Fast estimation of chlorophyll content on plant leaves using the light sensor of a smartphone
Estimación rápida del contenido de clorofila en hojas usando el sensor de luz de un teléfono celular
DOI:
https://doi.org/10.15446/dyna.v84n203.64316Palabras clave:
Plant nutrition, non-destructive chlorophyll meter, leaf light transmittance (en)Nutrición vegetal, medición no destructiva de clorofila, transmisión de luz por hojas (es)
Descargas
Recibido: 25 de abril de 2017; Revisión recibida: 10 de octubre de 2017; Aceptado: 20 de octubre de 2017
Abstract
Plant chlorophyll measurements can support nitrogen fertilization decisions. Using a 3D printed device and a red LED, here we tested the feasibility of using a smartphone ambient light sensor (ALS) to estimate leaf chlorophyll by light transmission. Its performance was evaluated by comparing 30 leaf sorghum (Sorghum bicolor (L.) Moench) transmission readings of the red LED (663 nm) obtained from the smartphone and a standard spectrometer, which showed a good coefficient of determination (r2 = 0.9067). Additionally, a comparison between the ALS and a SPAD 502TM (a commercial device for chlorophyll content estimation) was made with chrysanthemum (Dendranthema grandiflora Tzvelev) leaves, obtaining a good correlation between both measurements. Light transmission was also measured in S. bicolor flag leaves from plants growing in the greenhouse under increasing nitrogen fertilization. A clear fit between leaf light transmission and plant height was also observed, suggesting a simple, smartphone based estimation of plant’s chlorophyll.
Keywords:
Plant nutrition, non-destructive chlorophyll meter, leaf light transmittance..Resumen
Medir clorofila puede apoyar decisiones de fertilización nitrogenada de plantas. Usando un dispositivo impreso en 3D y un LED rojo, aquí probamos la factibilidad de usar un teléfono celular para estimar clorofila por transmisión de luz. Al comparar 30 lecturas de trasmisión de luz roja (663 nm) por hojas de sorgo Sorghum bicolor (L.) Moench, obtenidas del teléfono y de un espectrómetro estándar, se encontró un buen coeficiente de determinación (r2 = 0.9067). Adicionalmente, se hizo una comparación entre las lecturas del teléfono y un SPAD 502TM (un dispositivo comercial para la estimación del contenido de clorofila) en hojas de crisantemo Dendranthema grandiflora Tzvelev, obteniendo una buena correlación entre ambas medidas. La transmisión medida en hojas de S. bicolor de plantas de invernadero fertilizadas con nitrógeno también presentó buen ajuste con la altura de las plantas, sugiriendo la posibilidad de estimar fácilmente el contenido de clorofila en hojas usando teléfonos inteligentes.
Palabras clave:
Nutrición vegetal, medición no destructiva de clorofila, transmisión de luz por hojas..1. Introduction
Chlorophyll measurement in plants is widely used to monitor and control agricultural [1] and environmental processes [2]. Chlorophyll meters are mainly based on the analysis of fluorescence emissions [3], the transmission of specific light bands by leaves [4] or the analysis of leaf color in digital images. These images may come from devices such as cameras in smartphones [5] or from more sophisticated devices such as Google glasses, which send images through the internet to further receive chlorophyll related values from a remote server [6]. Because of the high correlation with nitrogen content, chlorophyll content is also used to address the nutritional status of N (nitrogen) in plants [7].
Analyses of leaf images from green or red channels are often used, since blue light can be also be absorbed by plant pigments other than chlorophyll. However, that approach can no longer be supported under the light of new discoveries. For example, blue colored leaves in begonia plants use green light bands that many other plants reflect [8]. Similarly, plant leaves can exhibit adaptations depending on the intensity of the light to which they are exposed, which result in differential reflectance [9] and would generate unexpected variations in the intensity of light reflected by leaves and hence in their images.
Plant growth is highly dependent on nitrogen availability, but excessively used it is responsible for greenhouse gas emissions and environmental concerns [7]. Therefore, the real access to devices for rapid assessment of plant nutritional status to further assist fertilizer decisions use are worldwide needed.
Because of the adsorptive properties of light by chlorophyll, leaf light transmission measurements are already used by portable chlorophyll meters. For example, the SPAD 502™ (Minolta) measure two leaf transmitted light bands centered at 650 and 940 nm, and its readings, then the so called SPAD numbers, are used to estimate nitrogen content in plants [10]. However, its cost can limit their use to well-funded farmers and laboratories. In any case, in order to maximize plant growth and reduce environmental concerns, the application of nitrogen should take into account the plant nitrogen status [11], which will be reached only by increasing the access of farmers to affordable and easy to use nitrogen meters.
Since instruments to diagnose plant nitrogen are based on well-established leaf transmittance properties [12] and light meters are already available in several types of mobile devices. Here we propose to use the smartphone light sensor to measure leaf chlorophyll content, along with the fact that chlorophyll in plant leaves results in great absorption of light in a band around 680 nm [13,15], this band is also absorbed by leaves and senescent fruits [16] and is commonly registered in almost any paper assessing visible light absorption by plant leaves.
This proposal is also supported by the well stablished association between chlorophyll and nitrogen concentration on leaves.
By using the light sensor on a smart phone, along with 3D printed accessories, we present a potentially new perspective to determine leaf chlorophyll content in a noninvasive way. The system is cheap and simple, and can be used directly in the field in remote locations with minimal training. The comparison of light transmission by leaves, obtained using the smartphone, the SPAD 502TM and the FLAMETM spectrometer readings, along with measurements in nitrogen supplemented plants indicate that this system has the potential to accurately estimate the content of leaf chlorophyll, and therefore the nutritional status of plants.
2. Materials and methods
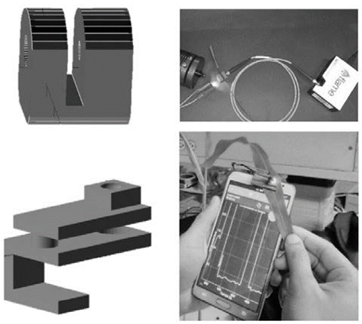
The general approach consisted in obtaining reference transmission spectra from sorghum (Sorghum bicolor (L.) Moench) plant leaves using a standard fiber optic spectrometer, in order to detect minimum transmission red bands, commonly linked to chlorophyll absorbance by intact leaves. Then, we picked a LED (light-emitting diode) with an emitting band peaking around the minimum red band transmission by these leaves. To obtain values of light transmission through plant leaves, two adapters were designed using the free software OpenScad, and further printed on a ROBO™ 3D printer using PLA (polylactic acid) filament. One of these adapters allowed to locate the leaves between a light source and the optical fiber coupled to the detector of a spectrometer. Another accessory allowed the positioning of a plant leaf between the ambient light sensor of the smartphone and the red emission LED (Fig. 1).
Figure 1: 3D printed accessories used to align optical elements and leaves during the tests. First row: support for the spectrometric measurement of leaf light transmission using a LED source or a halogen lamp. Second row: 3D printed device used to support the LED and leaves during light transmission tests using the smart-phone.
2.1. Relative performance of the smartphone light sensor compared to a fiber optic spectrometer and a gold standard SPAD 502™ chlorophyll meter
To evaluate the performance of the smartphone, the light transmission readings obtained from sorghum leaves were followed by the readings of the FLAMETM spectrometer (Ocean Optics Inc.) at the same leaf spot. These 663 nm LED transmission measurements were taken on 30 random points marked in the borders on different leaves. To further gain confidence on the performance of the smartphone based meter, 20 paired measurements on leaves of field grown chrysanthemum plants (Dendranthema grandiflora Tzvelev (cv Maisy)) were also obtained from a SPAD 502™ chlorophyll meter and from the smartphone.
2.2. Light transmission by sorghum leaves and red LED emission
The red LED was hereafter powered by a 5V external source and its emission spectra was measured using a FLAMETM (Ocean Optics Inc.) spectrometer with a spectral sensitivity from 350 to 1000 nm and a grating #3 (groove density 600). To measure the overall light transmitted by the leaves, the free app Physics Toolbox Sensor [17] was installed on a Samsung Galaxy Note 3Neo smartphone, which according to this application, is equipped with an environmental light sensor TMD27723 (AMS Corp.) [18]. It shows a spectral response between 300 and 1100 nm, and the readings between 300 and 700 nm are barely affected by temperature values between 5 and 75°C.
2.3. Soils, plant material, and data presentation
The experiment was conducted with plants grown under a polycarbonate roof in 232 cm3 pots for 64 days, and were amended (treatments) with increasing levels of biofertilizer (Abonaza™ g kg-1 soil): 0; 1.5; 3.0; 6,0 or 12. The soil consisted of a pooled mixture of low fertility soil subsamples representative of a large coffee producing area at the south west of Antioquia, Colombia, and is not representative of a particular taxonomic group. Relevant soil properties indicated values as follows: pH 4.6; CE (dSm-1) 0.08; OM (%) 2.7; Al, Ca, Mg and K (cmol c kg-1) 1.8, 1.6, 0.78 and 0.21 respectively; P, Fe, Mn, Cu, Zn, B (mg kg-1) 5, 51, 15, 1, 1 and 0.1 respectively. Additionally, lighting or temperature were not controlled, and these growth conditions are the ones naturally prevailing in the campus of “Universidad Nacional de Colombia” at Medellín. Each treatment included four replicates for plant height measurements. However, in some cases, only three replicates are presented for measurements of light transmission by leaves, due to involuntary mechanical damage to the flag leaf.
2.3.1. Smartphone based measurements of light transmission through sorghum and chrysanthemum leaves
The measurements were made on the flag leaf on sorghum (youngest leaf completely open), which was placed between the LED and the phone sensor (Fig 1)
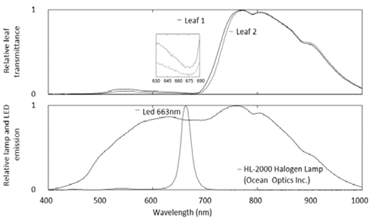
The lecture of light transmission values by the leaves were obtained from a position about one third of its length starting from the tip. The light was emitted by the 663 nm LED and was recorded from the Physics tool box app in the smartphone. The LED used here is a no name LED that we found at hand, whose emission peak is closer to spectral zones of greater light absorption by many plant species [15] (Fig. 2).
Figure 2: Transmission spectra of sorghum leaves (top) illuminated with a halogen lamp. Emission of the halogen lamp and the 663 nm LED source (bottom) used in this research. The insert in the upper figure shows a band of greater light absorption in two randomly chosen sorghum leaves.
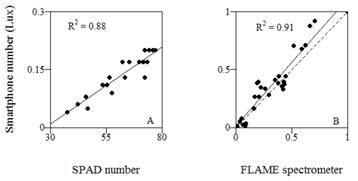
To compare the relative fit between the smartphone meter and the SPAD 502™ chlorophyll meter (Minolta corporation, Ltd., Osaka, Japan) or spectrometer masurements, these SPAD numbers or readings of the 663 nm band leaf transmission were correlated to further obtain a R 2 coefficient, and are presented in scatter plots (Fig. 3).
Figure 3: Relative performance of the smartphone based chlorophyll meter compared with two commercial devices on plant leaves. A: Smartphone readings compared with the readings of the gold standard SPAD 502™ chlorophyll meter (Minolta corporation, Ltd., Osaka, Japan) on chrysanthemum. B: Smartphone readings compared with a commercial spectrometer (FLAMETM, Ocean Optics Inc.) on sorghum. The solid line in each scatter plot represents the best fitted model. The dashed line shows a perfect fit reference line.
In the experiment with fertilized plants, the values of plant height and 663 nm band leaf transmission are presented in scatterplots, without further analysis. Light transmission values are presented in a standardized 0 to 1 scale. To overcome the limitations of a higher number of plant height values (20), as compared to a lower number of light transmission readings (17), the latest were taken randomly, without knowing the current height of the plants whose leaves were been assessed.
3. Results and discussion
The spectra of light transmission by sorghum leaves showed the lowest values of transmission of light in a band near 670 nm. This band of lower transmission, and greater adsorption of red light by chlorophyll, is consistent with the information available in the literature for a very large number of plants [13-15]. The overall spectra of visible light transmission by leaves and emission of the used LED are presented in Fig. 2.
The comparison between the transmission values of 663 nm light band obtained from the smartphone and the spectrometer showed a high correlation and an average squared error of less than 7% of the highest light transmission reading, which would represent the lower concentrations of chlorophyll in the leaf samples (Fig. 3). In chrysanthemum leaves, the Lux readings on the smartphone tended to stabilize, in front of ever increasing SPAD numbers.
Plant height showed an increase which was proportional to the amount of fertilizer applied, and a good fit to the values of 663 nm light transmission. The higher the values of plant height, the lower the transmission of the 663 nm LED (Fig. 4).
Figure 4: Leaf light transmission through sorghum leaves, using a LED diode peaking at 663 nm. The plants received increasing levels of a nitrogen biofertilizer. Light transmission values are presented on an inverted axes to ease the visualization of relationships between light transmission values through leaves and plant growth. The insert shows, from left to right, representative plants from each treatment with sequential increase in fertilizer application.
The values of sorghum leaf light transmission obtained from the ALS on the smartphone showed a good fit to readings obtained by using a standard fiber optic spectrometer. Furthermore, readings from the gold standard SPAD 502™ meter on field grown chrysanthemum leaves were highly correlated with those obtained from the ALS on the smartphone, the differences would be explained by a higher light intensity and sensitivity of the SPAD meter, and its general robust design, as compared to the loose parts making the experimental setup on the smartphone. However, these higher readings correspond to thicker leaves, in which the SPAD meter presented warning signals, perhaps associated to leaf thickness which is detected by its dual lighting system, which might be used to prevent false chlorophyll readings coming from low light transmission in thicker leaves. These smartphone values also showed a well agreement with the height of nitrogen fertilized plants. In spite of the simple arrangement of pieces, this system seems to be a promising tool for easy chlorophyll measurement to assist in nitrogen fertilizer use. Even though at this point it is not devoid of uncontrolled ambient noise and some simple optical adjustments might be user approached before final usage. Chlorophyll fluorescence also allows its analysis [3] and can be implemented under the same concept shown here. However, this technique involves the separation of excited and emitted bands in a more complex, larger, and expensive setup.
We did not test the performance of the smartphone as compared to other chlorophyll meters, since these are not easily available. Instead of that the readings of the ambient light sensor on the smartphone were compared to those obtained from the spectrometer using the same setup for leaf positioning. Additionally, spectrometers are the gold standard at hand to which any optical based instrument should be compared to. In the other hand, the low transmittance of red light by sorghum leaves in this study is in accordance with many reports in the scientific literature indicating a strong light absorption in a band between 650 nm and 680 nm, that was early attributed to plant chlorophyll [11]. This fact founds the development of some of the portable systems used today to estimate nitrogen fertilizer needs by crops, since leaf chlorophyll concentration is correlated to nitrogen content, as well as to red light absorbance, even in divergent groups of plant species such as legumes and cereals [16].
Here we did not pretend to create a model of nitrogen fertilization for sorghum plants. Instead of that, we show a potentially new way to craft new and affordable tools for farmers and perhaps scientists, in order to build these models, which are urgently needed to increase farmers’ profits while reducing environmental concerns.
Smartphones would help to make this possible, due to better and faster processors and sensors which turn them into handy meters ready for field use [5]. Since smartphones and free apps are available around the world today; this device might have applications beyond communications. For instance, by establishing simple field based tests, farmers or technicians can now create custom reference values for adequate chlorophyll concentration by simply applying known quantities of nitrogen fertilizers and recording plant growth.
Unlike chlorophyll determination based on leaf images, this simple array has the advantage of directly measuring the light band absorbed by leaf chlorophyll, and sensor response depends only on the 663 nm LED and the absorptive properties of the leaf, which is very high at this wavelength [18], although noisy light should be excluded. On image based analysis, Bayer filters in camera sensors have color overlaps, which would need to be removed to make measurements of specific light bands [19]. This means that in common cameras, RGB (Red, Green and Blue) channels represent broad and nonspecific sections of the light spectrum [20].
It is also important to note that leaves of different plants may show intrinsic conditions for light interference, like anatomy and chloroplast distribution, which might also be influenced by light exposure and time of the day [16]. These may all introduce variability, and should be accounted for when using this and any other chlorophyll meter to support nitrogen fertilization decisions. However, the most critical component of this and other leaf transmission based meters is perhaps a LED peaking around light bands which are most adsorbed by chlorophyll.
It is estimated that around 20 percent of the nitrogen fertilizer used worldwide is recovered as agricultural products [21]. It is clear that easing nitrogen diagnosing technologies would make a true contribution to a sustainable agricultural and environmental development. That can be easier by using the wide distribution of portable devices with optical sensors, like smartphones. In addition to sensors, smartphones commonly can access internet networks, which may also contribute to data exchange among users and may speed up the construction of databases with chlorophyll reference values under multiple environmental conditions, to support further fertilizer use decisions.
Although caution is needed until tools run perfect. Chlorophyll measurement is a commonly used approach to estimate N status in plants [22]. However, Mg [23] and Fe [24] deficiencies may also cause low chlorophyll levels since they are involved in chlorophyll biosynthesis. Therefore, high transmittance values of red light may also be obtained from Mg or Fe deficient plants, leading to erroneous diagnosis. Nevertheless, the visual symptoms of these deficiencies are radically different from each other, and may be used in conjunction with meters at hand, whereas new optical properties can be incorporated to this diagnosing tools.
In conclusion, the ambient light sensor of the smartphone showed a good performance to measure specific leaf light transmission, which is widely associated to chlorophyll presence. It can represent a new and affordable avenue to develop low cost chlorophyll meters and tune nitrogen fertilizer use in agriculture, which is urgently needed around the world.
Acknowledgements
This research was conducted at ¨Laboratorio de Suelos, Sección Microbiología, Universidad Nacional de Colombia - Sede Medellín”. Non logistic funding was provided by the authors. The final version of this manuscript was greatly improved by the comments of anonymous reviewers.
References
Referencias
Peng, S., Garcia, F.V., Laza, R.C., Sanico, A.L., Visperas, R M. and Cassman, K.G., Increased N-use efficiency using a chlorophyll meter on high-yielding irrigated rice, F. Crop. Res., 4 (2), pp. 243-252, 1996. DOI: 10.1016/0378-4290(96)00018-4.
Guanter, L., Zhang, Y., Jung, M., Joiner, J., Voigt, M. and Berry, J.A., Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence, Proc. Natl. Acad. Sci., 111(14), pp. E1327-E1333, 2014. DOI: 10.1073/pnas.1320008111.
Maxwell, K. and Johnson, G.N., Chlorophyll fluorescence: A practical guide, J. Exp. Bot., 51(345), pp. 659-668, 2000. DOI: 10.1093/jexbot/51.345.659
Mackinney, G., Absorption of light by chlorophyll solutions, J. Biol. Chem., 140, pp. 315-322, 1941.
Vesali, F., Omid, M., Kaleita, A. and Mobli, H., Development of an android app to estimate chlorophyll content of corn leaves based on contact imaging, Comput. Electron. Agric., 116, pp. 211-220, 2015. DOI: 10.1016/j.compag.2015.06.012
Cortazar, B., Koydemir, H.C., Tseng, D., Feng, S. and Ozcan, A., Quantification of plant chlorophyll content using google glass, HHS Public Access Author Manuscr., 15(7), pp. 1708-1716, 2015. DOI: 10.1039/c4lc01279h.Quantification.
Intaravanne, Y. and Sumriddetchkajorn, S., Android-based rice leaf color analyzer for estimating the needed amount of nitrogen fertilizer, Comput. Electron. Agric., 116, pp. 228-233, 2015. DOI: 10.1016/j.compag.2015.07.005.
Jacobs, M., Lopez-Garcia, M., Phrathep, O.P. and Lawson, T., Photonic crystal structure of Begonia chloroplasts enhances photosynthetic efficiency, Nat. Plants, October, pp. 1-16, 2016. DOI: 10.1038/nplants.2016.162.
Lenk, S. et al., Multispectral fluorescence and reflectance imaging at the leaf level and its possible applications, J. Exp. Bot., 58(4), pp. 807-814, 2007. DOI: 10.1093/jxb/erl207.
Markwell, J., Osterman, J.C. and Mitchell, J.L., Calibration of the Minolta SPAD-502 leaf chlorophyll meter, Photosynth. Res., 46(3), pp. 467-472, 1995. DOI: 10.1007/BF00032301.
Pinkard, E.A., Patel, V. and Mohammed, C., Chlorophyll and nitrogen determination for plantation-grown Eucalyptus nitens and E. globulus using a non-destructive meter, For. Ecol. Manage., 223(1), pp. 211-217, 2006. DOI: 10.1016/j.foreco.2005.11.003.
Xiong, D. et al., SPAD-based leaf nitrogen estimation is impacted by environmental factors and crop leaf characteristics., Sci. Rep., 5, August, p. 13389, 2015. DOI: 10.1038/srep13389.
Friedman, J.M., Hunt, E.R. and Mutters, R.G., Assessment of leaf color chart observations for estimating maize chlorophyll content by analysis of digital photographs, Agron. J., 108(2), pp. 822-829, 2016. DOI: 10.2134/agronj2015.0258
Gates, D.M., Keegan, H.J., Schleter, J.C. and Weidner, V.R., Spectral properties of plants, Appl. Opt., 4(1), p. 11, 1965. DOI: 10.1364/AO.4.000011.
Knapp, A.K. and Carter, G.A., Variability in leaf optical properties among 26 species from a broad range of habitats, Am. J. Bot., 85(7), pp. 940-946, 1998. DOI: 10.2307/2446360.
Merzlyak, M.N., Gitelson, A., Chivkunova, O.B. and Rakitin, V.Y.U., Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening, Physiologia Plantarum, 106(1), pp. 135-141, 1999. DOI: 10.1034/j.1399-3054.1999.106119.x.
Vieyra Software, Physics Toolbox Sensor Suite (Version 1.6.6). [online]. [Mobile application software]. 2017. Available at: https://play.google.com/store/apps/details?id=com.chrystianvieyra.physicstoolboxsuite&hl=es_419
AMS, TMD2772/ TMD2772WA Digital ALS and Proximity Module, [online]. pp. 1-59, 2016. Available at: https://ams.com/jpn/content/download/365023/1210677/file/TMD2772_TMD2772WA_Datasheet_EN_v2.pdf
Das, A.J., Wahi, A., Kothari, I. and Raskar, R., Ultra-portable, wireless smartphone spectrometer for rapid, non-destructive testing of fruit ripeness, Sci Rep, 6, p. 32504, 2016. DOI: 10.1038/srep32504.
Berra, E., Gibson-Poole, S., MacArthur, A., Gaulton, R. and Hamilton, A., Estimation of the spectral sensitivity functions of un-modified and modified commercial off-the-shelf digital cameras to enable their use as a multispectral imaging system for UAVs, Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci., 40(1), pp. 207-214, 2015.
Erisman, J.W., Sutton, M.A., Galloway, J., Klimont, Z. and Winiwarter, W., How a century of ammonia synthesis changed the world, Nat. Geosci., 1, pp. 636-639, 2008. DOI: 10.1038/ngeo325.
Muñoz-Huerta, R.F., Guevara-Gonzalez, R.G., Contreras-Medina, L.M., Torres-Pacheco, I., Prado-Olivarez, J. and Ocampo-Velazquez, R.V.. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances., Sensors (Basel)., 13(8), pp. 10823-10843, 2013. DOI: 10.3390/s130810823.
Guo, W., Nazim, H., Liang, Z. and Yang, D., Magnesium deficiency in plants: An urgent problem, Crop J., 4(2), pp. 83-91, 2016. DOI: 10.1016/j.cj.2015.11.003.
Miller, G.W., Pushnik, J.C. and Welkie, G.W., Iron chlorosis, a world wide problem, the relation of chlorophyll biosynthesis to iron, J. Plant Nutr., 7(1–5), pp. 1-22, 1984. DOI: 10.1080/01904168409363172
Cómo citar
IEEE
ACM
ACS
APA
ABNT
Chicago
Harvard
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Zlatin Zlatev, Vanya Stoykova, Galya Shivacheva, Miroslav Vasilev. (2023). Design and Implementation of a Measuring Device to Determine the Content of Pigments in Plant Leaves. Applied System Innovation, 6(4), p.64. https://doi.org/10.3390/asi6040064.
2. Karen Ospino-Villalba, Daniel Gaviria, Daniel Pineda, Juan Pérez. (2024). A 3D-Printable smartphone accessory for plant leaf chlorophyll measurement. HardwareX, 20, p.e00597. https://doi.org/10.1016/j.ohx.2024.e00597.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 DYNA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
El autor o autores de un artículo aceptado para publicación en cualquiera de las revistas editadas por la facultad de Minas cederán la totalidad de los derechos patrimoniales a la Universidad Nacional de Colombia de manera gratuita, dentro de los cuáles se incluyen: el derecho a editar, publicar, reproducir y distribuir tanto en medios impresos como digitales, además de incluir en artículo en índices internacionales y/o bases de datos, de igual manera, se faculta a la editorial para utilizar las imágenes, tablas y/o cualquier material gráfico presentado en el artículo para el diseño de carátulas o posters de la misma revista.