Impregnation Solution Influence on the Pulp Color of Plantains (Musa paradisiaca)
Influencia de Soluciones de Impregnación en el Color de la Pulpa de Plátano (Musa paradisiaca)
Keywords:
Lightness, sulphites, ascorbic acid, citric acid. (en)Luminosidad, sulfitos, ácido ascórbico, ácido cítrico. (es)
Downloads
IMPREGNATION SOLUTION INFLUENCE ON THE PULP COLOR OF PLANTAINS (musa paradisiaca)
INFLUENCIA DE SOLUCIONES DE IMPREGNACIÓN EN EL COLOR DE LA PULPA DE PLÁTANO (musa paradisiaca)
Misael Cortés Rodríguez1; René Mauricio Dávila Moreno2 and Jesús Humberto Gil González3
1 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Facultad de Ciencias Agrarias - Departamento de Ingeniería Agrícola y Alimentos. A.A. 1779, Medellín, Colombia. <mrcortes@unal.edu.co>
2 Auxiliary Professor. Instituto Universitario de La Paz. Escuela de Ingeniería Agroindustrial, km 14 vía Bucaramanga Vereda El Zarzal, Barrancabermeja, Colombia. <rene.davila@unipaz.edu.co>
3 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Facultad de Ciencias Agrarias - Departamento de Ingeniería Agrícola y Alimentos. A.A. 1779, Medellín, Colombia. <jhgilg@unal.edu.co>
Received: August 30, 2012; acepted: November 07, 2012.
Resumen. Se evaluó la influencia de soluciones antioxidantes (metabisulfito de sodio Na2S2O5 y ácido ascórbico C6H8O6) y acidulantes (ácido cítrico C6H8O7) sobre el color de la pulpa de plátano verde (PPV) impregnadas al vacío, almacenadas (0, 3, 6, 9, 12 y 15 días; 4, 20 y 30 °C) y empacadas con vacío (CV) y sin vacío (SV). El color se determinó mediante las coordenadas CIE-L*a*b*, siendo la luminosidad (L*) el parámetro de mayor importancia para el control del pardeamiento (L*crítico=70). Las concentraciones de los componentes en las soluciones de impregnación se fijaron para obtener en la PPV impregnado niveles de benzoato de sodio (1000 mg), EDTA (75 mg) y sulfitos (500, 1.000 y 1.500 mg), por kg de PPV fresco; y de ácido ascórbico (60, 90 y 120 mg) y ácido cítrico (100, 150 y 250 mg), por 100 g de PPV fresco. Se presentaron diferencias significativas (P<0,05) en L* con respecto al tratamiento y el tiempo, siendo la temperatura de 4 °C la más adecuada. Los tratamientos que cumplieron durante 15 días que L*>L*crítico fueron: metabisulfito de sodio (500 mg/kg PPV, SV y 0 mg/kg, CV), ácido ascórbico (90 mg/100 g PPV, SV y 60 mg/100 g PPV, CV), ácido cítrico (100 mg/100 g PPV, CV) y para la mezcla ácido ascórbico (90 mg): ácido cítrico (100 mg)/100 g PPV, a condiciones de empacado CV.
Palabras clave: Luminosidad, sulfitos, ácido ascórbico, ácido cítrico.
Abstract. The influence of antioxidant (sodium metabisulphite Na2S2O5 and ascorbic acid C6H8O6) and acidulant (citric acid C6H8O7) solutions on the color of vacuum impregnated green plantain pulp (GPP), stored (0, 3, 6, 9, 12 and 15 days; 4, 20 and 30 °C) and vacuum packed (VP) or packed without vacuuming (WV), was evaluated. The color was determined by the CIE-L*a*b* coordinates, where the lightness (L*) was the most important parameter for browning control (L*critical=70). Component concentrations in the impregnation solutions were established to achieve levels of sodium benzoate (1,000 mg), EDTA (75 mg) and sulphites (500, 1,000 y 1,500 mg), per kg of fresh GPP; and ascorbic acid (60, 90 and 120 mg) and citric acid (100, 150 and 250 mg), per 100 g of fresh GPP. Significant differences were seen (P<0.05) in L* regarding time and treatment, in which the temperature of 4 °C was the most adequate. Treatments with L*>L*critical for 15 days were: sodium metabisulphite (500 mg/kg of GPP, WV and 0 mg/kg of GPP, VP), ascorbic acid (90 mg/100 g of GPP, WV and 60 mg/100 g of GPP, VP), citric acid (100 mg/100 g of GPP, VP) and for the mixture of ascorbic acid (90 mg): citric acid (100 mg)/100 g of GPP, VP.
Key words: Lightness, sulphites, ascorbic acid, citric acid.
The plantain (Musa paradisiaca), belonging to the Musaceae family, is a pleasant-tasting fruit, highly caloric (85 Kcal/100 g), rich in carbohydrates and low in protein and fat. It is also a source of essential nutrients such as vitamins A, C, B1, B2 and B6, and small amounts of vitamin E; in addition, it provides minerals such as potassium, magnesium and phosphorus, as well as malic acid, folic acid and soluble and insoluble fiber (Hofsommer, 2001).
Colombia is the leading exporter of plantain in the world with 700,000 t/year; between the years 2007-2008, there was an increase in exports of 3.53% (in volume) and 10.18% (in value), from US $38.9 million to US $42.9 million (Augura, 2009). In Colombia, the harvest and post-harvest losses in plantain are estimated at 300,000 t/year, representing a value close to US $18.500. The causes of these losses are attributed to the low technology of crops, poor product handling from the production place to the final consumer, and include unsuitable harvests (Arrieta et al., 2006).
Green plantain pulp (GPP) has enzymatic browning reactions that adversely affect color, sensory characteristics and overall shelf life of processed foods (Millan and Roa, 2001). This reaction is started when monophenolic compounds present in tissues are hydroxylated in the presence of oxygen to o-diphenols by involving the enzyme polyphenol oxidase (PPO). Subsequently, the o-diphenols are oxidized to o-diquinones to produce melanoidins, compounds responsible for browning (Millan and Roa, 2001). These o-quinones can polymerize and covalently bind to nucleophilic amino acids to produce the dark brown or black pigment in fruits and vegetables. The use of browning inhibitors has been a common method to prevent darkening of the products. Different authors have studied browning control for GPP by immersion processes with ascorbic acid solutions (Pirone et al., 1998), lysine and glucose (Kwak et al., 2005), citric acid (Jiang et al., 2004), phosphates (McEvily et al., 1992) and sodium chloride (Lu et al., 2007) which have shown a capacity of inhibiting enzymatic browning (Yang and Shuji, 2000). Other researchers have studied banana browning control through treatment with N2O (Palomer et al., 2005), and in the longan fruit (Dimocarpus longan Lour), with sulfur dioxide (Jiang et al., 2002).
Sulfur dioxide and its salts are strong inhibitors of PPO; in Colombia, these compounds are approved by the Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) at maximum concentrations of 1,500 mg kg-1, as well as other institutions or food regulatory centers in other countries. Browning studies with these antioxidants have proven them effective for plantain (Tortoe et al., 2009), apple (Eissa et al., 2006), processed plantains (Jaworska et al., 2004; Li and Zhao, 2006) and potato (Limbo and Piergiovanni, 2006). However, there are some questions on the use of sulphites in foods due to possible health effects (Iyengar and McEvily, 1992; Lee, 2007).
The vacuum impregnation process (VI) has been described by Fito and Pastor (1994) as a mass transfer process in a porous solid-liquid system, through a hydrodynamic mechanism (HDM) action. The VI process has been applied as a mechanism for homogeneous incorporation of components into a structure with the purpose of improving organoleptic aspects, color control, and texture and as a methodology for obtaining functional foods (Cortés, 2004).
The objective of this investigation was to evaluate the influence of impregnating solutions (containing antioxidants and/or acidulants) on the color of green plantain pulp (Musa paradisiaca) preserved under different storage conditions.
MATERIALS AND METHODS
Raw material. The plantain fruits were obtained from "Los Robles", a farm located in the municipality of San Juan de Urabá (Antioquia, Colombia). Variety Dominico Harton (export type) plantains were used in a state of green maturity, which had reached the highest development in accordance with the variety to which they belong, and with totality of the green surface (ICONTEC - NTC 1190). Samples were formed into a cylindrical shape with a weight of 36.8 ± 0.54 g, a diameter of between 30-40 mm and a length of approximately 50 mm. Each batch of plantain evaluated corresponded to a bunch of plantains at different harvest times. Sodium metabisulphite (99% purity), ascorbic acid (99% purity), citric acid (99% purity), obtained from Chemicals Laboratories JM, and ethylenediaminetetraacetic acid (EDTA, 99.1% purity) from Biopack laboratories were used as components of the impregnating solutions.
Color. The tests were conducted in a spectrocolorimeter (X-Rite model SP-64) with an observation window of 4 mm, and illuminant D-65 with a 10° observer angle. From the reflection spectrum, the color coordinates CIE L*, a* and b* were obtained; where L* is a measure of brightness, a* represents green (-) to red (+) chromaticity, and b* stands for blue (-) to yellow (+) chromaticity. The color readings were made on the surface region of the GPP.
Vacuum impregnation process. A batch type vacuum impregnation device, with a capacity of 10 kg h-1, designed at the Universidad Nacional de Colombia, Sede Medellín, was used. The equipment consists of a stainless steel chamber, a vacuum pump and an electromechanical system which allows measuring mass changes in both the sample and the impregnating liquid, during the process. The dynamics of the impregnation were established in terms of the volume fraction of impregnation (m3 impregnated liquid /m3 initial sample) in the vacuum stage (X1) and the end of the process (X); the volumetric deformation (g1 and g) (m3 impregnated sample/m3 initial sample); and effective porosity (ee) (m3 gas/m3 initial sample). Processing times were 5 min, with a vacuum of 31.4 mm Hg and atmospheric pressure of 640 mm Hg.
Design of the solutions. The impregnation solutions (IS) were formulated from a NaCl (1.6%) solution with the same aw as the GPP (0.993 ± 0.001), to minimize other mechanisms of mass transfer during the VI process. The concentrations of the components in the IS were set under the discretion and authority of the research group on functional foods, and adjusted for both Colombian and industrial food additives regulations. The composition of the IS was determined with material balances (Cortés, 2004), which allow for obtaining different levels per kg of fresh GPP: sodium benzoate (1,000 mg), EDTA (75 mg) and sulphites (500, 1,000 and 1,500 mg); and per 100 g of fresh GPP: ascorbic acid (60, 90 and 120 mg) and citric acid (100, 150 and 250 mg). The mass fractions of the components in the is were determined with a material balance in the fruit-impregnation liquid system, with previous determination of X with the isotonic solution (Cortés, 2004).
Storage. The samples were stored at temperatures of 4, 20 and 30 °C, control times of 0, 3, 6, 9, 12 and 15 days and packaged with vacuuming (VP) and without vacuuming (WV) using plastic, multilayer polyamide-polyethylene bags (ALICO®) with water vapor (<15 g/m2/24 h/atm, T = 38 °C), O2 (60 cc/m2 /24 h/atm, T = 23 °C), N2 and CO2 barriers.
Experimental design. The experimental design used for the treatments with sulphites was a factorial randomized design with three factors: storage temperature (3 levels: 4, 20 and 30 °C), storage time (6 levels: 0, 3, 6, 9, 12 and 15 days) and treatment (8 levels: control VP and WV, sulphites at 500, 1,000 and 1,500 mg/kg of fresh GPP, VP and WV). All analyses were executed using Statgraphics Centurium (XV); the model used was two-way analysis of variance and the significant factors were analyzed by the multiple comparison technique "least significant difference (LSD)", with a confidence level of 95% and power of 80%. Three replicates were used per treatment. The experimental designs used for ascorbic acid and citric acid were similar to that of sulphites, where the concentrations used were: 60, 90 and 120 mg per 100 g of GPP, and 100, 150 and 250 mg per 100 g of GPP, respectively; the most favorable concentrations were mixed to evaluate synergy in controlling browning. The readings of the color parameters were performed in 3 batches, 5 samples/batch and 4 readings/sample, for a total of 60 measurements for each storage condition. To evaluate the effectiveness of the treatment, comparison controls corresponding to fresh GPP samples (without treatment) packaged in bags (VP and WV) were used. Figure 1 summarizes the treatments evaluated in this study.
RESULTS AND DISCUSSION
Table 1 shows the mean values and standard deviations of the parameters X, X1, g, g1 and ee in samples impregnated with the isotonic solution. X values of the GPP showed no significant differences with respect to the form, obtaining values of 9.52 ± 1.29, 10.13 ± 1.63 and 9.80 ± 1.27 for whole plantain pulp, trunk and sliced, respectively. These values are higher than those recorded for the cape gooseberry, 6.60 ± 1.16 (Marín, 2009; Restrepo et al., 2009), and the strawberry, 1.5 ± 0.3 (Restrepo, 2008), impregnated with isotonic solutions; and lower than those obtained in the mushroom Pleurotus ostreatus, 31.2 +10.7 (Ruiz, 2009). These values indicate that the structure of the plantain is sufficiently suitable for the incorporation of physiologically active compounds, because the matrix with the green physiological state provides intercellular spaces with air content and soluble compounds that facilitate the hydrodynamic mechanism (HDM) action in the VI process; these results validate the positive value obtained from ee (16.72 + 1.49 cm3 gas/cm3 GPP), representing the internal volume available for the incorporation of the isotonic solution.
Negative values of X1, g and g1 in the vacuum stage indicate the native liquid outlet from the plantain inner structure, generating a volumetric contraction for all forms, except for g1, which presented positive values for the cylindrical and whole plantain forms, where there was a response to the deformation effect. Negative values of g represent an overall contraction in the structure which is unable to recover its initial condition as a result of the coupling between the hydrodynamic mechanism and the strain-relaxation phenomenon.
Figure 2 shows the mean values, with LSD intervals (95%), of L*, a* and b* for the GPP impregnated with different concentrations of sulphites ([S2O5=]), and references the controls during storage. A preliminary evaluation of the color of fresh GPP during storage defined a critical L* value of 70 as a minimal commercial acceptance criterion (Davila, 2010). For all temperatures, the ANOVA showed significant differences (P<0.05) in L*, a* and b* with respect to time and treatment factors. In all cases, low coefficients of variability were observed, reflecting good reproducibility of the data for each storage condition.
S2O5= treatments and control, both at 4 °C and VP, showed values of L* in the range of 72-80 (> L*critical), achieving acceptable color for the maximum storage time (15 days); whereas, in the control sample (WV) the L*critical was obtained on day 6. At 20 and 30 °C, the same phenomenon was observed as that at 4 °C. However, the maximum time of acceptance was on day 9 (maximum time without the presence of fungal spores such as Aspergillus sp., Fusarium, and Rhizopus sp., which grow on the surface under low partial pressures of O2, and yeasts such as Sacharomices sp. and Candida sp. in anaerobic conditions; these mesophilic microorganisms have amylolytic characteristics, facilitating their proliferation in this type of food matrix). The results show that at 1,000 and 1,500 mg S2O5= kg-1 there is an increase of L*, which is attributed to the bleaching properties of sulphites, which act as reducing agents, converting quinones to diphenols (Li-Qin et al., 2009).
The parameters a* and b*, at storage conditions of 4, 20 and 30 °C, were located in the a* b* chromatic plane with two distinct groups: samples treated with S2O5= and control samples. In all cases, the control samples remained in the gray area, due to color changes produced by enzymatic oxidation reactions in storage. At 4 °C, the samples of the first group moved from the yellow area ≈ (a*, b*: 12, 37) to the limit of yellow, gray and red ≈ (a*, b*: 16, 34), because the matrices contain antioxidant compounds and low temperatures that slow enzymatic degradation processes, keeping these storage conditions in the yellow zone. At 20 °C, samples moved from the yellow area ≈ (a*, b*: 12, 38) to the limit of yellow and gray ≈ (a*, b*: 9, 32), because when temperature increases, enzymes are activated, leading to a change in the parameters a* and b* in the gray area; and at 30 °C, from the yellow area, ≈ a*, b*: 12, 37 to ≈ a*, b*: 16, 34, the samples retained the tendency to remain in the yellow zone until the ninth day of storage. However, samples impregnated with S2O5= (500 mg kg-1, VP and 30 °C) showed a tendency for displacement to the gray zone ≈ (a*, b*: 10, 25).
The food matrix, exposed to high temperatures and microbial activation, probably suffers degradation processes in tissues, leading to changes in color to the gray area, which are imperceptible to the human eye, recording a critical L* (70) at 9 days in storage.
The more effective results for the control of enzymatic browning using sodium metabisulphite were: 500 WV and 0 VP at 4 °C. However, selection of these will depend on operating costs and the effect on other quality attributes.
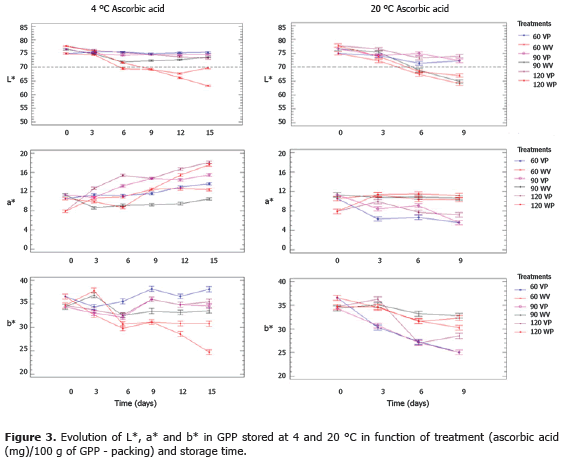
Figure 3 shows the mean values, with LSD intervals (95%), of L*, a* and b* for the impregnated GPP with different concentrations of ascorbic acid. For both temperatures (4 and 20 °C), the ANOVA showed significant differences (P<0.05) in L*, a* and b* with respect to time and treatment factors; however, coefficients of variation remained low in each trend of the evaluated parameters, leading to color changes in the tissues of the GPP as a result of enzymatic reactions, which were only slightly appreciable by the visual sensory method and the instrumental method with extensive assessment scales.
At 4 °C, L* showed two behaviors: first, where L* > L*critical remained during the 15 days of storage, which corresponded to all treatments with ascorbic acid and VP, and additionally 90 WV; second, where L* > L*critical lasted until approximately 7 d (samples WV 60 and WV 120). Ascorbic acid, in storage conditions, can be lixiviated when the contact surface of sliced food is higher, thus increasing the sensitivity to oxidation reactions by PPO, where ascorbate becomes dehydroascorbate (less stable compound) and transformed by hydrolysis to 2,3-diketogulonic acid, which is devoid of antioxidant properties (Robinson et al., 1991). At 20 °C, all treatments with VP showed values of L* > L*critical until day 9 (maximum time without the presence of fungi, yeast, and anaerobic bacterial agents such as Clostridium sp., which participate in starch degradation), and WV packaging treatments had values of L* > L*critical until approximately day 5.
At 4 °C, the chromaticity (a*) tends to increase over time, except in 90 WV which decreased slightly from 11 to 10. Meanwhile, the b* chromaticity fluctuated in the range of 31 to 37, except for 120 WV which decreased from 34 to 25. These variations in the chromatic plane (a* b*) identified a displacement from the yellow areas to the limits of yellow and red areas; and, in the case of treatment 60 WV, from the yellow zone to the limit of the yellow, gray and red areas. However, these displacements, although not seen in a large area of the chromatic plane, are due to the browning effect on the structure during storage. At 20 °C, the position in the chromatic plane (a* b*) changed from the yellow zone (8-11, a*; 34-36, b*) to the limits of yellow and gray areas in the ranges of 5-11 (a*) and 24-33 (b*).
The results obtained with ascorbic acid indicate that treatments 90 WV and 60 VP at 4 °C were the most effective for the control of enzymatic browning, and their selection depends on the balance of the costs of operation and the effect of treatments on other quality attributes.
Figure 4 shows the mean values, with LSD intervals (95%), of L*, a* and b* for the GPP impregnated with different concentrations of citric acid. For both temperatures (4 and 20 °C), the ANOVA showed statistically significant differences (P<0.05) in L*, a* and b* with respect to time and treatment factors, with low coefficients of variation.
In treatments with citric acid, L* similarly defined two groups at both temperatures. At 4 °C, a group consisted of VP samples with values of L* > L*critical until the last day of the study (15 days), which tended to decrease; and a second group, for WV samples with values of L* < L*critical until days 3, 6 and 7 at concentrations of 100, 250 and 150 (mg ascorbic acid/100 g of GPP), respectively. At 20 °C, VP samples reached the limits of L* > L*critical on day 9 (maximum study time without the presence of fungi and yeasts); and WV samples reached maximum commercial acceptance at 3 d (100 and 150 mg ascorbic acid/100 g of GPP) and 5 d (250 mg ascorbic acid/100 g of GPP).
At temperatures of 4 and 20 °C, changes in the a* b* chromatic plane were similar, maintaining a* in the range 7-10 during storage, while b* changed from 30-32 to 22-27. This situation is due to slight changes in the matrix structure of the plantain, which decreased from the limits of the yellow-gray to gray area. The results obtained with citric acid indicate that the treatment 100 VP at 4 °C was the most effective at controlling enzymatic browning.
Figure 5 shows the mean values, with LSD intervals (95%), of the color parameters L*, a and b* for the GPP impregnated with a mixture of ascorbic acid (90 mg): citric acid (100 mg)/100 g of GPP, stored at 4 and 20 °C and VP and WV. The ANOVA showed, for both temperatures, statistical differences (P<0.05) in L*, a* and b* with respect to time and treatment factors, except for the parameter b* at 20 °C; however, the coefficients of variability were low.
Under VP and WV conditions, at 4 °C, it was found that L* > L*critical lasted until 15 and 11 d, respectively; whereas at 20 °C, L> L*critical lasted until 9 and 6 d, respectively. As in previous treatments, at 20 °C, the maximum time without observing the presence of fungi and yeasts was 9 days. Chromaticity a* did not present significant changes, keeping between 12-13 (4 °C) and 10-13 (20 °C). For the b* parameter, changes in both temperature values ranged from 24 to 27. In the a* b* chromatic plane, the displacement of the samples at 4 and 20 °C ranged from the limit of the yellow and gray area to the gray area.
The interactions between these compounds points to an antagonistic effect only under the WV conditions, reducing the commercial acceptance time from 15 days (treatment achieved with ascorbic acid (90 mg)/100 g of GPP) to 11 days (achieved with the mixture of acids). The results obtained with the mixture indicated that the most effective treatment for the control of enzymatic browning is the VP condition.
CONCLUSIONS
The color parameters CIE L* a* b* are of greater importance for browning control in GPP as represented by the lightness (L*). Values of L*> L*critical =70, which are considered as commercially acceptable.
The best treatments for controlling browning were obtained under storage conditions of 4 °C and VP. Individual treatments that fulfilled the criterion of L*critical during 15 days of storage were: sodium metabisulphite (500 WV and 0 VP), ascorbic acid (90 WV and 60 VP) and citric acid (100 VP).
The mixture of ascorbic acid (90 mg): citric acid (100 mg)/100 g of GPP fulfilled the acceptance criterion (L*> L*critical = 70) in VP conditions for 15 days; while under WV conditions, an antagonistic effect was observed, limiting the acceptance time to 11 days.
BIBLIOGRAPHY
Arrieta, A., U. Baquero y J. Barrera. 2006. Caracterización fisicoquímica del proceso de maduración del plátano `papocho' (Musa ABB Simmonds). Agronomía Colombiana 24(1): 48-53.
Augura. 2009. Coyuntura bananera colombiana. Asociación de Bananeros de Colombia. 32 p.
Cortés, M. 2004. Desarrollo de productos de manzana deshidratados enriquecidos con vitamina E. Tesis Doctoral. Universidad Politécnica de Valencia. Valencia, España. 254 p.
Dávila, R. 2010. Evaluación del color en la pulpa de plátano (Musa paradisiaca) y banano (Musa cavendish) fresco durante el almacenamiento. Tesis de Maestría. Facultad de Ciencias Agropecuarias. Universidad Nacional de Colombia. Medellín. 20 p.
Eissa, H.A., H.H. Fadel, G.E. Ibrahim, I. M. Hassan and A.A. Elrashid. 2006. Thiol containing compounds as controlling agents of enzymatic browning in some apple products. Food Research International 39(8): 855-863.
Hofsommer, H.J. 2001. The composition of banana puree. Fruit Processing 11(1): 6-11.
Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC). 1976. NTC1190. Plátanos. Clasificación. Icontec, Bogotá. 4 p.ff
Iyengar, R. and A. J. McEvily, 1992. Anti-browning agents: alternatives to the use of sulfites in foods. Trends in Food Science & Technology 3: 60-64.
Jaworska, G., W. Kmiecik and J. Slupski 2004. Effect of technological measures on the quality of canned banana desserts. Food Science and Technology 7(1): 1-10.
Jiang, Y., L. Pen and J. Li. 2004. Use of citric acid for shelf life and quality maintenance of fresh-cut Chinese water chestnut. Journal of Food Engineering 63(3): 325-328.
Jiang, Y., Z. Zhang, D.C. Joyce and S. Ketsa. 2002. Postharvest biology and handling of longan fruit (Dimocarpus longan Lour.). Postharvest Biology and Technology 26(3): 241-252
Fito, P. and R. Pastor. 1994. Non - diffusional mechanisms ocurring during vacuum osmotic dehydration (VOD). Journal of Food Engineering 21(4): 513-519
Kwak, E.J., S.Y. Lee, M. Murata and S. Homma. 2005. Effect of pH control on the intermediates and melanoidins of nonenzymatic browning reaction. LWT - Food Science and Technology 38(1): 1-6.
Lee, M.K. 2007. Inhibitory effect of banana polyphenol oxidase during ripening of banana by onion extract and Maillard reaction products. Food Chemistry 102(1): 146-149.
Li, Y. and Zhao M. 2006. Simple methods for rapid determination of sulfite in food products. Food Control 17(12): 975-980.
Li-Qin, Z., Z. Jie, Z. Shu-Hua and G. Lai-Hui S. 2009. Inhibition of browning on the surface of peach slices by short-term exposure to nitric oxide and ascorbic acid. Food Chemistry 114(1): 174-179.
Limbo, S. and L. Piergiovanni. 2006. Shelf life of minimally processed potatoes: Part 1. Effects of high oxygen partial pressures in combination with ascorbic and citric acids on enzymatic browning. Postharvest Biology and Technology 39: 254-264.
Lu, S., Y. Luo, E. Turner and H. Feng. 2007. Efficacy of sodium chlorite as an inhibitor of enzymatic browning in apple slices. Food Chemistry 104(2): 824-829.
Marin, Z. 2009. Viabilidad de desarrollo de uchuva (Physalis peruviana L.) minimamente procesada enriquecida con microorganismos probióticos a partir de la ingenieria de matrices. Tesis Magister en Ciencia y Tecnología de Alimentos.Departamento de Ingeniería Agrícola y de Alimentos. Universidad Nacional de Colombia, Medellín. 152 p.
McEvily, A., R. Iyengar and W.S. Otwell. 1992. Inhibition of enzymatic browning in foods and beverages. Critical Reviews in Food Science and Nutrition 32(3): 253-273.
Millan, F. y V. Roa. 2001. Uso de la metodología de superficie de respuesta en la evaluación del pardeamiento en Cambur procesado por impregnación al vacío. Interciencia 26(7): 290-295.
Palomer, X. I. Roig-Villanova, D. Grima-Calvo and M. Vendrell. 2005. Effects of nitrous oxide (N2O) treatment on the postharvest ripening of banana fruit. Postharvest Biology and Technology 36(2): 167-175.
Pirone, B., M. Ochoa, A. Kesseler y A. Michelis. 1998. Evolución de la concentración de ácido ascórbico durante el proceso de deshidratación de frutos de la rosa mosqueta (Rosa eglanteria L.) Revista de Investigaciones Agropecuarias 31(1): 85-98.
Restrepo, A., M. Cortes y C. Márquez. 2009. Uchuvas (Physalis peruviana L.) mínimamente procesadas fortificadas con vitamin E. Vitae 16(1): 19-30.
Restrepo, A. 2008. Nuevas perspectivas de consumo de frutas: Uchuvas (Physalis peruviana L.) y fresa (Fragaria vesca L.) mínimamente procesadas fortificadas con vitamina E. Universidad Nacional de Colombia, Sede Medellín. 109 p.
Ruiz, M. 2009. Aplicación de la ingeniería de matrices en el desarrollo de hongos comestibles (Pleorotus ostreatus) mínimamente procesados, fortificados con vitamina C, E y minerales calcio y zinc. Tesis de Maestría en Ciencia y Tecnología de Alimentos. Facultad de Ciencias Agropecuarias. Universidad Nacional de Colombia, Sede Medellín. 109 p.
Tortoe, C., P. Johnson and A. Nyarko. 2009. Effects of osmo-dehydration, blanching and semi-ripening on the viscoelastic, water activity and colorimetry properties of flour from three cultivars of plantain (Musa AAB). Innovative Food Science and Emerging Technologies 10(1): 82-86.
Yang, C.P., M. Ashrafuzzaman, S. Fujita, N. Nakamura and N. Hayashi. 2000. Purification and characterizacion of polyphenol oxidase from banana (Musa sapientum L.). Journal of Agricultural and Food Chemistry 48(7): 2732-2735.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
Article abstract page views
Downloads
License
Copyright (c) 2015 Revista Facultad Nacional de Agronomía Medellín

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.