Larvicidal activity of ethanolic extract of Azadirachta indica against Aedes aegypti larvae
Actividad larvicida del extracto etanólico de Azadirachta indica contra larvas de Aedes aegypti
DOI:
https://doi.org/10.15446/rfnam.v73n3.80501Keywords:
Bio-larvicide, Crude ethanolic extract, GC-MS, Mosquito, Neem, Phytol (en)Biolarvicida, Extracto crudo de etanol, GC-MS, Mosquito, Neem, Fitol (es)
Downloads
Aedes aegypti is a mosquito that carries dengue virus, yellow fever and other diseases transmitted to humans. Organophosphorus larvicides are used to control the proliferation of this mosquito, which has generated a high degree of resistance; hence, new alternatives such as bio-larvicides formulated with plant extracts are of great interest. The aims of this study were to evaluate the ethanolic extract of Azadirachta indica leaves as a larvicide against Aedes aegypti and to determine the main compounds present in it by GC-MS. In the assay, three concentrations of ethanolic extract were used (10 mg L-1, 20 mg L-1, and 50 mg L-1). This was performed thrice against a positive control (commercial larvicide: spores and endotoxic crystals of Bacillus thuringiensis var. israelensis Serotype H-14) and negative control (water). After 72 h of incubation, it was observed higher larval mortality (93%) in the ethanolic extract at a concentration of 50 mg L-1; the extracts at 10 mg L-1 and 20 mg L-1 shown larval mortality of 47% and 70%, respectively. The majority compound determined by the GC-MS analysis was phytol (14.4% area). The results obtained in this study demonstrated the larvicidal potential of the ethanolic extract of A. indica against larvae of A. aegypti.
Aedes aegypti es un mosquito portador del virus del dengue, la fiebre amarilla y otras enfermedades transmitidas a los humanos. Los larvicidas organofosforados se utilizan para controlar la proliferación de este mosquito, el cual ha generado un alto grado de resistencia, por lo que las nuevas alternativas de biolarvicidas formulados con extractos de plantas son de gran interés. Los objetivos de este estudio fueron evaluar el extracto etanólico de hojas de Azadirachta indica como larvicida contra Aedes aegypti e identificar los principales compuestos químicos GC-MS. En el ensayo, se utilizaron tres concentraciones de extracto de etanol (10 mg L-1, 20 mg L-1 y 50 mg L-1), este se realizó por triplicado contra un control positivo (larvicida comercial: Esporas y cristales endotóxicos de Bacillus thuringiensis var. israelensis Serotipo H-14) y un control negativo (agua). Después de 72 horas de incubación, se observó una mayor mortalidad larval (93%) en el extracto etanólico a una concentración de 50 mg L-1, y los extractos a 10 mg L-1 y 20 mg L-1 mostraron una mortalidad larval del 47% y 70% respectivamente. El compuesto con mayor abundancia determinado por el análisis CG-MS fue el fitol (área del 14,4%). Los resultados obtenidos en este estudio demostraron el potencial larvicida del extracto de etanol de A. indica contra las larvas de A. aegypti.
Recibido: 18 de noviembre de 2019; Aceptado: 9 de julio de 2020
ABSTRACT
Aedes aegypti is a mosquito that carries dengue virus, yellow fever and other diseases transmitted to humans. Organophosphorus larvicides are used to control the proliferation of this mosquito, which has generated a high degree of resistance; hence, new alternatives such as bio-larvicides formulated with plant extracts are of great interest. The aims of this study were to evaluate the ethanolic extract of Azadirachta indica leaves as a larvicide against Aedes aegypti and to determine the main compounds present in it by GC-MS. In the assay, three concentrations of ethanolic extract were used (10 mg L-1, 20 mg L-1, and 50 mg L-1). This was performed thrice against a positive control (commercial larvicide: spores and endotoxic crystals of Bacillus thuringiensis var. israelensis Serotype H-14) and negative control (water). After 72 h of incubation, it was observed higher larval mortality (93%) in the ethanolic extract at a concentration of 50 mg L-1; the extracts at 10 mg L-1 and 20 mg L-1 shown larval mortality of 47% and 70%, respectively. The majority compound determined by the GC-MS analysis was phytol (14.4% area). The results obtained in this study demonstrated the larvicidal potential of the ethanolic extract of A. indica against larvae of A. aegypti.
Keywords:
Bio-larvicide, Crude ethanolic extract, GC-MS, Mosquito, Neem, Phytol.RESUMEN
Aedes aegypti es un mosquito portador del virus del dengue, la fiebre amarilla y otras enfermedades transmitidas a los humanos. Los larvicidas organofosforados se utilizan para controlar la proliferación de este mosquito, el cual ha generado un alto grado de resistencia, por lo que las nuevas alternativas de biolarvicidas formulados con extractos de plantas son de gran interés. Los objetivos de este estudio fueron evaluar el extracto etanólico de hojas de Azadirachta indica como larvicida contra Aedes aegypti e identificar los principales compuestos químicos GC-MS. En el ensayo, se utilizaron tres concentraciones de extracto de etanol (10 mg L-1, 20 mg L-1 y 50 mg L-1), este se realizó por triplicado contra un control positivo (larvicida comercial: Esporas y cristales endotóxicos de Bacillus thuringiensis var. israelensis Serotipo H-14) y un control negativo (agua). Después de 72 horas de incubación, se observó una mayor mortalidad larval (93%) en el extracto etanólico a una concentración de 50 mg L-1, y los extractos a 10 mg L-1 y 20 mg L-1 mostraron una mortalidad larval del 47% y 70% respectivamente. El compuesto con mayor abundancia determinado por el análisis CG-MS fue el fitol (área del 14,4%). Los resultados obtenidos en este estudio demostraron el potencial larvicida del extracto de etanol de A. indica contra las larvas de A. aegypti.
Palabras clave:
Biolarvicida, Extracto crudo de etanol, GC-MS, Mosquito, Neem, Fitolhttp://www.revistas.unal.edu.co/index.php/refame.Aedes aegypti, known as mosquito, is widely distributed in America, except Canada and Chile. It is also the vector of several diseases transmitted to humans, including dengue, malaria, and, more recently, chikungunya and zika. Vector-borne diseases are the second group of pathologies with the highest morbidity in Ecuador, led by dengue and chikungunya (Ministerio de Salud Pública, 2015).
Dengue is caused by a virus of the Flaviviridae family, which presents different epidemiological patterns associated with four serotypes that cause this disease (DENV-1, DENV-2, DENV-3, and DENV-4) (WHO, 2020b). It has an alarming impact on human health worldwide due to its easy transportation from one place to another by infected travelers. In 2019, there were more than 3.1 million cases from which 28,000 were severe, and 1,534 ended up in death; currently, around 500 million people are at risk of contracting dengue in Latin America (PAHO, 2020). The WHO has reported several compounds to improve mosquito larvicides, among them are chemical and synthetic organic oils (WHO, 2009). Chemical and biological larvicides are insecticides widely used to kill insects as A. aegypti in the larval life stage and to control the pests. Synthetic organophosphate insecticides (temephos and malathion), and pyrethroids (deltamethrin, lambda-cyhalothrin, and cypermethrin) are used for the control of adults of Aedes aegypti, especially in the emerging stage; however, the pyrethroid and cypermethrin have become ineffective (Vargas-Miranda et al., 2019). Recently, it has been reported that A. aegypti has developed resistance to one of the four classes of insecticides most commonly used for its control (pyrethroids, organochlorines, carbamates, and organophosphates) (PAHO, 2020), and 26 countries have detected resistance to all four classes (WHO, 2020a).
Concerns about environmental pollution and the development of insect resistance to chemical larvicides have stimulated the search for natural insecticides derived from plants (Howard et al., 2010). Larvicides from botanical origin have increased the general interest, like the ones derived from Azadirachta indica (Meliaceae family, commonly known as neem tree), whose insecticidal, pesticidal and larvicidal extracts have been widely studied (Vietmeyer, 1992).
The neem tree is considered one of the most versatile trees in the world, thanks to its ability to grow in areas that reach high temperatures. Its extract composition is considered one of the richest and most complete because of the presence of a great variety of alkaloids, fIavonoids, phenolic compounds, steroids and ketones (Imam et al., 2012). These compounds can be extracted from the entire tree, seeds, stem, fIowers and fruits, varying in concentration depending on the region and the time of year the tree is collected (Fernandes et al., 2019). Extracts of Azadirachta indica mixed with other plant extracts and synthetic products have been evaluated against different mosquitoes, such as Cx. quinquefasciatus, Cx. pipiens, Ae. aegypti, Ae. togoi, and An. stephensi; showing synergistic, additive and antagonistic effects (Shaalan et al., 2005).
Schneider et al. (2017) reported the high potential of neem oil to control pupae and adults of D. saccharalis present in sugarcane. The study conducted by Lin et al. (2016) provided insight into the gene expression of Monochamus alternatus (vector of the destructive forest pest pinewood nematode) at the transcriptional level when subjected to azadirachtin, an active compound of neem, confirming its potential against the pest. This enhances the value of azadirachtin as a potential insecticide of natural origin. Besides, the neem extract is considered a growth regulator insecticide for the control of the lesser mealworm beetle Alphitobius diaperinus (Coleoptera: Tenebrionidae) (Zorzetti et al., 2015). In other applications, Forim et al. (2013) developed a method to prepare nanoparticles loaded with neem (A. indica) extracts, which presented a promissory larvicidal activity against Plutella xylostella with 100% larval mortality.
Azadirachtin's terpenoids and limonoids are the chemicals responsible for the insecticide, larvicide and antibacterial activity, as was reported by Su and Mulla (2003), Ndione et al. (2007) and Liu et al. (2014). The insecticide activity by the limonoids in azadirachtin is related to the direct inhibition of chitin synthesis and changes of pupation and metamorphosis related to the hormone ecdysone (Wandscheer et al., 2004).
Considering the above mentioned, the present study aimed to evaluate the ethanolic extracts of Azadirachta indica leaves, which grows in the Ecuadorian coastal zone, against Aedes aegypti larvae and to identify the compounds present in the extract responsible for the larvicidal activity.
MATERIALS AND METHODS
Plant material
Leaves of A. indica from Guayaquil - Ecuador (2°07'32" S, 79°50'48" W) were collected in February 2015, during the tree fIowering stage. A sample of the plant material was taken for botanical identification, being herborized in the National Herbarium of Ecuador (QCNE), keeping an herbal control (Code: CIBE019) in the Bioproducts laboratory of CIBE-ESPOL, Guayaquil, Ecuador.
Plant extract
The plant extract was obtained according to the procedure described by Maragathavalli et al. (2012). Fresh leaves were dried in an oven with air recirculation for 24 h at 55 °C for subsequent manual milling and sieving, the selected fraction was the one remaining on the 2-mm mesh sieve. Three successive macerations were performed with ethanol at 96% (100 g dry sample per 500 mL of solvent) under stirring at room temperature for 48 h and then filtered. The solvent of the combined maceration was removed by roto-evaporation at 40 °C, and the extracted material was kept at 4 °C until use (Heidolph, 40001). Three samples (100, 200, 300 mg) were dissolved on 100 mL of distilled water, giving an initial concentration of 1, 2, and 5 mg mL-1.
GC-MS analysis
The plant extract was analyzed by GC-MS according to the method of Umar et al. (2014) with some modifications, using Agilent Technologies gas chromatography and mass spectrometry equipment (7890A GC and 5975C XL MSD inert with triple-axis detector). The samples dissolved in distilled water were filtered or centrifuged to remove any insoluble matter. The injection of 2.0 µL of a sample (10 mg mL-1) was performed at 250 °C with no division mode; the detector temperature was 280 °C, and the oven was equipped with an HP-5 capillary column (30 m×0.25 mm ID×0.25 µm film thickness). The GC-MS was programmed with a ramp of 70 °C for 2 min, at a speed of 5 °C min-1 until reaching 285 °C. The carrier fIow of helium was adjusted at a speed of 1.2 mL min-1 to have a run of 45 min. Electronic ionization was set at 70 eV (135 and 230 °C), and the data were collected in full scan mode (40-1000 amu). Lastly, the compounds were identified by comparing their retention index and the Wiley 9th mass spectrum data with the NIST 2011 MS Library.
Larvicidal assay
The Chemistry Faculty of Universidad de Guayaquil provided the A. aegypti larvae. The assay was developed according to the methodology of Wandscheer et al. (2004) and Cruz-Estrada et al. (2013). Three concentrations of neem extract were prepared by diluting 1 mL of the initial extracts (1, 2, and 5 mg mL-1) in 100 mL of water, obtaining final doses of 10 mg L-1, 20 mg L-1, and 50 mg L-1; dose range was selected based on Wandscheer et al. (2004). Ten larvae (III and IV instar stage) were placed in each polyethylene plastic containers with test solutions (100 mL) at a temperature from 25 to 30 °C and 12 h photoperiod. The tests were performed thrice. Negative control (NC) was water, and positive control (PC) was Bactivec (Labiofam), bio-larvicide which active ingredient (0.6%) is Bacillus thuringiensis var. israelensis; for the assay, it was diluted to the recommended application (1% v/v). After 24, 48, and 72 h, the percentage of mortality was registered.
Statistical analysis
Assumptions of normality and homogeneity of variances were corroborated, then the data was submitted to 2 way ANOVA. Tukey's test at a 5% probability was used for the comparison of means. The statistical software use was MINITAB 16.
RESULTS AND DISCUSSION
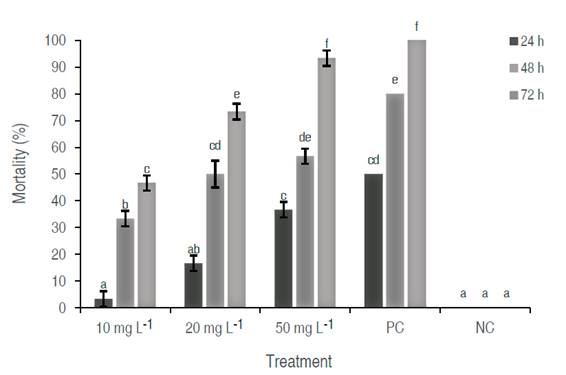
In the comparative study of the percentage of mortality (%) of each extract vs. the exposure time, the mortality was proportional to the exposure time (P<0.05). Among the different concentrations, significant differences were found (P<0.05), by comparing each independent extract, differences between extracts 10 mg L-1 and 20 mg L-1, 10 mg L-1 and 50 mg L-1, 20 mg L-1 and 50 mg L-1 were demonstrated (P<0.05), indicating that a higher concentration greater the mortality over time, observing a dose-dependent behavior. The extract 50 mg L-1 exhibited the highest mortality with values close to the positive control. The mortality percentages of positive control were 50%, 80%, and 100 % at 24, 48, and 72 h, respectively. The negative control had no larvicidal effect, ensuring the results of this study (Figure 1).
Figure 1: Larval mortality (%) of A. aegypti treated with the ethanolic extract of A. indica at different concentrations 10 mg L-1, 20 mg L-1, and 50 mg L-1, and Positve (PC, Bactivec) and Negative (NC, water) Control, observed at 24, 48 and 72 h of exposure. Different lowercase letters indicate significant differences among the concentrations by the Tukey test (P<0.05).
The results of this research demonstrate the larvicidal action of neem extract on larvae of A. aegypti. The best larvicidal activity (mortality 93%) was obtained at 50 mg L-1 of the extract at 72 h. Nour et al. (2012) reported the larvicidal activity against Aedes aegypti mosquitoes' larvae of extracts from different parts of A. indica (leaves, stem, root, and seed), being the leaves the ones that produced the greatest activity. In their study, they reported a larvicidal activity between 60-70% in 48 h using a concentration of 50 mg L-1 of ethanolic A. indica leaf extract, similar to those reported in this study. However, here, the result was superior compared to the ethanolic extract obtained by refIux (50% mortality).
Previous research of larvicidal action of ethanolic extract of A. indica seeds against Aedes aegypti was reported by Wandscheer et al. (2004), obtained an LC50 value of 440 mg L-1. On the other hand, Shaima et al. (2006) reported a larvicidal activity of the methanolic A. indica leaf extract against Anopheles stephensi with LC50 values of 18.2 and 13.1 ppm, after 24 and 48 h, respectively. This demonstrates the larvicidal potential of A. indica extract for different species of mosquitoes.
Differences between larvicidal activities could be attributed to the different methods of drying employed. The oven with recirculating air used in this research could cause thermal degradation of the active compounds present in the leaves, reducing the larvicidal activity of extract compared to those reported by Maragathavalli et al. (2012) for the same species in another geographical ecological environment.
GC-MS analysis
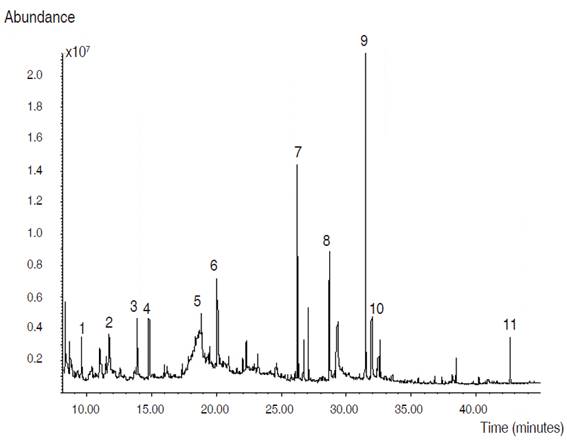
In Figure 2, the gas analytical chromatogram of the compounds identified by GC-MS of the ethanolic extract is shown. The significant component detected in the extract was phytol (14.24%). The presence of terpenes and fatty acids was also reported (Table 1).
Figure 2: Chromatogram of the ethanolic extract of the leaves of Azadirachta indica.
Table 1: Chemical Compounds identified by GC-MS in the ethanolic extract of Azadirachta indica leaves.

The presence of the terpene phytol, as the main component of the ethanolic extract of A. indica is consistent with that reported by Cruz-Estrada et al. (2013). Furthermore, previous reports suggest that phytol extracted from plants have larvicidal activity against mosquitoes (Renjana and Thoppil, 2013). Phytol presented in the leaf extract of Lantara chamber, Azadirachta indica and Ocimum gratissimum possess larvicidal properties against Aedes aegypti and Culex quinquefasciatus (Maneemegalai and Sathish, 2008; Maragathavalli et al., 2012; Pratheeba et al., 2015), and Premna latifolia extract against Aedes albopictus as observed in the studies conducted by Krishnaveni and Ramamurthy (2014). The combination of neophytadiene and phytol has been shown to have higher insecticidal activity than its components alone (Cáceres et al., 2015). Additionally, potent toxicity of 2-Furancarboxaldehyde has been shown against Drosophila melanogaster larvae (Miyazawa et al., 2003). On the other hand, linoleic acid has larvicidal activity against Aedes aegypti with LC50 and LC90 values of 35.39 and 96.33 ppm, respectively (Rahuman et al., 2008), so this compound in the present study could be contributing to larvicidal activity.
According to the results mentioned above may be noted that the neem tree leaves have a high amount of terpenes, which is corroborated by this study where phytol was the most abundant metabolite, a component that confers the insecticide property and possibly the responsible of the reported larvicidal action.
CONCLUSIONS
In this research, it was observed higher larval mortality (93%) after 72 h incubation in the ethanolic extract at a concentration of 50 mg L-1, compared with the extracts at 10 mg L-1 and 20 mg L-1 shown larval mortality of 47% and 70%, respectively. In the analysis by GC-MS, the presence of phytol was evident as a majority component in the ethanolic extract (14.24%).
ACKNOWLEDGEMENTS
The authors thank the Escuela Superior Politecnica del Litoral (ESPOL) for the financial support to perform this study, and Dr. Maria C. Villacrés, PROMETEO-SENESCYT, for helping in the correction of the manuscript.
REFERENCES
References
Cáceres LA, McGarvey BD, Briens C, Berruti F, Yeung KKC and Scott IM, 2015. Insecticidal properties of pyrolysis bio-oil from greenhouse tomato residue biomass. Journal of Analytical and Applied Pyrolysis 112: 333-340. doi: 10.1016/j.jaap.2015.01.003
Cruz-Estrada A, Gamboa-Angulo M, Borges-Argáez R and RuizSánchez E. 2013. Insecticidal effects of plant extracts on immature whitefIy Bemisia tabaci Genn. (Hemiptera: Aleyroideae). Electronic Journal of Biotechnology 16(1): 6. doi: 10.2225/vol16-issue1-fulltext-6
Fernandes SR, Barreiros L, Oliveira RF, Cruz A, Prudêncio C, Oliveira AI, Pinho C, Santos N and Morgado J. 2019. Chemistry, bioactivity, extraction and analysis of azadirachtin: State-of-the-art. Fitoterapia 134: 141-150 doi: 10.1016/j.fitote.2019.02.006
Forim MR, Costa ES, Da Silva MFDGF, Fernandes JB, Mondego JM and Boiça Junior AL. 2013. Development of a new method to prepare nano-/microparticles loaded with extracts of Azadirachta indica, their characterization and use in controlling Plutella xylostella. Journal of Agricultural and Food Chemistry. 61(38): 9131–9139. doi: 10.1021/jf403187y
Imam H, Azad H and Ahmed A. 2012. Neem (Azadirachta indica A. Juss) - A Nature’s Drugstore: An overview. International Research Journal of Biological Sciences 1(6): 76-79.
Krishnaveni S and Ramamurthy V. 2014. Larvicidal efficacy of leaf extracts of Heliotropium indicum and Mukia maderaspatana against the dengue fever mosquito vector Aedes aegypti. Journal of Entomology and Zoology Studies 2(5): 40-45.
Lin T, Liu Q and Chen J. 2016. Identification of differentially expressed genes in Monochamus alternatus digested with azadirachtin. Scientific Reports 6: 33484. doi: 10.1038/srep33484
Liu L, Zhao YL, Cheng GG, Chen YY, Qin XJ, Song CW, Yang XW, Liu YP and Luo XD. 2014. Limonoid and steroidal saponin from Azadirachta indica. Natural Products and Bioprospecting 4: 335-340. doi: 10.1007/s13659-014-0042-2
Maneemegalai S and Sathish M. 2008. Evaluation of larvicidal effect of Lantana camara Linn against mosquito species Aedes aegypti and Culex quinquefasciatus. Advances in Biological Research 2(3-4): 39–43.
Maragathavalli S, Brindha S, Kaviyarasi NS, Annadurai B and Gangwar SK. 2012. Mosquitoes larvicidal activity of leaf extract of Neem (Azadirachta Indica). Advanced Biological Research 2(1): 138-142.
Ministerio de Salud Pública. 2015. Gaceta Epidemiológica Semanal No. 40. Ministerio de Salud Pública, Quito. 48p
Miyazawa M, Anzai J, Fujioka J and Isikawa Y. 2003. Insecticidal compounds against Drosophila melanogaster from Cornus officinalis Sieb. et Zucc. Natural Product Research 17(5): 337–339. doi: 10.1080/1057563031000072587
Ndione R, Faye O, Ndiaye M, Dieye A and Afoutou J. 2007. Toxic effects of neem products (Azadirachta indica A. Juss) on Aedes aegypti Linnaeus 1762 larvae. African Journal of Biotechnology 6(24): 2846-2854.
Nour AH, Sandanasamy JDO and Nour AH. 2012. Larvicidal activity of extracts from different parts of Neem (Azadirachta indica) against Aedes aegypti mosquitoes’ larvae. Scientific Research and Essays 7(31): 2810-2815. doi: 10.5897/SRE12.133
PAHO – Pan American Health Organization. 2020. Dengue. In https://www.paho.org/es/temas/dengue Accessed: August 2020.
Pratheeba T, Ragavendran C and Natarajan D 2015. Larvicidal, pupicidal and adulticidal potential of Ocimum gratissimum plant leaf extracts against filariasis inducing vector. International Journal of Mosquito Research 2(2): 1–8.
Rahuman AA, Venkatesan P and Gopalakrishnan G, 2008. Mosquito larvicidal activity of oleic and linoleic acids isolated from Citrullus colocynthis (Linn.) Schrad. Parasitology Research 103(6): 1383–1390. doi: 10.1007/s00436-008-1146-6
Renjana PK and Thoppil JE. 2013. Larvicidal activities of the leaf extracts and essential oil of Premna latifolia Roxb. (Verbenaceae) against Aedes albopictus Skuse (Diptera: Culicidae). Journal of Applied Pharmaceutical Science 3(6): 101–105. doi: 10.7324/JAPS.2013.3616
Schneider LCL, Silva CV and Conte H. 2017. Toxic effect of commercial formulations of neem oil, Azadirachta indica A. Juss., in pupae and adults of the sugarcane borer, Diatraea saccharalis F. (Lepidoptera: Crambidae). Arquivos do Instituto Biológico 84: e0432014. doi: 10.1590/1808-1657000432014
Shaalan EAS, Canyon D, Younes MWF, Abdel-Wahab H and Mansour AH. 2005. A review of botanical phytochemicals with mosquitocidal potential. Environment International 31(8): 1149-1166. doi: 10.1016/j.envint.2005.03.003
Shamia P, Mohan L and Srivastava CN. 2006. Impact Analysis of Neem Kernel Extracts on the Developmental Profile of Anopheles stephensi. Journal of Asia-Pacific Entomology 9(1): 11-17. doi: 10.1016/S1226-8615(08)60270-8
Su T and Mulla MS. 2003. Oviposition bioassay responses of Culex tarsalis and Culex quinquefasciatus to neem products containing azadirachtin. Entomologia Experimentalis et Applicata 91(2): 337-345. doi: 10.1046/j.1570-7458.1999.00500.x
Umar MI, Asmawi MZ, Sadikun A, Abdul Majid AMS, Atangwho IJ Item M, Khadeer Ahamed MB, Altaf R and Ahmad A. 2014. Multiconstituent synergism is responsible for anti-infIammatory effect of Azadirachta indica leaf extract. Pharmaceutical Biology 52(11):1-12. doi: 10.3109/13880209.2014.895017
Vargas-Miranda K, Troyo A y Calderón-Arguedas Ó. 2019. Resistencia de Aedes aegypti (Diptera: Culicidae) a insecticidas organofosforados y piretroides en la localidad de Orotina, Alajuela, Costa Rica. Revista Costarricense de Salud Pública 28(1): 15-24.
Vietmeyer ND. 1992. Neem: a tree for solving global problems. Report of an ad hoc panel of the Board on Science and Technology for International Development. National Research Council. National Academy Press, Washington DC.
Wandscheer CB, Duque JE, Da Silva MAN, Fukuyama Y, Wohlke JL, Adelmann J and Fontana JD. 2004. Larvicidal action of ethanolic extracts from fruit endocarps of Melia azedarach and Azadirachta indica against the dengue mosquito Aedes aegypti. Toxicon 44(8): 829-835. doi: 10.1016/j.toxicon.2004.07.009
WHO – World Health Organization. 2009. Dengue Guidelines For Diagnosis, Treatment, Prevention And Control: new edition. WHO Press, Geneva. 147 p.
WHO – World Health Organization. 2020a. Malaria. Insecticide resistance. In. http://www.who.int/malaria/areas/vector_control/insecticide_resistance/en/ Accessed: August, 2020. WHO – World Health Organization. 2020b. Dengue and severe dengue. In: https://www.who.int/news-room/fact-sheets/detail/dengueand-severe-dengue Accessed: August, 2020.
Zorzetti J, Constanski K, Santoro PH, Fonseca ICB and Neves PMOJ. 2015. Growth regulator insecticides for the control of the lesser mealworm beetle Alphitobius diaperinus (Coleoptera: Tenebrionidae). Revista Colombiana de Entomología 41(1): 24–32.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Anmut Assemie, Temam Gemeda, Kazim Husain. (2023). Larvicidal Activities of Allium sativum L. and Zingiber officinale Rosc. Extracts against Filariasis Vectors in Hadiya Zone, Ethiopia. BioMed Research International, 2023(1) https://doi.org/10.1155/2023/6636837.
2. Gabriel Goetten de Lima, Tainá Wilke Sivek, Mailson Matos, Emanoela Lundgren Thá, Ketelen Michele Guilherme de Oliveira, Irisdoris Rodrigues de Souza, Tielidy Angelina de Morais de Lima, Marta Margarete Cestari, Washington Luiz Esteves Magalhães, Fabrício Augusto Hansel, Daniela Morais Leme. (2022). A biocide delivery system composed of nanosilica loaded with neem oil is effective in reducing plant toxicity of this biocide. Environmental Pollution, 294, p.118660. https://doi.org/10.1016/j.envpol.2021.118660.
3. Nina Difla Muflikhah. (2023). Larvacidal Activity of the Mulberry (Morus alba L.) Leaf Extract Against Larvae of Aedes aegypti. Indonesian Journal of Tropical and Infectious Disease, 11(2) https://doi.org/10.20473/ijtid.v11i2.37328.
4. Asrianto Asrianto, Suarna Samai, Muhamad Sahidin, Indra Taufik Sahli, Risda Hartati, Wiwiek Mulyani. (2025). Efikasi Biolarvasida Berbagai Tanaman Untuk Pengendalian Vektor Nyamuk Anopheles. Jurnal Sains dan Kesehatan, 5(2), p.224. https://doi.org/10.30872/jsk.v5i2.p224-235.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2020 Revista Facultad Nacional de Agronomía Medellín, Autores

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.