Immobilization of recognition elements on a self-assembled monolayers bio-platform
Inmovilización de elementos de reconocimiento en una bio-plataforma basada en capas auto-ensambladas
DOI:
https://doi.org/10.15446/dyna.v84n202.63963Palabras clave:
functionalized surface, biosensor, polydiacetylene liposome, biotin-streptavidin, 3-aminopropyltriethoxysilane (en)superficie funcionalizada, biosensor, liposomas de polidiacetileno, biotina-estreptavidina, aminopropiltrietoxisilano (es)
Descargas
Recibido: 9 de abril de 2017; Revisión recibida: 8 de agosto de 2017; Aceptado: 24 de agosto de 2017
Abstract
Tailored materials formed by spontaneous two-dimensional arrangement of 3-aminopropyltriethoxysilane self-assembled monolayer on glass (amino-functionalized glass) has been exploited to attach biomolecules in well-organized structures useful in biosensing. Succinimidyl ester of both unpolymerized diacetylene liposome (NHS-DA-liposome) layer and PEGylated biotin (Bt-PEG-NHS) matrix were covalently bonded to the amino-functionalized glass by the NHS linker, and exposed to either Tyrosinase (Ty) or Streptavidin (SAV) solution. The interaction between Ty and polymerized NHS-PDA-liposome transformed the planarity of the PDA backbone, and a blue-to-red transition occurred; Bt-PEG attached to the fluorescent-SAV by bioaffinity. Sensing capability of bioplatform systems was evaluated by Uv-vis spectroscopy or fluorescence microscopy. Biomolecule functionalized SAMs retained the recognition potential of colorimetric Ty-PDA-liposome after biological interaction, and also facilitated the fabrication of a protein-resistant matrix with a particular affinity property. This surface chemistry is accessible to depositing proteins on both SAM-coated glass surface, and tethered to SAM, resulting in optical bioplatform arrays.
Keywords:
functionalized surface, biosensor, polydiacetylene liposome, biotin-streptavidin, 3-aminopropyltriethoxysilane..Resumen
Los materiales funcionalizados por adsorción sobre capas autoensambladas de 3-aminopropiltrietoxisilano (APTS) en vidrio (vidrio amino-funcionalizado) sirven para inmovilizar biomoléculas en estructuras usadas para biosensores. Liposomas de polidiacetileno (lip-PDA-NHS) y Biotin PEGilado con NHS se inmovilizaron aprovechando el éster de succinimidilo; y sirvieron para reconocer Tirosinasa o streptavidina (SAV). Debido a la interacción liposoma-PDA-Ty, ocurrió un cambio en la planaridad de la cadena polimérica PDA, percibido como una transición de azul-a-rojo; mientras que, la biotina inmovilizada interactúo con SAV por afinidad biológica. La capacidad de detección del sistema se evaluó por espectroscopia UV-vis o por microscopia de fluorescencia. Las capas de APTS funcionalizadas con biomoléculas retuvieron la capacidad de reconocimiento colorimétrico después de la interacción biológica, permitiendo la fabricación de una matriz resistente a proteínas con una propiedad de bioafinidad. Esta química de superficies es simple y accesible para la modificación de substratos de vidrio, útiles en arreglos de bioplataformas ópticas.
Palabras clave:
superficie funcionalizada, biosensor, liposomas de polidiacetileno, biotina-estreptavidina, aminopropiltrietoxisilano..1. Introduction
The analytical signal of biosensors is generally proportional to the number of recognition events occurring at the interface, involving the interactions between biomolecules and ligands. Accordingly, the surface coverage contributes to regulate the extension of the interfacial interactions with target molecules, resulting in a change of a specific physical property; which is then perceived by the transducer element, turning it into a measurable analytical signal. [1]. In an optical biosensor, tuning the interplay of specific response can be reached by surface functionalization with chemical or biological coupling agents, to keep the recognition elements on top of the surface. The nanostructures self-assembled monolayers (SAMs) are formed by appropriate interactions between molecular constituents that organize themself in a stable and well-defined array on different substrates. [2]. The surface-induced order and stability of SAMs impart definite properties on a molecular level, making them useful for improving control of adhesion, wettability, detergency, isolating, friction and lubrication processes, as well as for detecting biological activity in biosensors. [3]
SAMs on glass, a translucent substrate, provide the tailored surface required to sense the analyte, leading a change of colorimetric or fluorescence emission properties. Alkyl trichlorosilanes have been used to form SAMs on glass, a cheap and widely available material; however, considering that they are susceptible to hydrolysis, alkyl triethoxysilanes substitute them in fabrication of SAMs on silica, metal and glass substrates. [4]. For example since 1969, a simple preparation method based on 3-Aminopropyltriethoxysilane (APTS) is used to form SAMs on glass. [5,6] APTS molecule contains the siloxane group that anchors the molecule on the hydroxylated glass by in situ formation of polysiloxane, which is connected to surficial silanol groups (-SiOH) via Si-O-Si bonds; the attained APTS-SAMs exhibit high stability, and resistance to increased temperature; but they have a strong trend to form multilayers. [7]
The functionalized glass displays a terminal amine group capable of direct coupling with N-hydroxysuccinimidyl linker (NHS), operating as scaffolds to control the spontaneous formation of more complex structures of determined composition. [8]. Thus, amino-functionalized glass provides a path for chemical derivatizion, offering the possibility to carry hydrophobic, polar, or reactive functional groups for selective binding of guest molecules. [9]. Covalent immobilization of ligands to evaluate both biomolecule detection, and attachment points of biomolecules, provided by SAMs, has direct implications for the development of optical biosensors. Resulting in application of two biosensing approaches, for detecting protein targets, and for studying processes dependent on immobilized protein. [10]
This paper presents amino-functionalized glass surface for supporting both optical sensing approaches: Attachment of colorimetric polydiacetylene (PDA) liposomes modified with an enzyme, that are capable to bind the substrate and undergo a color transformation after stimuli, as a result of changes in the planarity of the polymer backbone; [11] and immobilization of a ligand that recognizes by bioaffinity a particular fluorescent-labeled enzyme, it system shows resistance to the unwanted adsorption of other proteins based on a passive surface of polyethylenglycol (PEG). [12]
2. Materials and methods
2.1. Chemical reagents
All 3- aminopropyltriethoxysilane (APTS), ethanol (EtOH), hydrochloric acid (HCl), sulfuric acid (H2SO4), 30% hydrogen peroxide (H2O2), tyrosine, sodium carbonate (Na2CO3) and sodium bicarbonate (NaHCO3), and 10 mM sodium phosphate buffer (PBS), pH 7.4 was prepared from sodium hydrogen phosphate, potassium dihydrogen phosphate, and 150 mM sodium chloride which were purchased from Sigma Aldrich. 10,12-pentacosadiynoic acid (DA) was acquired to FSG inc., and Dimiristoyl Phosphocholine was acquired to Avanti Polar. Biotin-PEG5000-succinimidyl ester (Bt-PEG-NHS) was obtained from Pierce Biotechnology. Tyrosinase (Ty) and streptavidin Fluorescein isothiocyanate conjugated (FITC-SAV) was brought in Invitrogen, Molecular Probes. Nanopure water was supplied from a Simplicity MilliporeQ system (Conductivity 6 µS/cm). Diacetylene-succinimidyl ester (DA-NHS) was obtained as a kind gift from Dr. Punit Kohli.
2.2. Preparation of amino-functionalized glass slide
The surface of the glass slides previously activated in solution piranha (H2SO4 and H2O2, 7:3 v/v) for 1 hour, was rinsed with nanopure water and dried under a nitrogen stream. Then, it was silanized in a beaker containing APTS in acid ethanol solution APTS:EtOH:HCl of 98.8:0.2: 1, for one and a half hours at room temperature. [13] Two aliquots of 5 mL of EtOH were used to eliminate excessive reagent from the surface and a nitrogen stream was applied. The slides were rinsed with 5 mL of 10 mM PBS, pH 7.3. Contact angle (CA) was evaluated by the advancing drop DMS-200 and calculated by the tangent method.
2.3. Immobilization of biostructures on functionalized glass slide for protein binding
2.3.1. Preparation of NHS-PDA liposome on amino-glass slide
1 mM liposome modified with NHS in 10 mM PBS, pH 7.3 solution was synthesized by a literature method. [14] Briefly, 0.0123 g of DA and 0.0040 g of DA-NHS dissolved in 25 mL dichloromethane were mixed and filtered. The filtrate was transferred into a round bottom flask containing 0.0060 g of DMPC in 25 mL of dichloromethane. Then the solid obtained by rotoevaporation, was dissolved with 50 mL of 10 mM PBS (pH 7.3), sonicated at 75 ºC for 15 minutes and the NHS-DA-liposome solution was kept at the refrigerator for eight hours. 250 µL of this unpolimerized liposome solution were incubated by 60 minutes on the amino-functionalized glass. [15]. The immobilized NHS-DA-liposomes on glass slide were polymerized by 1, 3, and 5 minutes under a 254 nm UV light in an ice bath. The attachment of polymerized liposomes (NHS-PDA-liposome) on the glass surface was evidenced by heating the slide at 100 °C on a hot plate instrument. Uv-vis spectra were obtained with a Genesys 10 spectrophotometer before and after of thermal stimulus, in the range 400-700 nm.
2.3.2. Preparation of Bt-PEG on amino-glass slide
The biotin was covalently tagged to the amino-functionalized glass slides by adding 100 μL of 2 mM Biotin-PEG5000-NHS dissolved in 50 mM carbonate buffer, pH 8.3, onto amino-functionalized glass, for 30 min at room temperature. The biotin modified glass slides were then washed sequentially with 10 mL aliquots of 10 mM PBS solution, nanopure water and dried under a stream of nitrogen. [16]
2.4. Colorimetric detection of proteins by NHS-PDA-liposome-glass slide
250 µL of 10 µM of Ty was deposited onto NHS-PDA-liposome-functionalized glass slide, at room temperature for 30 minutes. Uv-vis spectra before and after binding event were obtained. [17]
Activity of immobilized Ty onto PDA-liposome-functionalized glass slide was evaluated for substrate detection. In a separated experiment, unpolymerized and immobilized liposomes on SAM glass interacted with Tyrosinase solution under similar conditions. Then, polymerization of Ty-DA-liposomes on glass slide occurred under 254 nm UV light. The resultant system Ty-PDA-liposome functionalized glass slide was exposed to 250 µL of 1 mM Tyrosine in 0.1M phosphate buffer (pH 7.0) at 25°C. UV-vis spectral changes before and after interaction were monitored spectrophotometrically. [18]
2.5. Detecting proteins by Fluorescent emission using Bt functionalized glass slide.
The coating of Biotin-PEG5000-functionalized glass slide was in contact with 200 μL of 2 μM FITC-SAV solution prepared in 0.1 M PBS, pH 7.3 for 30 minutes. [19] Resultant FITC-SAV-Bt at top of the functionalized glass was evaluated by fluorescence emission of the FITC in the air using an inverted fluorescent microscope (Leica DMIRB) equipped with a Nuance (Perkin-Elmer) multispectral imaging system and 515 long pass emission filter (N2.1, 513 832) under the 20X objective and filter No 2.
3. Results and Discussion
3.1. Silanized glass surface
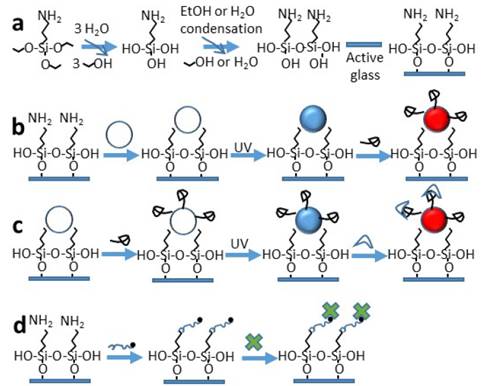
Different solid supports could be used to prepare optical sensing surfaces. Especially glass is a readily available and inexpensive support media, holding a relatively homogeneous chemical surface susceptible to chemical modification by silanization chemistry. SAM on glass provides detection capabilities in the UV-visible range and also in fluorescence emission for biosensors. The importance of proper surface modification are displayed by SAM-glass, keeping active the probe molecules such as immobilized proteins, and suppressing of non-specific binding in optical sensors. Fig. 1 presents the scheme for gradual adaptation by chemisorption and self-organization of alkyl molecules on the surface of a glass substrate depending of the sensing use of this surface. Fig. 1, scheme a) shows the activation step by hydroxylation of glass and silanization by chemisorption, the amine terminal group is exposed at top; scheme b) presents the preparation step of NHS-PDA-liposome-functionalized glass slide, immobilization of NHS-DA-liposome, followed by UV polymerization; then Ty detection with transformation blue-to-red PDA; scheme c) shows activity of Ty onto NHS- PDA-liposome-functionalized glass slide for substrate detection, immobilization of NHS-DA-liposome, followed by modification with Ty and UV polymerization, then enzyme-substrate interaction, with change from blue-to-red color; and scheme d) presents the preparation of Bt-PEG functionalized glass slide for protein binding, then recognition of Fl-SAV with simultaneous blocking of the surface to proteins different to analyte of interest, visualized by the green fluorescent emission.
Figure 1: Probe biomolecules functionalized SAM on glass surface for sensing purposes. a) Preparing a glass surface by chemisorption of SAMs, b) Immobilized liposome, then polymerization (PDA-liposome) for sensing enzyme binding, leading a transformation blue-to-red PDA, c) Covalent attachment of liposome modified with enzyme, then polymerization (Ty-PDA-liposome) for substrate-ligand recognition resulting in blue-to-red colorimetric change, and d) Immobilized Bt-PEG-functionalized glass for affinity recognition of fluorescent streptavidin.
In this study, functionalized glass was obtained by modification with the coupling agent APTES, a bifunctional organosilane that provides a chemical bond between two different materials, the glass and amine-linkers such as N-hydroxysuccinimidil reagents. The initial self-assembled structure is formed by chemisorption on hydroxylated glass; reaching an equilibrium to the lowest free-energy state of molecules of APTES, which demands a balance between attractive and repulsive molecular forces. [21]
3.2. Ligand immobilization by displacement of the NHS ester on the biomolecule with amino group of APTS
Table 1 presents the contact angle (CA) calculated of functionalized SAM glass.
Source: The authors.Table 1: Contact angle of functionalized SAM glass.

The resultant APTS-glass shows the CA value of 56° with increased hydrophobicity in presence of amino group; in contrast the hydroxylated glass holds the CA of 20°. If the CA parameter of water is less than 30°, the surface is designated hydrophilic since the forces of interaction between water and the surface are close to the cohesive forces of bulk water. [22] The hydrophobicity of short chain of APTS can be attributed to films with less ordered extension because three possible reasons, i) the reduced van der Waals interactions between adjacent hydrocarbon chains; [23] ii) the produced monolayer consists of a cross-polymerized network of molecules with only a few bonds to the surface; [24] and iii) some amino groups can interact with the silica surface instead to water. [25]
Next, APTS-glass interacted with either reactive liposomes containing NHS at surface or the heterobifunctional polymer Bt-PEG-NHS with the polyethylenglycol as a flexible linker and biocompatible spacer. The remain NHS groups on the attached liposome are chemical ligands available to interact with amine groups from lysine residues; the ligand Bt directs the specific recognition sites with the streptavidin protein, by affinity or electrostatic interaction and PEG keeps the surface free of another interactions. [26] Wettability of surface was evaluated by CA parameter, in presence of liposomes, or with the polymer PEG, the resultant CA values of 54° and 51°, respectively agree with lower hydrophobic property of the surface. CA data compare well with the values found on similar SAM, high hydrophobicity of 58° for APTS and a lighter hydrophobicity accounted for PEG of 45°. [27] For NHS-PDA-liposome functionalized glass, the calculated CA value of 54° is close to the polydiacetylene film range of 57-78° found in literature due to presence of hydrophilic carboxylic and NHS groups. [28]
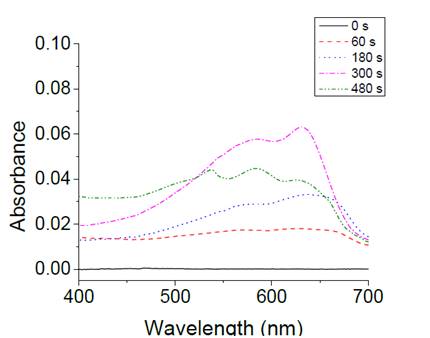
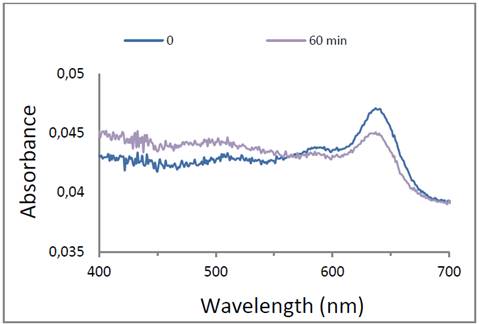
Also the structure NHS-PDA-liposome was monitored by UV-vis spectra obtained after polymerization. Fig. 2 shows the binding of NHS-DA-liposome functionalized glass and the development of the polymerization process at different times of exposition, 1, 3 and 5 minutes by 254 nm light. UV-vis spectra was changing during the polymerization process. Initially unpolymerized diacetylenes do not absorb light in the measured range, 400-700 nm. After the first minute under irradiation, the surface displayed a characteristic band at 640 nm, attributed to π-π* electronic promotion with a vibronic shoulder at 580 nm, the blue PDA, resulting of the overlap of the adjacent π- orbitals, a conjugated and planar arrangement that accumulates stress in the structural backbone. [29] The spectra kept the shape, and intensity of blue PDA was increased as polymerization time was prolonged. After 5 minutes of UV irradiation, the spectra shows a new band around 540 nm, excessive irradiation energy contributes to rotation of C-C bonds of the conjugated backbone, thus modifying the planarity of the polymer arrangement and promoting a partial distortion of conjugated π -orbitals, that results in higher π-π* band gap and reduces the effective conjugation length of the backbone. This transformation is perceived as an optical change assigned to the red PDA phase. [30]
Figure 2: UV-vis spectra of PDA-NHS -lip immobilized on APTS-glass through the polymerization process.
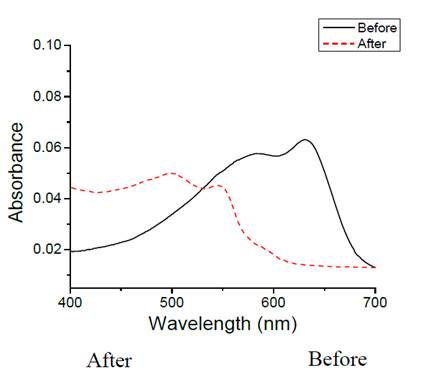
As a proof of PDA-liposome binding to APTS-glass, the UV-vis spectra before and after an experiment by heating the slide of Fig. 3 shows the spectral absorption changes due to heating at 100 oC of the NHS-PDA-liposome functionalized glass sensing surface.
Figure 3: UV-vis spectra of PDA-NHS liposomes sensing thermochromic effect, A) Before, and B) After heating.
The resultant UV-vis spectra changed at 640 nm, where the band intensity decreased and a new band appeared at 540 nm with a shoulder at 490 nm, assigned to emerging of a new red PDA. [31] Fig. 4 shows the Liposome-SAM-glass slide before and after heat process at 100 oC.
Figure 4: Image of PDA-liposome-functionalized glass slide A) Before, and B) After heating.
This immobilization method on APTS SAM provides an alternative to the covalent bond formation between liposomes and glass surface. [32]
3.3. Colorimetric detection of biological activity of functionalized glass
In order to evaluate the activity of immobilized liposomes, the enzyme Ty was covalently attached on the top of the PDA-liposome functionalized glass surface. Fig. 5 presents the typical spectra of Ty-PDA liposome after polymerization for 5 minutes. The Ty-PDA-liposome-functionalized glass system was immersed into a 1 mM tyrosine solution and the spectral changes were followed spectroscopically for 60 minutes. The intensity of blue PDA at 640 nm decreased, and a broad band between 480 to 550 nm increased, including the tyrosine oxidation product and the red PDA phase.
Figure 5: UV-vis spectra of immobilized Ty-PDA-liposome on APTS-glass in presence of substrate tyrosine.
As colorimetric detection is a versatile inexpensive and portable method because digital photographic imaging. This approach should be exploited to design reliably and robustly attachment of specific biomolecules as the sensing system based on active immobilized Ty. Regulated by the properties of both the functionalized surface and the targeting agent will reach specific targeting relying on lock and key interactions, where the targeting moiety interacts with solely the target. In addition, PDA-liposome functionalized SAM system offers high accessibility of substrate molecules to the immobilized biomolecule and specially, after the recognition event, a small rotation of C-C bond to the polymer backbone occurred. This conformational change disturbed the effective conjugated length and it was transduced through the alkyl chain length. Thus, the Ty-PDA-liposome functionalized glass system is a nanostructured platform upon which a colorimetric sensor, based on tailored surfaces, which were obtained by amino-self-assembled monolayers can be produced. [32]
3.4. Fluorescence emission detection of biological activity by Bt-PEG-fuctionalized glass
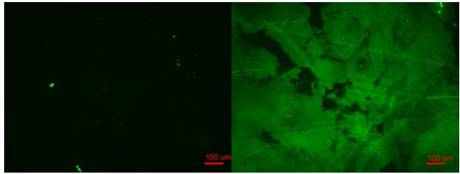
The second approach involves the immobilized Bt ligand on the top of SAM glass system interacting with fluorescein labeled Streptavidin to demonstrate detection of protein binding. The Fig. 6 shows the fluorescence imaging changing from a not fluorescent structure to a fluorescent attached Bt-SVA on the top of the SAM layer. [34]
Figure 6: Fluorescence microimage of SAV-Biotin-PG5000-SAM on glass (Right side). Control shows null fluorescence (Left side). Scale bar 100 µm.
CA value of Pegylated-Bt surface was 51°, in contrast to hydrophobic immobilized compounds including stearic acid, paraffin wax, myristoyl alcohol, and cetyl palmitate which exhibits a large hydrophobic contact angle around 110°. [35] The combination of Bt-functionalized glass for targeting the molecule of interest and protein resistance for blocking all other molecules, and high number of EG moieties improves protein repulsion enhancing the mechanical stability of this surface. [36]
The Fig. 6 shows a poor quality of monolayers, which was perturbed by cross-link of head groups Si-O-Si which causes chain distortion and the lack of long range order. [37] The quality of monolayers depends on several factors such as nature and roughness of the substrate, solvent used, temperature, concentration of the adsorbate. [38]
Advantages of this nanostructured bioplatform includes a much greater open area enabling a much faster contact of the measured species with optical or luminescent sensor and, thus, a potentially faster measurement; as well as different layer can grow on top of amino-functionalized glass, producing ordered arrangement able to give an optical signal without use expensive instruments as SPR, optical fiber, Raman, or difficult systems as nanofluids or Langmuir films.
4. Conclusion
The characterization and identification of interactions between ligands and biomolecules provides a significant basis for understanding the biological cell. Biomolecular recognition events at solid surfaces demand effective immobilization of biomolecules or ligands. This study used a binding system supported on SAMs, which determines the response of optical biosensor using glass material. It was shown that adsorption from organic solutions on polar solid surfaces can be used to produce a bioplatform of homogeneous and compact amino monolayers. The nanostructure liposome was immobilized on the top of the sensing surface as PDA displayed thermochromic behavior after heating, from intensity changes at the 640 nm band, attributed to the blue band formation appeared in the UV-vis spectra and chromic response after an enzyme binding. Furthermore the immobilized Ty enzyme retained its activity, as the sensor showed chromic response in presence of the natural ligand tyrosine. The second immobilization strategy involved Bt-PEG capable to bridge with fluorescent SVA, which was observed under fluorescence imaging. Both systems, Enzyme-PDA-liposome-functionalized glass offers to recognize natural substrates of immobilized enzyme giving out a colorimetric response; and the Biotin-streptavidin system is accessible to interact with different biotinylated proteins because SAV has four binding sites, facilitating the fluorescence measurement. In addition, SAV can be covalently coupled with different available ligands such as fluorochromes, enzymes and other tethers which make the biotin-streptavidin system widely used in the study of a variety of biological structures and processes. Both sensing mechanism emerges as a promising tools in biosensor development where one of the couple, either the biotin molecule or the colorimetric PDA liposome containing the active enzyme, is immobilized on the transducer element. . In addition, Pegylated surface minimizes nonspecific adsorptions, as hydrophobicity increased.
References
Referencias
Leech, D., Affinity biosensors, Chem &. Soc. Rev., 23, pp. 205-213, 1994. DOI: 10.1039/CS9942300205
Whitesides, G.M. and Boncheva, M., Beyond molecules: Self-assembly of mesoscopic and macroscopic components, Proc. Natl. Acad. Sci. USA, 99(8), pp. 4769-4774, 2002. DOI: 10.1073/pnas.082065899
Myers, D., Surfaces, interfaces, and colloids: Principles and applications, Second Edition. New York:John Wiley & Sons, 1999.
Wang, W. and Vaughn, M.W., Morphology and amine accessibility of (3-Aminopropyl) triethoxysilane films on glass surfaces. Scanning, 30(2), pp. 65-77, 2008. DOI: 10.1002/sca.20097.
Messing, R.A, Weisz, P.F. and Baum, G., Surface deactivation of porous glass membranes. J Biomed Mat Res, 3(3), pp. 425-430, 1969. DOI: 10.1002/jbm.820030304.
Fodor, S.P.A., Read, J.L., Pirrung, M.C., Stryer, L., Lu, A.T. and Solas, D., Light-directed addressable parallel chemical synthesis. Science, 251(4995), pp. 767-773, 1991. DOI: 10.1126/science.1990438.
Wen, K., Maoz, R., Cohen, H., Sagiv, J., Gibaud, A., Desert, A. and Ocko, B.M., Postassembly chemical Modification of a highly ordered organosilane multilayer: New insights into the structure, bonding, and dynamics of self-assembling silane monolayers. ACS Nano, 2(3), pp. 579-599, 2008. DOI: 10.1021/nn800011t.
Tallawi, M., Rosellini, E., Barbani, N., Cascone, M.G., Rai, R., Saint-Pierre, G. and Boccaccini, A.R., Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. Journal of the Royal Society Interface, 12(108), pp. 1-24, 2015. DOI: 10.1098/rsif.2015.0254.
Ulman, A., Formation and structure of self-assembled monolayers, Chem. Rev., 96(4), pp. 1533-1554, 1996. DOI: 10.1021/cr9502357.
Newman, R.H. and Zhang, J., Small molecules and chemical tools at the interface. Nature Chemical Biology, 4, pp. 382-386, 2008.
Dogra, N., Li, X. and Kohli, P., Investigating ligand-receptor interactions at bilayer surface using electronic absorption spectroscopy and fluorescence resonance energy transfer. Langmuir, 28(36), pp. 12989-12998, 2012. DOI: 10.1038/nchembio0708-382
Lee, S.W. and Laibinis, P.E., Protein- resistant coatings for glass and metal oxide surfaces derived from oligo (ethylene glycol)- terminated alkytrichlorosilanes. Biomaterials, 19(18), pp. 1669-1675, 1998.
Verné, E., Vitale-Brovarone, C., Bui, E., Bianchi, C.L. and Boccaccini, A.R., Surface functionalization of bioactive glasses. Biomed Mater Res A, 90(4), pp. 981-992, 2009. DOI: 10.1002/jbm.a.32153.
Moreno, Y.L., Becerra, N.P., Chaparro, S. y Reyes, J., Evaluación de la sensibilidad colorimétrica para la determinación de nanoestructuras compuestas de polidiacétileno/lípidos, usando espectroscopia de absorción electrónica y UV-Vis fluorescencia. Acta Agronómica, 65(3), pp. 268-275, 2016. DOI: 10.15446/acag.v65n3.45689.
Shim, H.Y., Lee, S.H., Ahn, D.J., Ahn, K.D. and Kim, J.M., Micropatterning of diacetylenic liposome on glass surfaces. Materials Science and Engineering C, 24(1-2), pp. 157-161, 2004. DOI: 10.1016/j.msec.2003.09.067
Nicholas, M.P., Rao, L. and Gennerich, A., Covalent immobilization of microtubules on glass surfaces for molecular motor force measurements and other single-molecule assays. Methods in Molecular Biology, 1136, pp. 137-169, 2014. DOI: 10.1007/978-1-4939-0329-0_9
Kim, K.W., Choi, H., Lee, G.S., Ahn, D.J., Oh, M.K. and Kim, J.M., Micro-patterned polydiacetylene vesicle chip for detecting protein-protein interactions. Macromolecular Research, 14(4), pp. 483-485, 2006. DOI: 10.1007/BF03219115
Cieńsk, A.M., Labus, K., Lewańczuk, M., Koźlecki, T., Liesiene, J. and Bryjak, J., Effective L-Tyrosine hydroxylation by native and immobilized tyrosinase. PLoS ONE, 11(10), e0164213, 2016. DOI: 10.1371/journal.pone.0164213
Ruiz-Taylor, L.A., Martin, T.L., Zaugg, F.G., Witte, K., Indermuhle, P., Nock, S. and Wagner P., Monolayers of derivatized poly(L-lysine)-grafted poly(ethylene glycol) on metal oxides as a class of biomolecular interfaces. Proc. Natl. Acad. Sci. USA, 98(3), pp. 852-857, 2001. DOI: 10.1073/pnas.98.3.852
Vendra, V.K., Wu, L. and Krishnan, S., Polymer thin films for biomedical applications in Kumar C.S.S.R. Nanomaterials for the Life Sciences, 5, Nanostructured Thin Films and Surfaces. Weinheim: WILEY-VCH, 2010.
Naik, V.V., Städler, R. and Spencer, N.D., Effect of leaving group on the structures of alkylsilane SAM. Langmuir, 30(49), pp. 1482-14831, 2014. DOI: 10.1021/la503739j
Ho, T.A., Papavassilioua, D.V., Lee, L.L. and Striolo, A., Liquid water can slip on a hydrophilic surface. Proc. Natl. Acad. Sci. USA, 108(39), pp. 16170-16175, 2011. DOI: 10.1073/pnas.1105189108
Vallant, T., Kattner, J., Brunner, H., Mayer, U. and Hoffmann, H., Investigation of the formation and structure of self-assembled alkylsiloxane monolayers on silicon using in situ attenuated total reflection infrared spectroscopy. Langmuir, 15(16), pp. 5339-5346, 1999. DOI: 10.1021/la9900977
Onclin, S., Ravoo, B.J. and Reinhoudt, D.N., Engineering silicon oxide surfaces using self-assembled monolayers. Angew. Chem., Int. Ed., 44(39), pp. 6282-6304, 2005. DOI: 10.1002/anie.200500633
Chiang, C.H., Ishida, H. and Koenig, J.L., The structure of γ-aminopropyltriethoxysilane on glass surfaces. Journal of Colloid and Interface Science, 74(2), pp. 396-404, 1980. DOI: 10.1016/0021-9797(80)90209-X
Chivers, C.E., Koner, A.L., Lowe, E.D. and Howarth, M., How the biotin–streptavidin interaction was made even stronger: Investigation via crystallography and a chimeric tetramer. Biochemical Journal, 435(1), pp. 55-63, 2011. DOI: 10.1042/BJ20101593
Scott, M.A., Wissner-Gross, Z.D. and Yanik, M.F., Ultra-rapid laser protein micropatterningL screening for directed polarization of single neurons. Lab on a Chip, 12(12), pp. 2265-2276, 2012. DOI: 10.1039/c2lc21105j.
Lee, J., Pyo, M., Lee, S.H., Kim, J., Ra, M., Kim, W.Y., Park, B.J., Lee, C.W. and Kim, J.M., Hydrochromic conjugated polymers for human sweat pore mapping, Nature Communications, 5(3736), pp. 1-10. 2014. DOI: 10.1038/ncomms4736
Jelinek, R. and Ritenberg, M., Polydiacetylenes–recent molecular advances and applications. RSC Adv., 3, pp. 21192-21201, 2013. DOI: 10.1039/C3RA42639D
Chance, R., Baughman, R., Müller, H. and Eckhardt, C.J., Thermochromism in a polydiacetylene crystal, J. Chem. Phys., 67(8), pp. 3616-3618, 1977. DOI: 10.1063/1.435361
Lee, Y.B., Kim, J.M., Park, L.H., Son, Y.G., Ahn, D.J. and Kim, J.M., Immobilization of polydiacetylene sensor on solid substrate. NSTI-Nanotech, 2, pp. 434-437, 2005.
Carpick, R.W., Sasaki, D.Y., Marcus, M.S., Eriksson, M.A. and Burns, A.R., Polydiacetylene films: A review of recent investigations into chromogenic transitions and nanomechanical properties. Phys.: Condens. Matter, 16, pp. R679-R697, 2004. DOI: 10.1088/0953-8984/16/23/R01
Roym D. and Park, J.W., Spatially nanoscale-controlled functional surfaces toward efficient bioactive platforms. J. Mater. Chem. B, 3(26), pp. 5135-5149, 2015. DOI: 10.1039/C5TB00529A
Darst, S.A., Ahlers, M., Meller, P.H., Kuba- lek, E.W., Blankenburg, R., Ribi, H.O., Ringsdorf, H. and Kornberg, R.D., Two-dimensional crystals of streptavidin on biotinylated lipid layers and their interactions with biotinylated macromolecules. Biophys. J., 59(2), pp. 387-396, 1991. DOI: 10.1016/S0006-3495(91)82232-9
Zisman, W.A., Relation of the equilibrium contact angle to liquid and solid constitution. Contact angle, wettability, and adhesion advances in chemistry; American Chemical Society: Washington, DC, 1964.
Beneschet, J., Svedhem, S., Svensson, S.T., Valiokas, R., Liedberg, L. and Tengvall, P., Protein adsorption to oligo (ethylene glycol) self-assembled monolayers: Experiments with fibrinogen, heparinized plasma, and serum. J. Biomater. Sci. Polymer Ed., 12, pp. 581-597, 2001.
Lio, A., Charych, D. and Salmeron, M., Comparative atomic force microscopy study of the chain length dependence of frictional properties of alkanethiols on gold and alkylsilanes on mica. Journal of Physical Chemistry B, 101(19), pp. 3800-3805, 1997. DOI: 10.1021/jp963918e
Ulman, A., Formation and structure of self-assembled monolayers. Chemical Reviews, 96(4), pp. 1533-1554, 1996. DOI: 10.1021/cr9502357
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Isabel G. Vazquez-Gutierrez, Miguel A. Reyes-López, Sara A. Ochoa, Ariadnna Cruz-Córdova, Rigoberto Hernández-Castro, Abdú Orduña-Díaz, Juan Xicohtencatl-Cortes. (2024). Specific Detection of Uropathogenic Escherichia coli via Fourier Transform Infrared Spectroscopy Using an Optical Biosensor. ACS Omega, 9(25), p.27528. https://doi.org/10.1021/acsomega.4c02794.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 DYNA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
El autor o autores de un artículo aceptado para publicación en cualquiera de las revistas editadas por la facultad de Minas cederán la totalidad de los derechos patrimoniales a la Universidad Nacional de Colombia de manera gratuita, dentro de los cuáles se incluyen: el derecho a editar, publicar, reproducir y distribuir tanto en medios impresos como digitales, además de incluir en artículo en índices internacionales y/o bases de datos, de igual manera, se faculta a la editorial para utilizar las imágenes, tablas y/o cualquier material gráfico presentado en el artículo para el diseño de carátulas o posters de la misma revista.