4 show the action of Aloe Vera in the cross-linked network (down), which changes completely their morphology and appearance when compared to microscopy obtained in trials with the membranes (above images). Hydrogels imbibed in a 2% Aloe Vera solution show a highly porous morphology in the 50/50 PVA/CH -10% PVA hydrogel. Pores show different sizes, ovoid shapes and interconnections among them, which presumably means this hydrogel has good features to promote cell growth.
The 30/70 PVA/CH -5% PVA hydrogel shows flake-type morphology, and it is more widespread in the whole sample of the material; it also shows that the hydrogel can promote cell growth due to its morphology. It can be seen that 30/70 PVA/CH -10% PVA hydrogel also has a porous morphology, although this is not homogeneously distributed throughout the material. If compared to that observed before immersion, the change observed in the internal structure of these hydrogels is significant, because when entering the Aloe Vera solution on the network of each hydrogel, its porosity and morphology are not so flat and homogenous, and shows the structure formed by the PVA/CH.
All this indicates that, although the variation is small, the PVA/CH relation used can cause morphological changes. In addition, the structure obtained in these hydrogels enables cell growth, thanks to its porosity, as is described by De la Torre [4] and Sanchez [15]. When crosslink of PVA/CH increases; the porosity of hydrogel and the water diffusion into hydrogel also increases in agreement with Wikanta [16].
3.3. Mechanical tests
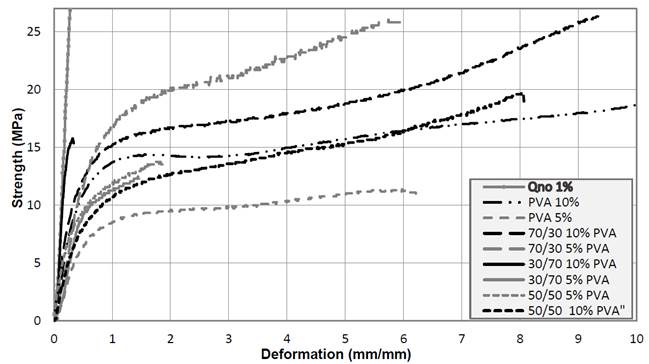
Different changes in chitosan’s crosslinking degree have been used to achieve desired mechanical properties in the membranes. Increasing the system’s crosslinking degree will result in stronger membranes. However, a higher crosslinking degree produces a more brittle structure. Hence, there is an optimum crosslinking degree to achieve relatively strong and elastic hydrogels [17]. Mechanical tests of chitosan membranes are showed in Fig. 5 and their properties in Table 1, both comparing pure chitosan and cross-linked membranes.
Figure 5: Stress-strain curve of PVA/CH membranes
Source: The Authors.Table 1: Mechanical properties of PVA/CH membranes

PVA/CH membranes for 5 and 10% PVA exhibited lower tensile strength, compared to pure chitosan, which can be attributed to the three-dimensional porous structure of the membrane for obtained crosslinking, in accordance with Zhao [11].
Similarly, to other tensile properties, it was found that the modulus generally increased with the increasing of chitosan percentage. Samples prepared at 5% PVA showed a higher Young modulus for 30/70 PVA/CH and 70/30 PVA/CH membranes, followed by 50/50 PVA/CH; this can be attributed to a less efficient crosslinking rate between PVA and chitosan. This means that as the chitosan network increases, the mechanical properties increase becoming most rigid the membrane, which is consistent with results reported by Cabrera [18] and De Souza [19].
In agreement with Drury [20], PVA/CH stress-strain curves were highly dependent on the strain rate, which is indicative of a viscoelastic material, which exhibits a nonlinear behavior, generally observed in tensile tests of synthetic fibers, tendons, and ligaments.
It is relevant to compare the mechanical properties of chitosan membranes and cross-linked membranes with the mechanical properties of tissues. The absolute values for the
ultimate stress and modulus of cross-linked membranes are several orders of magnitude smaller than non-mineralized tissues, as skin or muscle (KPa versus MPa) [21]. This suggests that chitosan is mechanically too weak to be used as a replacement for tissues, so it can be improved with PVA addition.
The results of the tensile test show that the CH membranes are more rigid, have higher Young's modulus, yield strength and Tensile Strength compared to pure PVA samples. While for PVA/CH membranes, values of yield strength and Tensile Strength are lower, but when the PVA/CH relation increases, tensile strength increases according as PVA concentration increases. Samples with PVA/CH -10% PVA showed a yield rate similar to the pattern of 10% PVA, as shown in the Table 1.
3.4. Aloe Vera absorption
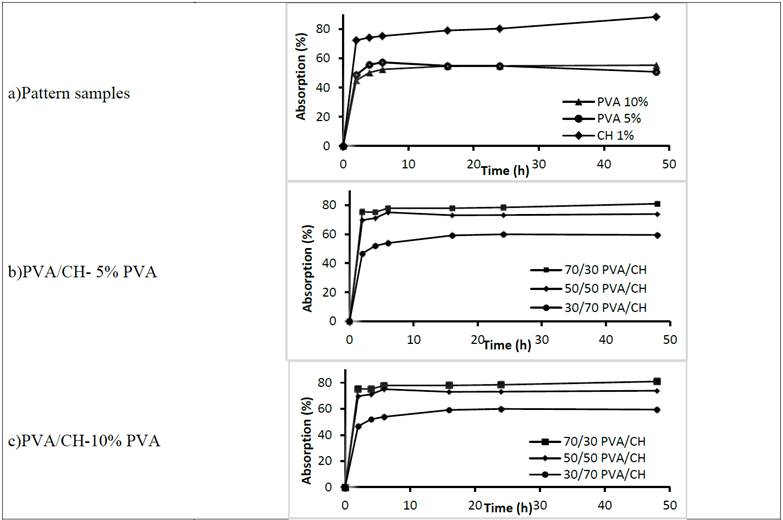
Fig. 6 shows the absorption capacity of evaluated membranes. According to swelling experiments, membranes absorbed water very quickly, reaching a swelling equilibrium during the first 3 hours, with an increase in volume appreciably noticed by visual inspection of the samples, in agreement with Tang [22].
Figure 6: Absorption percentage of hydrogels at 2% Aloe Vera through time
Water absorption of hydrogels may be supported by hydroxyl groups of PVA and amino groups of chitosan, which interact with water molecules through hydrogen bond, and by the presence of a porous network in the hydrogel. Chitosan’s absorbing rate is highly improved by the presence of PVA, and its value is not dependent of the PVA/CH ratio, in agreement with Borzacchiello [23].
Fig. 6a shows that the CH sample absorbs more water in its network (almost 8 times its weight). PVA patterns showed an increase in absorption of approximately 50%, the 10% PVA hydrogel was more stable through time.
The CH hydrogel showed a tendency to continue absorbing, and the 5% PVA hydrogel showed a tendency towards desorption, with lower absorption values at 48 hours. This water absorption may be supported by hydroxyl groups of PVA and amino groups of CH which interacted with water molecules through hydrogen bond, and by the presence of a porous network in the hydrogel.
Cross-linked hydrogels (Fig. 6b and 6c) show greater absorption capacity when the PVA is at higher concentrations, indicating a suitable cross-linking among molecules, in agreement with Wikanta [16]. Fig. 6b for 5% PVA hydrogels shows that the 30/70 PVA/CH hydrogel is the worst of the three hydrogels with other PVA/CH relationships, as the other two fractured in 6 hours after starting absorption, while 70/30 and 50/50 PVA/CH -5% achieving a 75% water gain, with an appropriate texture and transparency suitable for medical use.
Fig. 6c shows the good performance of 50/50 PVA/CH and 70/30 PVA/CH at 10% PVA hydrogels. The absorption curve of the 70/30 PVA/CH hydrogel achieves high absorption values near 80%, but shows a tendency to continue absorbing; despite having achieved a 60% absorption, the 30/70 PVA/CH hydrogel is stable after 48 hours, reaching stability value at 16 hours.
While 50/50 PVA/CH hydrogel showed a good performance and stability at 48 hours, its texture is less rigid than the 70/30 PVA/CH, and takes a whitish, translucent and is not restored once with the solution of Aloe Vera, a situation that is not considered feasible for the observation of the wound during treatment, enforcement is sought with this study.
The comparison between chitosan membrane and PVA/CH crosslinked membranes has shown a water percentage decrease in the total mass of wet membrane. An average of 80% for chitosan membrane versus 50% for crosslinked PVA/CH membrane could be observed. This result confirms how water absorption is drastically changed by the PVA percentage incorporated, in agreement with that reported by Beppu [24].
3.5. Antibacterial activity
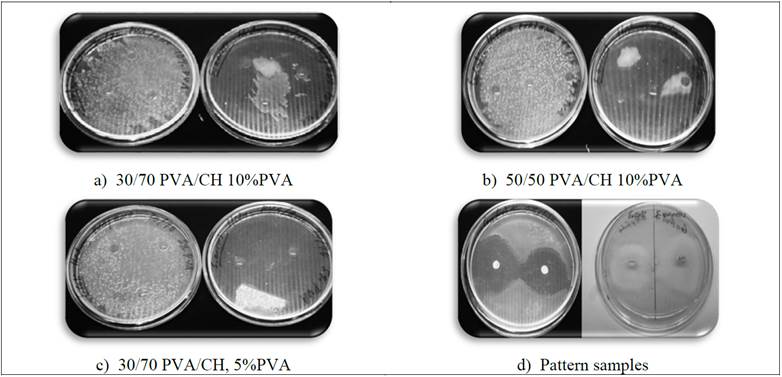
Fig 7 shows Inoculation of PVA/CH discs in bacterial cultures: Left: E. Coli, right: S. Aureus for 24 hours.
The clear zones diameter formed represent PVA/CH hydrogel the inhibition activity tested. Samples were analyzed in order to reveal the antibacterial activity of 30/70 PVA/CH for PVA 5 and 10%, and 50/50 PVA/CH-10% PVA embedded in a 2% Aloe Vera solution. After incubation, it was only possible to see bacteriostatic activity for S. Aureus, for a period of 8 hours in lower content PVA (30/70 PVA/CH -5%), as can be seen in Fig. 7c.
Figure 7: Inoculation of PVA/CH discs in bacterial cultures: Left: E. Coli, right: S. Aureus
After this period, S. Aureus proliferates normally. After 24 hours, all samples showed no bacterial growth inhibitory halo, or bacteriostatic, both for S. Aureus and E. Coli, according to that reported by Wikanta [16], also pattern culture medium solution Aloe Vera showed a slight bacterial growth could be due to environmental pollution, despite all the media were sterilized and previously worked in older aseptically.
Those results differ from findings reported by Leon [12], in which other zones of inhibition of bacterial growth of S. Aureus in PVA/CH hydrogels with Dragon's blood solution were presented, and these films only exhibited activity against S. Aureus, while in this study no bacteriostatic or bactericidal activity was not presented to E. Coli.
The positive and negative patterns behaved as expected; there was growth in negative pattern and showed the halo of inhibition in the positive pattern for S. Aureus and E. Coli.
Results showed that all hydrogels containing PVA/CH inhibited the growth of the bacteria tested with bigger inhibition zone than pattern hydrogel. The antibacterial properties of hydrogel containing chitosan are due to a positive charge of the amino groups in chitosan, and to its ability to bind to a negative charge in bacteria, and its effectiveness did not depend solely on the chitosan formulation but also on the type of fungus [25,26].
4. Conclusions
It was possible to obtain a hydrogel suitable to be used in tissue engineering (30/70 PVA/CH -10% PVA) with appropriate crosslinking degree, good mechanical properties, absorption capacity, morphological, and that managed to retain bacterial growth during 8 hours, thanks to the incorporation of Aloe Vera in the network.
The hydrogels obtained with PVA/CH mixtures have properties that are different to pure PVA hydrogel mechanical. The latter is very flexible and has low absorbency. Instead, mixtures hydrogels have good flexibility and good absorption capacity.
The study of these polymeric media for the controlled release of active ingredients, it marks for the development of new biocompatible devices. This study generated a hydrogel which has good features allowing it to be implemented in the future as a releasing medium and chitosan Aloe Vera, however, should kinetic release studies should be performed soon, in vivo studies and improve the bactericidal properties of the device for cutaneous applications.
Acknowledgment
The authors wish to thank to CODI of University of Antioquia for the financial support given to the project, and Biomaterials Research Group BIOMAT of University of Antioquia.
References
Referencias
Tapan, K, Amrita, T, Amit, A., Ajazuddin, H. and Krishna, T., Modified chitosan hydrogels as drug delivery and tissue engineering systems: Present status and applications. Acta Pharmaceutica Sinica B. 2(5), pp. 439-449, 2012. DOI: 10.1016/j.apsb.2012.07.004
Arredondo, P. y Londoño, M., Hidrogeles potenciales biomateriales para la liberación controlada de medicamentos. Revista Ingeniería Biomédica. [online]. 3(5), pp. 83-94, 2009. Available at: http://www.scielo.org.co/scielo.php?script=sci_abstract&pid=S1909-97622009000100013&lng=es&nrm=iso
Arredondo, A, Patiño, J, Londoño, M. y Echeverri, C., Matriz a partir de un hidrogel de alcohol polivinílico combinada con sulfadiazina de plata con potencial aplicación en el manejo y control de la sepsis en heridas dérmicas. Revista Iberoamericana de Polímeros. [online]. 12(4), pp. 178-187, 2011. Availabel at: http://www.reviberpol.iibcaudo.com.ve/pdf/AGO11/arredondo.pdf
De la Torre, M., Hidrogeles poliónicos de chitosán y ácido poliacrílico como sistema de liberación de amoxicilina para el tratamiento de h. pylori. Tesis Doctoral, Facultad de Farmacia. Universidad Complutense, Madrid, España. 2003, 310 P. Disponible en: http://biblioteca.ucm.es/tesis/far/ucm-t26645.pdf
Katime, I., Katime, O. y Katime, D., Materiales inteligentes: Hidrogeles macromoleculares, algunas aplicaciones biomédicas. Anales de la Real Sociedad Española de Química, [en línea]. pp. 35-50, 2005. Disponible en: http://dialnet.unirioja.es/servlet/ articulo?codigo=1354681
Madhumathi, K., Shalumon, K., Divya-Rani, V., Tamura, H., Furuike, T. and Selvamurugan, N., Wet chemical synthesis of chitosan hydrogel–hydroxyapatite composite membranes for tissue engineering applications. International Journal of Biological Macromolecules. 45, pp. 12-15, 2009. DOI: 10.1016/j.ijbiomac.2009.03.011
Echeverri, C., Vallejo, C. y Londoño, M., Síntesis y caracterización de hidrogeles de Polivinil Alcohol por la técnica congelamiento/descongelamiento para aplicaciones médicas. Revista EIA, [en línea]. 12, pp. 59-66, 2009. Disponible en: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S1794-12372009000200005
Escobar, J., Katime, I. y Zaldivar, D., Hidrogeles principales características en el diseño de sistemas de liberación controlada de fármaco. Revista Iberoamericana de Polímeros, [en línea]. 3(3), pp. 1-25. 2002. Disponible en: http://www.reviberpol.iibcaudo. com.ve/pdf/publicados/escobar2.pdf
Bustamante B,J.E. y Carrascal, L.M., Estandarización de la tecnica espectrofotométrica (UV-VIS) para la cuantificación de antraquinonas presentes en productos a base de Aloe Vera. Trabajo de Grado, Escuela de Química, Programa de Tecnologia Química. Universidad Tecnológica de Pereira, Pereira, Colombia. [en línea]. 2010, 68 P. Disponible en: http://repositorio.utp.edu.co/dspace/bitstream/ handle/11059/1800/54308520218B982.pdf?sequence=1
ASTM International. ASTM D88212 Standard Test Method for Tensile Properties of thin Plastic Sheeting. ASTM. International, [online]. 2012. Available at: https://www.astm.org/Standards/ D882.htm
Zhao, S., Ji, Q., Xing, K., Li, X., Liu, C. and Chen, X., Preparation and characteristics of novel porous hydrogel films based on chitosan and glycerophosphate. Carbohydrate polymers, 76, pp. 410-416, 2009. DOI: 10.1016/j.carbpol.2008.11.020
Leon, K. y Santiago, J., Propiedades antimicrobianas de películas de quitosano/PVA embebidas en extracto de sangre de grado. Revista Sociedad Química de Perú. [en línea]. 73, pp. 158-165, 2007. Disponible en: http://www.scielo.org.pe/scielo.php?script=sci_ arttext&pid=S1810-634X2007000300005
Dos Santos, B, Da Silva, R. y Farias, I., Morfologia e propriedades térmicas de blendas de poli(álcool vinílico)/quitosano. Revista Iberoamericana de Polímeros. [en línea]. 17(3), pp. 139-144, 2016. Disponible en: http://www.reviberpol.iibcaudo.com.ve/pdf/ MAY16/santos.pdf
Nagahama, H., Maeda, H., Kashiki, T., Jayakumar, R. and Tamura, H., Preparation and characterizacion of novel chitosan/gelatin membranes using chitosan hydrogel. Carbohydrate polymers, 76, pp. 255-260, 2009. DOI: 10.1016/j.carbpol.2008.10.015
Sánchez, B., Sibaja, B., Vega, J. y Madrigal, C., Síntesis y caracterización de hidrogeles de quitosano obtenido a partir del camarón langostino (Pleuroncodes Planipes) con potenciales aplicaciones biomédicas. Revista Iberoamericana de Polímeros, [en línea]. 8(4), pp. 241-267, 2007. Disponible en: http://www.reviberpol.iibcaudo.com.ve/pdf/SEP07/sanchez.pdf
Wikanta, T, Erizal, Tjahyono and Sugiyono, Synthesis of polyvinyl alcohol-chitosan hydrogel and study of its swelling and antibacterial properties Squalen, [online]. 7(1), pp. 1-10, 2012. Available at: http://www.bbp4b.litbang.kkp.go.id/squalen-bulletin/index.php/squalen/article/download/10/8
Peppas, N, Buresa, P, Leobandung, W. and Ichikawa, H., Hydrogels in pharmaceutical formulations. European Journal of Pharmaceutics and Biopharmaceutics, 50, pp. 27-46, 2000. DOI: 10.1016/S0939-6411(00)00090-4
Cabrera, J, Paredes, C. y, Urday, E., Preparación y caracterización de películas de Polivinil Alcohol conteniendo nanoparticulas de TiO2. Revista Iberoamericana de Polímeros, [en línea]. 8, pp. 323-333, 2007. Disponible en: http://www.reviberpol.iibcaudo.com.ve/ pdf/SEP07/cabrera.pdf
De Souza, E. and Sander, H., Preparação e caracterização de blendas de quitosana/PVA reticuladas quimicamente com glutaraldeído para aplicação em engenharia de tecido. Quimica Nova, 31(6), pp. 1460-1466, 2008. DOI: 10.1590/S0100-40422008000600034
Drury, J., Dennis, R. and Mooney, D., The tensile properties of alginate hydrogels. Biomaterials. 25, pp. 3187-3199, 2004. DOI: 10.1016/j.biomaterials.2003.10.002
Park, J.B. and Lakes, R.S., Chapter 9: Structure-property relationships of biological materials. In: Biomaterials: An introduction, 2ª ed. New York: Plenum Press; pp: 185-222, 2001. DOI: 10.1007/978-0-387-37880-0_9
Tang, Y., Du, Y., Li, Y. and Wang, X., A thermosensitive chitosan/ polyvinyl alcohol hydrogel containing hydroxyapatite for protein delivery. Journal of Biomedical Materials Research (A) pp. 953-963, 2008. DOI: 10.1002/jbm.a.32240.
Borzacchiello, A., Ambrosio, L., Netti, P., Peniche, C., Gallardo, A. and San Román, J., Chitosan based hydrogels: Synthesis and characterization. Journal of Materials Science: Materials in Medicine. [online]. 12, pp: 861-864, 2001. Available at: https://link.springer.com/article/10.1023/A:1012851402759
Beppu, M., Vieira, R., Aimoli, C. and Santana, C., Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. Journal of Membrane Science, 301, pp. 126-130, 2007. DOI: 10.1016/j.memsci.2007.06.015
Ing, L., Zin, N., Sarwar, A. and Katas. H., Antifungal activity of chitosan nanoparticles and correlation with their physical properties. International Journal of Biomaterials, 2012, pp. 1-9, 2012. DOI: 10.1155/2012/632698
Tikhonov, V., Stepnova, E., Babak, V., Yamskov, I., Palma, J., Jansson, H. and Salinas, J., Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydrate Polymers. 64, pp. 66-72, 2006. DOI: 10.1016/j.carbpol.2005.10.021
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Lorena Gonçalves Ribeiro, Jéssica de Brito Mota, Tainá Elizabete Campos Silva, Thais F.R. Alves, Marco Vinícius Chaud, Xirley Pereira Nunes, Joyce Kelly Marinheiro da Cunha Gonsalves. (2023). Influence of Triplaris gardneriana Wedd ethanolic extract in the chemic-mechanics properties of chitosan: Polyvinyl alcohol membranes as intelligent curatives. Materials Today Communications, 34, p.105153. https://doi.org/10.1016/j.mtcomm.2022.105153.
2. Lucia Uribe-Herrera, Diana Marcela Escobar-Sierra, Manuela Cardona-Franco, Laura Arango-Arroyave. (2022). Fabricación de membranas sensibles al cambio de pH para posibles usos en tratamiento de heridas cutáneas. Revista ION, 35(2) https://doi.org/10.18273/revion.v35n2-2022002.
3. Dure Najaf Iqbal, Asia Ashraf, Arif Nazir, Samar Z. Alshawwa, Munawar Iqbal, Naveed Ahmad. (2023). Fabrication, Properties, and Stability of Oregano Essential Oil and Sodium Alginate-Based Wound-Healing Hydrogels. Dose-Response, 21(4) https://doi.org/10.1177/15593258231204186.
4. Harini Sosiati, Muhammad Dirga Rianto, Aris Widyo Nugroho, Sudarisman. (2020). Fabrication by Electrospinning Technique and Characterization of Curcuma Mangga Val Reinforced PVA Fibrous Membranes. IOP Conference Series: Materials Science and Engineering, 846(1), p.012001. https://doi.org/10.1088/1757-899X/846/1/012001.
5. Shirin Nour, Rana Imani, Ali Mohammad Sharifi. (2022). Angiogenic Effect of a Nanoniosomal Deferoxamine-Loaded Poly(vinyl alcohol)–Egg White Film as a Promising Wound Dressing. ACS Biomaterials Science & Engineering, 8(8), p.3485. https://doi.org/10.1021/acsbiomaterials.2c00046.
6. Dong-Hee Lee, Hamin Park, Won-Ju Cho. (2023). Synaptic Transistors Based on PVA: Chitosan Biopolymer Blended Electric-Double-Layer with High Ionic Conductivity. Polymers, 15(4), p.896. https://doi.org/10.3390/polym15040896.
7. Indira M, Sudarsini B, Naga Lakshmi A, Venkateswarulu T.C. (2025). Tissue Engineering Applications of Aloe vera L. Burm. F.: A Comprehensive Review. Regenerative Engineering and Translational Medicine, https://doi.org/10.1007/s40883-025-00436-7.
8. Dyah Hikmawati, Elia Fernanda Adiputri, Alfian Pramudita Putra, Jan Ady. (2019). The Role of Relative Humidity on Physical Characteristics of Poly Vinyl Alcohol-Aloe vera Fiber Membrane by Using Electrospinning Methods. Materials Science Forum, 966, p.157. https://doi.org/10.4028/www.scientific.net/MSF.966.157.
9. Fabian Avila-Salas, Adolfo Marican, Jorge Villaseñor, Mauricio Arenas-Salinas, Yerko Argandoña, Julio Caballero, Esteban Durán-Lara. (2018). In-Silico Design, Synthesis and Evaluation of a Nanostructured Hydrogel as a Dimethoate Removal Agent. Nanomaterials, 8(1), p.23. https://doi.org/10.3390/nano8010023.
10. Hala A. El-Maghawry, Marwa Ali Tawfek, Hana M. Sawan. (2024). Assessment of the Mechanical Properties and Antimicrobial Activity of Chitosan-Coated Elastomeric Orthodontic Modules. Annals of Orthodontics and Periodontics Specialty, 4(1), p.54. https://doi.org/10.51847/EncFzk1BSm.
11. Chukwuebuka Egbuna, Ena Gupta, Shahira M. Ezzat, Jaison Jeevanandam, Neha Mishra, Muhammad Akram, N. Sudharani, Charles Oluwaseun Adetunji, Priyanka Singh, Jonathan C. Ifemeje, S. Deepak, A. Bhavana, Angelo Mark P. Walag, Rumaisa Ansari, Juliana Bunmi Adetunji, Umme Laila, Michael Chinedu Olisah, Peculiar Feenna Onyekere. (2020). Functional Foods and Nutraceuticals. , p.337. https://doi.org/10.1007/978-3-030-42319-3_18.
12. Dyah Hikmawati, Noveni Putri Maharani, Alfian Pramudita Putra, Siswanto. (2020). The Effect of Ultraviolet Exposure on Physical Properties of Electrospun Nanofiber Membrane Based on Polyvinyl Alcohol and Aloe vera. Key Engineering Materials, 860, p.244. https://doi.org/10.4028/www.scientific.net/KEM.860.244.
13. Yavuz Yagizatli, Alpay Sahin, Irfan Ar. (2022). Effect of thermal crosslinking process on membrane structure and PEM fuel cell applications performed with SPEEK-PVA blend membranes. International Journal of Hydrogen Energy, 47(95), p.40445. https://doi.org/10.1016/j.ijhydene.2022.04.183.
14. Sabiha Sultana, Sohail Imran, Amir Naveed, Sardar Hussain, Rozina Khattak, Luqman Ali Shah, Kamran Rehan, Imran Rehan, Mujeeb Ur Rehman, Uzma Hashmat, Farzana Haider. (2024). Fabrication of nano filler doped PVA/starch biodegradable composites with enhanced thermal conduction, water barrier and antimicrobial performance for food industry. Heliyon, 10(7), p.e28290. https://doi.org/10.1016/j.heliyon.2024.e28290.
15. Lucía Gabriela Beltrán-Novelo, Fernando Javier Aguilar-Pérez, Myriam Angélica De La Garza-Ramos, Arturo Abraham Cienfuegos-Sarmiento, José Rubén Herrera-Atoche, Martha Gabriela Chuc-Gamboa, Jacqueline Adelina Rodríguez-Chávez, Juan Valerio Cauich-Rodríguez. (2025). Mechanical and Antimicrobial Evaluation of Chitosan-Coated Elastomeric Orthodontic Modules. Dentistry Journal, 13(10), p.447. https://doi.org/10.3390/dj13100447.
16. Zahraa A. Abdul Muhsin, Ahmed Saad Aldhamin, Shafik S. Shafik. (2022). Effect of Gamma Radiation and Borax on Mechanical Properties of Polyvinyl Alcohol and Chitosan Blend Film. 2022 International Symposium on Multidisciplinary Studies and Innovative Technologies (ISMSIT). , p.293. https://doi.org/10.1109/ISMSIT56059.2022.9932830.
17. Dure N. Iqbal, Atira Munir, Mazhar Abbas, Arif Nazir, Zahid Ali, Samar Z. Alshawwa, Munawar Iqbal, Naveed Ahmad. (2023). Polymeric Membranes of Chitosan/Aloe Vera Gel Fabrication With Enhanced Swelling and Antimicrobial Properties for Biomedical Applications. Dose-Response, 21(2) https://doi.org/10.1177/15593258231169387.
18. Isis Siqueira Fernandes, Laís da Costa Saboia, Vinicius Soares Gonçalves, Jorge Luiz Siqueira da Costa Neto, Ana Paula Duarte Moreira, Natália Dias Souza, Alexandre Miguel do Nascimento, Douglas Siqueira de Almeida Chaves, Luiz Henrique Guerreiro Rosado, Leonardo Duarte Batista da Silva, Glauco Favilla Bauerfeldt, Antonieta Middea, Renata Nunes Oliveira. (2023). Delivery kinetics of natural active agents by PVA hydrogels intended for wound care. Matéria (Rio de Janeiro), 28(3) https://doi.org/10.1590/1517-7076-rmat-2023-0071.
19. Sabna Kotta, Hibah Mubarak Aldawsari, Nabil A. Alhakamy, Mahmoud Abdelkhalek Elfaky, Shaimaa M. Badr-Eldin. (2024). Synthesis, Structural Characterization, and Antimicrobial Evaluation of Silver Nanoparticle-embedded Layered Double Hydroxides for Delivery in Polymeric Hydrogel Matrices. Journal of Pharmaceutical Innovation, 19(6) https://doi.org/10.1007/s12247-024-09901-2.
20. Adolfo Marican, Fabián Avila-Salas, Oscar Valdés, Sergio Wehinger, Jorge Villaseñor, Natalia Fuentealba, Mauricio Arenas-Salinas, Yerko Argandoña, Verónica Carrasco-Sánchez, Esteban Durán-Lara. (2018). Rational Design, Synthesis and Evaluation of γ-CD-Containing Cross-Linked Polyvinyl Alcohol Hydrogel as a Prednisone Delivery Platform. Pharmaceutics, 10(1), p.30. https://doi.org/10.3390/pharmaceutics10010030.
21. Cecilia Valdez-Alegría, Rosa Fuentes-Rivas, José García-Rivas, Rosa Zavala Arce, María Jiménez Núñez, Beatriz García-Gaitán. (2020). Synthesis of Chitosan-Polyvinyl Alcohol Biopolymers to Eliminate Fluorides from Water. Biomolecules, 10(1), p.156. https://doi.org/10.3390/biom10010156.
22. Leimapokpam Sophia Devi, Roy Paily Palathinkal, Ashok Kumar Dasmahapatra. (2024). Preparation of cross-linked PANI/PVA conductive hydrogels for electrochemical energy storage and sensing applications. Polymer, 293, p.126673. https://doi.org/10.1016/j.polymer.2024.126673.
23. Juan Camilo Zárate-Moreno, Diana Marcela Escobar-Sierra, Rigoberto Ríos-Estepa. (2023). Development and Evaluation of Chitosan-Based Food Coatings for Exotic Fruit Preservation. BioTech, 12(1), p.20. https://doi.org/10.3390/biotech12010020.
24. Hosna Alvandi, Hajar Rajati, Tahereh Naseriyeh, Seyyed Soheil Rahmatabadi, Leila Hosseinzadeh, Elham Arkan. (2024). Incorporation of Aloe vera and green synthesized ZnO nanoparticles into the chitosan/PVA nanocomposite hydrogel for wound dressing application. Polymer Bulletin, 81(5), p.4123. https://doi.org/10.1007/s00289-023-04874-7.
25. Fabián Avila-Salas, Yeray A. Rodriguez Nuñez, Adolfo Marican, Ricardo I. Castro, Jorge Villaseñor, Leonardo S. Santos, Sergio Wehinger, Esteban F. Durán-Lara. (2018). Rational Development of a Novel Hydrogel as a pH-Sensitive Controlled Release System for Nifedipine. Polymers, 10(7), p.806. https://doi.org/10.3390/polym10070806.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 DYNA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
El autor o autores de un artículo aceptado para publicación en cualquiera de las revistas editadas por la facultad de Minas cederán la totalidad de los derechos patrimoniales a la Universidad Nacional de Colombia de manera gratuita, dentro de los cuáles se incluyen: el derecho a editar, publicar, reproducir y distribuir tanto en medios impresos como digitales, además de incluir en artículo en índices internacionales y/o bases de datos, de igual manera, se faculta a la editorial para utilizar las imágenes, tablas y/o cualquier material gráfico presentado en el artículo para el diseño de carátulas o posters de la misma revista.