Correlations and path analysis between fruit characteristics and seeds of Pachira aquatica Aubl.
Correlaciones y análisis de sendero entre caracteres del fruto y semillas de Pachira aquatica Aubl.
DOI:
https://doi.org/10.15446/rfna.v71n1.67027Keywords:
Ornamental tree, Indirect selection, Forest genetic breeding, Genetic resources (en)Árbol ornamental, Selección indirecta, Mejoramiento genético forestal, Recursos genéticos (es)
Pachira aquatica Aubl. is an important fruit-ornamental tree of Sinú valley at risk of genetic erosion. The objective was to estimate correlations between fruit characteristics, seeds, and path analyses
for fresh fruit weight, for the purpose of genetic improvement. The treatments corresponded to 10 trees; 10 random fruits of each tree were taken. Analysis of variance were performed for fixed effects
models, correlations and path analysis among the variables: fresh fruit weight, fruit length, fruit peel weight, number of seed per fruit, weight of seed per fruit and weight and volume and density of a seed. Statistical differences between trees were presented for all the characteristics, except for volume of one seed. Genetic correlations were greater in magnitude (32.8%) and statistical significance than
phenotypic ones (29.7%). Seed weight showed high genetic correlation with seven characteristics (rG>0.53). The fresh fruit weight showed significant and direct genetic correlations with five variables, with correlation coefficients between 0.65* and 1.00**. The indirect effects explained the level of association between fruit length, seed/fruit weight, and percentage of seed/fruit with fresh fruit weight. The weight of peel/fruit exhibited greater direct and indirect effects on the fresh fruit weight in the two path analysis. It is possible to increase the weight of seed/fruit, weight and volume of a seed indirectly through the selection of trees with higher fresh fruit weight and/or peel/fruit weight, to improve yield and physiological quality of the seed.
Pachira aquatica Aubl. es un árbol frutal-ornamental importante del Valle del Sinú con riesgo de erosión genética. El objetivo fue estimar correlaciones entre características del fruto, semillas, y análisis de sendero para peso de fruto, con fines de mejoramiento genético. Los tratamientos correspondieron a 10 árboles; de cada uno se tomaron 10 frutos al azar. Se realizaron análisis de varianza para modelos de efectos fijos, correlaciones y análisis de sendero entre las variables: peso de fruto, largo de fruto, peso de cáscara del fruto, número de semillas/fruto, peso de semillas/fruto, porcentaje de semilla/fruto, y peso, volumen y densidad de una semilla. Se presentaron diferencias entre árboles en todas las características, excepto para volumen de una semilla. Las correlaciones genéticas resultaron mayores en magnitud (32,8%) y significancia estadística que las fenotípicas (29,7%). El peso de una semilla acusó alta correlación genética con siete características (rG>0,53). El peso fresco de fruto mostró correlaciones genéticas significativas y directas con cinco variables, con coeficientes de correlación entre 0,65* y 1,00**. Los efectos indirectos explicaron el nivel de asociación entre los caracteres largo de fruto, peso de semillas/fruto y porcentaje de semilla/fruto con peso fresco de fruto. El peso de cáscara/fruto exhibió mayores efectos directos e indirectos sobre el peso fresco del fruto en los análisis de sendero. Es posible aumentar el peso de semillas/fruto, peso y volumen de una semilla indirectamente, al seleccionar árboles con mayor peso fresco de fruto y/o peso de cáscara/fruto, para mejorar rendimiento y calidad fisiológica de la semilla.
Recibido: 8 de julio de 2017; Aceptado: 27 de octubre de 2017
ABSTRACT
Pachira aquatica Aubl. is an important fruit-ornamental tree of Sinú valley at risk of genetic erosion. The objective of this work was to estimate correlations between fruit characteristics, seeds, and path analyses for fresh fruit weight, for the purpose of genetic improvement. The treatments corresponded to 10 trees; 10 random fruits of each tree were taken. Analysis of variance were performed for fixed effects models, correlations and path analysis among the variables: fresh fruit weight, fruit length, fruit peel weight, number of seed per fruit, weight of seed per fruit and weight and volume and density of a seed. Statistical differences between trees were presented for all the characteristics, except for volume of one seed. Genetic correlations were greater in magnitude (32.8%) and statistical significance than phenotypic ones (29.7%). Seed weight showed high genetic correlation with seven characteristics (rG>0.53). The fresh fruit weight showed significant and direct genetic correlations with five variables, with correlation coefficients between 0.65* and 1.00**. The indirect effects explained the level of association between fruit length, seed/fruit weight, and percentage of seed/fruit with fresh fruit weight. The weight of peel/fruit exhibited greater direct and indirect effects on the fresh fruit weight in the two path analysis. It is possible to increase the weight of seed/fruit, weight and volume of a seed indirectly through the selection of trees with higher fresh fruit weight and/or peel/fruit weight, to improve yield and physiological quality of the seed.
Keywords:
Ornamental tree, Indirect selection, Forest genetic breeding, Genetic resources.RESUMEN
Pachira aquatica Aubl. es un árbol frutal-ornamental importante del Valle del Sinú con riesgo de erosión genética. El objetivo de este trabajo fue estimar correlaciones entre características del fruto, semillas, y análisis de sendero para peso de fruto, con fines de mejoramiento genético. Los tratamientos correspondieron a 10 árboles; de cada uno se tomaron 10 frutos al azar. Se realizaron análisis de varianza para modelos de efectos fijos, correlaciones y análisis de sendero entre las variables: peso de fruto, largo de fruto, peso de cáscara del fruto, número de semillas/fruto, peso de semillas/fruto, porcentaje de semilla/fruto, y peso, volumen y densidad de una semilla. Se presentaron diferencias entre árboles en todas las características, excepto para volumen de una semilla. Las correlaciones genéticas resultaron mayores en magnitud (32,8%) y significancia estadística que las fenotípicas (29,7%). El peso de una semilla acusó alta correlación genética con siete características (rG>0,53). El peso fresco de fruto mostró correlaciones genéticas significativas y directas con cinco variables, con coeficientes de correlación entre 0,65* y 1,00**. Los efectos indirectos explicaron el nivel de asociación entre los caracteres largo de fruto, peso de semillas/fruto y porcentaje de semilla/fruto con peso fresco de fruto. El peso de cáscara/fruto exhibió mayores efectos directos e indirectos sobre el peso fresco del fruto en los análisis de sendero. Es posible aumentar el peso de semillas/fruto, peso y volumen de una semilla indirectamente, al seleccionar árboles con mayor peso fresco de fruto y/o peso de cáscara/fruto, para mejorar rendimiento y calidad fisiológica de la semilla.
Palabras clave:
Árbol ornamental, Selección indirecta, Mejoramiento genético forestal, Recursos genéticos.Paquira aquatica is a tropical tree known as ‘cacao de monte’. It is important because of its potential use in the restoration of wetlands, degraded soils and forests (Hernández-Montero and Sosa, 2016) and as a fruit-ornamental tree (Li et al., 2009). Fast-growing, dominant native and characteristic of freshwater wetlands vegetation from Mexico to northern Brazil (Infante-Mata et al., 2011). In Colombia, the wide diversity of endemic, native, wild and exotic species of shrub and forest of interest have been little researched (Rivera-Martin et al., 2013). The research on P. aquatica has focused mainly on the morphology of the fruit, seeds, germination and seedlings (De Oliveira et al., 2007; Silva et al., 2012a), effects of light intensity and paclobutrazol on tree growth (Hernández-Montero and Sosa, 2016), characterization of seed oil (Jorge and Luzia, 2012), potential fungicidal effect of seed (Souza et al., 2014) and floral morphometric variation (Ramírez et al., 2010), among others. However, no studies were found related to the correlations and path analysis between the biometric characters of the fruit and the seeds of this species.
In the department of Córdoba, Colombia, there are no reports of P. aquatica in natural or planted populations. Only isolated and dispersed trees have been observed in urban, peri-urban or rural areas, which have grown and developed spontaneously due to the dispersal of their seeds and seedlings by animals and humans. As a plant genetic resource, an increase in its use as a fruit-ornamental tree is still to be seen.
In genetic improvement programs, the knowledge of the magnitude, meaning, and nature of correlations, as well as path analysis (Cruz, 2006; Cruz and Regazzi, 1997), are necessary to increase the efficiency of selection and estimate the genetic progress for several desirable traits (Shafique et al., 2016, Marcal et al., 2015, Salla et al., 2015). In addition, it is indispensable for the understanding of the mechanisms of autecology, reproduction, dispersion, succession, natural regeneration, collection and silvicultural practices (Machado et al., 2016; Rodrigues et al., 2015).
The objective of this study was to estimate the correlations between the biometric characteristics of the fruit and the seeds, as well as the direct and indirect causal effects of such variables with respect to the weight of the fruit in P. aquatica Aubl.
MATERIALS AND METHODS
Location
The study was carried out in 2016, in the plant breeding laboratory of the Universidad de Córdoba, located in Montería-Córdoba, Colombia, in the middle of the Sinú Valley, at 8°52'N latitude and 76°48'W longitude at a altitude of 13 m (Palencia et al., 2006). The 10 trees of P. aquatica are located in the urban and peri-urban area of the same city.
Plant material
Ten trees of P. aquatica were taken; from each one random samples of 10 fruits of free pollination were selected, for a total of 100 fruits. The trees were from 5 to 15 m tall, 10 to 25 old.
Evaluated traits
Nine biometric characteristics of fruit and seed were measured: fresh fruit weight (FFW), fruit length (FL), fruit peel weight (FPW), number of seeds per fruit (NSF), weight of seeds per fruit (WSF), percentage of seeds per fruit (PSF), weight of a seed (SW), volume of a seed (SV) and density of a seed (SD).
FFW, FPW and WSF were measured in grams with a precision balance; while FL and NSF, in centimeters and units, respectively. The PSF was calculated as the WSF/FFW ratio, expressed as a percentage. The SW as the average in grams of a sample of five seeds taken at random from each fruit. The SV in milliliters, as the average volume of distilled water displaced by a sample of five seeds taken at random from each fruit, and the SD by the ratio (SW/SV).
Statistical analyses
Ten trees (treatments) were chosen for availability and 10 fruits (repetitions) were randomly picked from each tree, variance analysis (fixed effects model) were performed for the nine response variables. In addition, estimates of the coefficients of phenotypic, genotypic and pathway correlations were made with the GENES software version Windows GENES V.2016.6.0 (Cruz, 2016). Once the correlation coefficients "r" were estimated, the statistical significance was tested by the t test (Steel and Torrie, 1980) at significance levels of 0.05 and 0.01.
Two path analyses were performed for the system: FFW as variable effect (Y) in function of the causes variables: FL (X1), FPW (X2), NSF (X3), WSF (X4) and PSF (X5). The two path analyses were originated with the matrices of phenotypic and genotypic correlations between such variables.
For the estimation of the direct effects in each path analysis, GENES software uses a correlation matrix (phenotypic or genetic: according to the interest), decomposes it and organizes it into P = A-1 R, where: A-1 is the inverse of the correlation matrix (between each of the cause variables), R is the vector of correlation coefficients between the cause variables with the effect variable, and P is the vector path coefficients.
The decomposition of the correlation coefficients of each of the causal variables with the effect variable (rxiy; i = 1,2, …,5) in its components: direct effect (Pi) and the indirect effect (Ei), allows the estimation of the indirect effects of each cause variable (Ei), from each of the following equations:
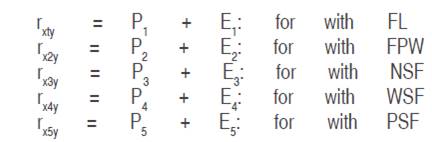
The path coefficient due to residual effects or other variables not considered in the study (h) is estimated by the following equation:

RESULTS AND DISCUSSION
Analysis of variance and average values
The analysis of variance and average values for the nine characteristics of the P. aquatica fruit appear in Table 1. Except for weight of a seed (SW), the table shows significant statistical differences (P<0.05 and highly significant P<0.01) among the 10 trees, for the variables: fresh fruit weight (FFW), fruit length (FL), fruit peel weight (FPW), number of seeds per fruit (NSF), weight of seeds per fruit (WSF), percentage of seeds per fruit (PSF), volume of a seed (SV) and density of a seed (SD), which shows genetic differences between trees, a consistent result with the reported by Silva et al. (2012a) en P. aquatica and in agreement with the concepts of Pereira et al. (2011) in Hymenaea stigonocarpa, with different number of tree, who point out that tropical trees show great variability in the mentioned characteristics.
Table 1: Mean values and levels of statistical significance of the analysis of variance for nine biometric characteristics of the fruit and seeds of P. aquatica trees.
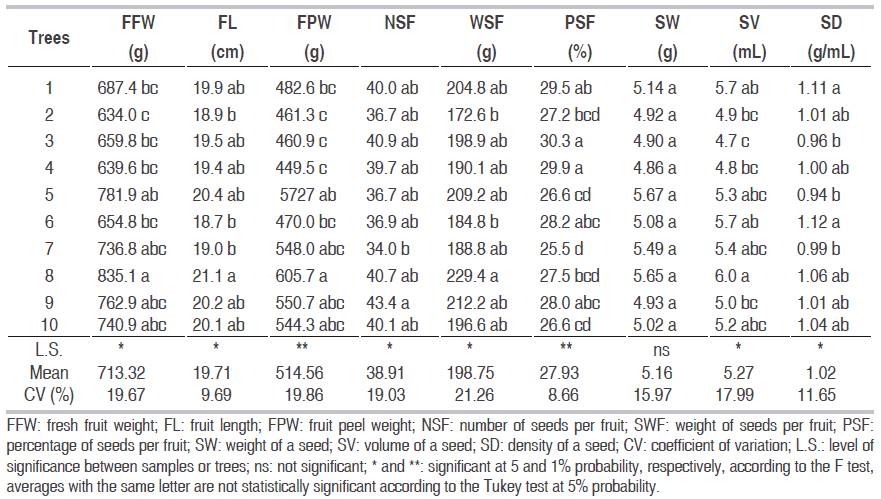
The mean of FFW and FPW of tree number eight was significantly higher than those of trees one, two, three and four, but similar to those of trees five, nine and ten. The FL of tree eight exceeded that of trees two, six and seven, but was similar to that of the remaining ones. The SNF presented only significant difference between tree seven and tree nine. The WSF of tree eight only exceeded that of trees two and six. The PSF of trees three and four was superior to that of trees two, five, seven, eight and ten. The SD of tree six was greater than that of trees three and five. The averages presented in Table 1 are superior to those found by De Oliveira et al. (2007) and Silva et al. (2012a) in Brazil, who reported 317 g, 13 cm, 39 and 12.5 g, for FFW, FL, NSF, SW, respectively (differences in fertility, precipitation and pollination, among others). This was expressed in a greater filling of fruits and seeds as indicated by Hernández-Montero and Sosa (2016). Also, the genotype x environment interaction, which was due to the location of the trees in different places of the same selection area. The phenotypic variability presented at the average level and the low coefficients of variation obtained (<21.26%) demonstrate the reliability of the results and corroborates the statements by Balcorta-Martínez and Vargas-Hernández (2004), in Gmelina arborea, when their coefficients of variation are less than 25%.
Phenotypic and genotypic correlations
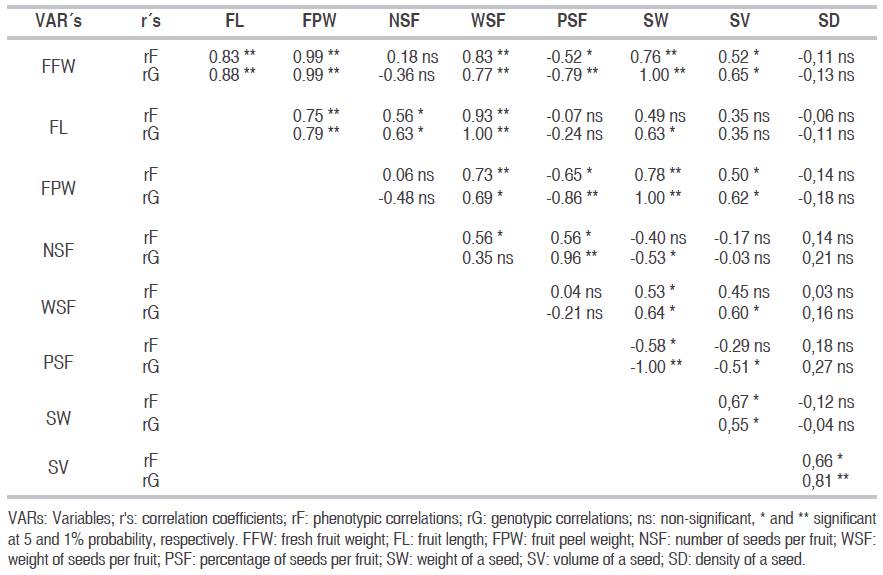
Only 19 phenotypic correlations (29.7%) and 21 genetic correlations (32.8%) were significant at 1% or 5% probability, either directly or inversely (Table 2). Positive degrees of association were more numerous in both types of correlations. Significant genetic correlations were estimated between pairs of variables among which there were no significant phenotypic correlations: SW with FL and NSF and SV with WSF. This is explained by a moderate influence of environmental factors and/or non-additive genetic factors, which negatively affect the true expression of the level of correlation between the variables (Binotto et al., 2010; Marcal et al., 2015).
Table 2: Phenotypic (rF) and genetic (rG) correlations between nine biometric characteristics of the fruit and seeds of P. aquatica.
These results partially agree with those reported by Marcal et al. (2015), Cardona-Medina and Muriel (2015) and corroborated by Cruz (2006) in the sense that the phenotypic correlations have little practical value and can lead to errors, considering genetic as environmental effects, which according to these authors can lead to an inefficient selection due to the masking effect of the environment.
The FFW simultaneously exhibited significant phenotypic and genetic correlations with six variables, while SW correlated with other genetic variables except SD. The correlation coefficients were positive and fluctuated between 0.55* with DS and 1.00** with FFW, PSF and FPW; while the correlation with NSF and PSF was negative, with levels of genetic association of -0.53* and -1.00**, respectively. On the other hand, SV presented significant and direct correlations with SW, WSF, FPW and FFW (rG>0.55*) and inverse with PSF (rG=-0.51*), whereas with FL and NSF, the correlation was not significant. The FFW showed significant and direct genetic correlations with FL, FPW, WSF, SW and SV, with coefficients ranging from 0.65* (SV) to 1.00** (SW), while the correlation with PSF was significant and inverse (rG=-0.79**). The variable with the lowest degree of association with others was SD, but had a high, significant and direct genetic correlation with SV (rG=0.81**); these results are consistent with those reported by Marcal et al. (2015) and Cardona-Medina and Muriel (2015).
According to Vallejo et al. (2010), the significant and positive correlation between two variables (SW with SV, FFW, PSF and FPW; SV with SW, WSF, FPW and FFW; FFW with FL, FPW, WSF, SW and SV) means that when one increases or decreases, the other does it in the same sense. In contrast, the significant and inverse correlation between two variables (SW with NSF and PSF, SV with PSF, FFW with PSF) indicates that when one increases or decreases, the other does it in the opposite sense. These genetic correlations suggest that the control of the associated variables is given by one gene with pleiotropic effects or by several genes that are linked. The absence of genetic association between two characters (SW with SD, SV with FL and NSF, FFW with NSF and SD, etc.) shows that the genetic control of such variables is given by genes. These genes act independently and by other variables that mask the true level of correlation between these characters, as was reported by Marcal et al. (2015) and Cardona-Medina and Muriel (2015). The genetic correlation of the SW with the other characteristics of the fruit, allows to improve the seed physiological quality and the selection of trees with heavier seeds. The absence of significant genetic correlation between FFW and NSF (rG=-0.36) is not very common because the number of seeds per fruit (NSF), being one of the important primary components of fresh fruit weight (FFW), was expected with a high, direct and significant genetic correlation, similar to what happened with fruit peel weight (FPW). This may be due to indirect masked effects of other variables on the actual level of association between them (Araméndiz et al., 2010).
The magnitude, sense, and significance of the genetic correlations between FFW with FL, FPW, PSF, SW and SV (with rG>0.65*) are important in the process of selection, management and conservation of seeds, due to the fact that it is possible to select trees with high PSF, SW and SV indirectly through higher FFW and/or FL. These are much easier to measure in less time and cost, than PSF, SW and SV, which are more difficult and expensive to estimate in fruit or forest trees for seed consumption and/or seedling production in nurseries. This is especially true of native species, where low germination rates, recalcitrant seeds and dormancy problems are generally present, which require pre-germination treatments to achieve high germination. Silva et al. (2012b) and Barboza-Nogueira et al. (2014) have reported similar results.
The value and level of the genetic correlations estimated between the characteristics of the fruit and its seeds allows inferring that the selection of trees with higher FFW and/or FL, allows an increase of the production in terms of WSF, SW and SV. This situation is very advantageous for the breeder and the producer, since their interest is the selection of genotypes of high seed production (Souza et al., 2014) and species survival (Hernández-Montero and Sosa, 2016).
The size and weight of the seeds of the same tree are usually associated with a higher percentage of germination and seedling performance. Large and heavy seeds contain higher amounts of carbohydrates in the endosperm or cotyledons, and are available as a source of energy to stimulate seedling germination, emergence, survival and growth (Pereira et al., 2011). These correlations have been reported by Huerta-Paniagua and Rodríguez-Trejo (2011). However, other studies do not report such associations (Binotto et al., 2010) and may vary between species and between regions (Pereira et al., 2011).
Falconer and Mackay (1996) point out that the difference between genetic correlations for the same group of variables, even in the same species, are explained by an influence of environmental factors and/or non-additive genetic factors (genetic interactions) which underestimate the true expression of the level of correlation between the variables studied, since these environmental factors can affect the variables under study through the same or different physiological mechanisms.
Path analysis for fresh fruit weight
The decomposition of the phenotypic and genetic correlations of FFW (variable effect) was performed for several models that included different sets of cause variables (primary and secondary components of the FFW). The estimates of the determination coefficients (R2) and the residual effects (h) for each model allowed the inclusion of FL, FPW, NSF, WSF and PSF as causes or explanatory variables (Table 3).
Table 3: Analysis of the direct (diagonal in bold) and indirect effects of the phenotypic and genotypic correlations of FL, WPF, NSF, WSF, PSF, on the FFW of P. aquatica.
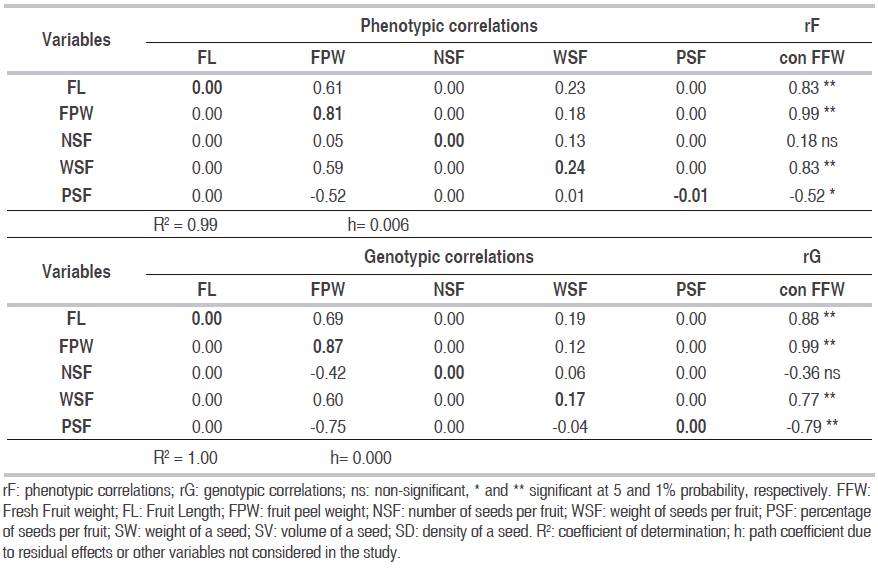
The R2 in the two path analysis was between 99% and 100%, with phenotypic and genetic correlations, which is excellent and indicates the importance of the primary components in the definition of FFW. There is clear tendency of the model to improve the explanation of FFW as a function of FL, FPW, NSF, WSF and PSF, with the analysis of genetic correlations (R2=1.00). Likewise, there is a trend in the definition of direct and indirect effects for each of the associations which is slightly higher when using genetic correlations, because the effects of non-additive environmental and genetic factors are not considered, which maximizes the expression of the true relationship between the explanatory variables FL, FPW, NSF, WSF and PSF and the variable effect of the model FFW.
The results in both types of correlation are very similar in terms of the importance of the indirect effects on the direct ones in the explanatory variables, except for FPW, since the indirect effects are those that mainly explain the degree of association between the characters FL, WSF and PSF with FFW, in the two path analysis. The degree and sense of correlation between FL and FFW at phenotypic (0.83**) and genetic (0.88**) levels is explained indirectly through the FPW (0.61 and 0.69), in 73.5% and 78.4%, respectively. Likewise, the magnitude and direction of the correlation between WSF and FFW at the phenotypic (0.83**) and genetic (0.77**) level is supported by indirect effect through the FPW (0.59 and 0.60), by 71.1% and 77.9%, respectively. We found a similar situation for the magnitude and direction of the correlation between PSF and FFW at the phenotypic (-0.52*) and genetic (-0.79**) levels, which is explained by indirect effect through the FPW (-0.52 and -0.75), in 100% and 94.9%, respectively. The direct effects were only important to explain the degree of correlation between FPW and FFW (0.99**), both in path analysis with phenotypic correlations (0.81) and with genetic correlations (0.87), this explains the two levels of association in 81.8% and 87.9%, respectively, confirming the direct association between these two variables.
The FPW exhibited greater direct and indirect effects on the FFW, in comparison with the other cause variables in the two types of path analysis, which is consistent with the reports of Salla et al. (2015) and Marcal et al. (2015).
It can be inferred that FFW and WPF can be used as selection criteria in the genetic improvement of P. aquatica to obtain trees and/or clones with higher production of WSF, SW and SV. This is favorable to the breeder and producer in the selection of genotypes of high seed production (Souza et al., 2014) and vigorous seedlings (Hernández-Montero and Sosa, 2016). However, it is necessary to consider the constraint imposed by the inverse relationship between PSF and FFW, since it is possible to obtain genotypes with higher FFW, but with lower PSF, which is a disadvantage when it is desired to increase the physiological efficiency in the production of seeds.
CONCLUSIONS
Differences in fruit biometric characteristics on ten trees showed genetic variability among P. aquatica trees, which can be used in the genetic improvement of the species.
The FFW showed significant and direct genetic correlations with FL, FPW, WSF, SW and SV, with coefficients ranging from 0.65* (SV) to 1.00** (SW), while the correlation with PSF was significant and inverse (rG = -0.79**). This allows selecting a character of interest through of another of easier expression.
The FPW exhibited the greatest direct and indirect effects on the FFW in the two types of path analyses; therefore it is possible to increase the FPW, SW and SV indirectly through the selection of trees with higher FPW and/or FFW, to improve the yield and physiological quality of the seed.
REFERENCES
References
Araméndiz H, Espitia M y Cardona C. 2010. Análisis de sendero en berenjena (Solanum melongena L.). Revista U.D.C.A Actualidad & Divulgación Científica 13(1): 115-123.
Balcorta-Martínez HC, Vargas-Hernández JJ. 2004. Variación fenotípica y selección de árboles en una plantación de melina (Gmelina arbórea Linn., Roxb.) de tres años de edad. Revista Chapingo Serie Ciencias Forestales y del Ambiente 9(2): 13-19.
Barboza-Nogueira FC, Lobo-Pinheiro CH, Medeiros-Filho S and Da Silva Matos DM. 2014. Seed germination and seedling development of Anadenanthera colubrina in response to weight and temperature conditions. Journal of Plant Sciences 2(1): 37-42. doi: 10.11648/j.jps.20140201.17.
Binotto AF, Col-Lúcio AD and Lopes SJ. 2010. Correlations between growth variables and the dickson quality index in forest seedlings. Cerne 16(4): 457-464. doi: 10.1590/S0104-77602010000400005.
Cardona-Medina E and Muriel SB. 2015. Seed germination and plant development in Escobedia grandiflora (Orobanchae): Evidence of obligate hemiparasitism? Acta Biológica Colombiana 20(3): 133-140. doi: 10.15446/abc.v20n2.43776.
Cruz CD e Regazzi AJ. 1997. Modelos Biométricos Aplicados ao Melhoramento Genético. 2da. ed. Editora UFV. Brasil. p.390.
Cruz CD. 2006. Programa Genes: Biometria. Editora UFV. Viçosa (MG). p.382.
Cruz CD. 2016. Programa Genes V.2016.6.0 – Aplicativo computacional em genética e estatística. http://www.ufv.br/dbg/genes/genes.htm;accessed: September 2016.
De Oliveira LZ, Cesarino F, Moro FV, Pantoja, TDF e Silva BMDS. 2007. Morfologia do fruto, da semente, germinação e plántula de Pachira aquatica Aubl. (Bombacaceae). Revista Brasileira de Biociências 5(suplemento 1): 840-842.
Falconer DS and Mackay T. 1996. Introduction to Quantitative Genetics. Fourth edition. Prentice Hall. p.464.
Hernández-Montero JR and Sosa VJ. 2016. Reproductive biology of Pachira aquatica Aubl. (Malvaceae: Bombacoideae): a tropical tree pollinated by bats, sphingid moths and honey bees. Plant Species Biology 31(2): 125–134. doi: 10.1111/1442-1984.12096.
Huerta-Paniagua R y Rodríguez-Trejo D. 2011. Efecto del tamaño de semilla y la temperatura en la germinación de Quercus rugosa Née, Revista Chapingo Serie Ciencias Forestales y del Ambiente 17(2): 179-187. doi: 10.5154/r.rchscfa.2010.08.053.
Infante-Mata D, Moreno-Casasola P, Madero-Vega C, Castillo-Campos G and Warner BG. 2011. Floristic composition and soil characteristics of tropical freshwater forested wetlands of Veracruz on the coastal plain of the Gulf of Mexico. Forest Ecology and Management 262(1): 1514–1531. doi: 10.1016/j.foreco.2011.06.053.
Jorge N y Luzia DMM. 2012. Caracterização do óleo das sementes de Pachira aquatica Aublet para aproveitamento alimentar. Acta Amazônica, Manaus 42(1): 149-156. doi: 10.1590/S0044-59672012000100017.
Li Q, Deng M, Chen J and Henny RJ. 2009. Effects of Light Intensity and Paclobutrazol on Growth and Interior Performance of Pachira aquatica Aubl. Hortscience 44(5): 1291–1295.
Machado EL, Silva SA, Fernandes LDS e Brasileiro HS. 2016. Variabilidade genética e homozigose em uma população F4 de mamoneira por meio de marcadores microssatélites. Bragantia 75(3): 307-313. doi: 10.1590/1678-4499.536.
Marcal TS, Ferreira A, Dos Santos WBO, Soler JHG e Da Silva MFF. 2015. Correlações genéticas e análise de trilha para caracteres de fruto da palmeira juçara. Revista Brasileira de Fruticultura 37(3): 692-698. doi: 10.1590/0100-2945-163/14.
Palencia G, Mercado T y Combatt E. 2006. Estudio agroclimático del departamento de Córdoba. Facultad de Ciencias Agrícolas. Ed. UNICOR. P.126.
Pereira SR, Giraldelli GR, Laura VA e De Souza AL. 2011. Tamanho de frutos e de sementes e sua influência na germinação de jatobá-do-cerrado (Hymenaea stigonocarpa var. stigonocarpa ex Hayne, Leguminosae – Caesalpinoideae). Revista Brasileira de Sementes 33(1): 141–148. doi: 10.1590/S0101-31222011000100017.
Ramírez N, Nasar JM, Valera L, Garay V, Briceño, H, Quijada M, Moret YA, Montilla J. 2010. Variación morfométrica floral en Pachira quinata (Jacq.) W. Alverson (Bombacaceae). Acta Botánica Venezuelica 33(1): 83-102.
Rivera-Martin LE, Peñuela-Mora MC, Jiménez-Rojas EM, Vargas-Jaramillo M. 2013. Ecología y silvicultura de especies útiles amazónicas: Abarco (Cariniana micrantha Ducke), Quinilla (Manilkara bidentata (A. DC.) A. Chev.) y violeta (Peltogyne paniculata Benth). Universidad Nacional de Colombia (Sede Amazonas - Colombia) – Inst. Amazónico de Investigaciones, IMANI. 180p.
Rodrigues JK, Mendonca MS e Gentil DFO. 2015. Aspectos biométricos, morfoanatômicos e histoquímicos do pirênio de Bactris maraja (Arecaceae). Rodriguésia 66(1): 75-85. doi: 10.1590/2175-7860201566105.
Salla VP, Danner MA, Citadin I. Zolet SAS, Donazzolo J e Gil BV. 2015. Análise de trilha em caracteres de frutos de jabuticabeira. Pesquisa Agropecuária Brasileira 50(3): 218-223. doi: 10.1590/S0100-204X2015000300005.
Shafique MS, Ahsan M, Mehmood Z, Abdullah M, Shakoor A. and Ahmad MI. 2016. Genetic variability and interrelationship of various agronomic traits using correlation and path analysis in Chickpea (Cicer arietinum L.). Academia Journal of Agricultural Research 4(2): 082-085.
Silva KB, Alves EU, Alcantara RD. e Pontes-Matos V. 2012b. Caracterização morfológica de frutos, sementes e germinação de Sideroxylon obtusifolium (Roem. e Schult.) Penn. (Sapotaceae). Revista Árvore 36(1): 59-64. doi: 10.1590/S0100-67622012000100007.
Silva KB, Alves EU, Matos VP e Bruno RL. 2012a. Caracterização morfológica de frutos, sementes e fases da germinação de Pachira aquatica Aubl, (Bombacaceae), Revista de Ciencias Agrícolas 33(3): 891-898. doi: 10.5433/1679-0359.2012v33n3p891.
Souza DK, Lima R.A, Domingues CA, Pedroso LA, Facundo VA, Gama FC. E Alves MR. 2014. Potencial fungicida do extrato etanólico obtido das sementes de Pachira aquatica Aubl. sobre Fusarium sp. Ciência e Natura 36(2): 114–119. doi: 10.5902/2179460X13611O.
Steel M and Torrie J. 1980. Principles and procedures of statistics. McGraw-Hill. New York. United States. p. 633.
Vallejo F, Espitia M, Estrada E. y Ramírez H. 2010. Genética Vegetal. Universidad Nacional de Colombia – Palmira – Valle del Cauca. Talleres Gráficos de Impresora Feriva S. A. p. 384.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Adriana M. Gallego, Romer A. Zambrano, Martha Zuluaga, Anyela V. Camargo Rodríguez, Mariana S. Candamil Cortés, Angela P. Romero Vergel, Jorge W. Arboleda Valencia. (2022). Analysis of fruit ripening in Theobroma cacao pod husk based on untargeted metabolomics. Phytochemistry, 203, p.113412. https://doi.org/10.1016/j.phytochem.2022.113412.
2. Talita L. S. Nascimento, Karine F. S. Oliveira, Joemil O. D. Junior, Alexandre S. Pimenta, Dulce M. A. Melo, Marcus A. F. Melo, Renata M. Braga. (2024). Biosorption of nickel and cadmium using Pachira aquatica Aubl. peel biochar. Scientific Reports, 14(1) https://doi.org/10.1038/s41598-024-54442-w.
3. Lyvia Daim Costa, Renata Pereira Trindade, Patrick da Silva Cardoso, Nelson Barros Colauto, Giani Andrea Linde, Deborah Murowaniecki Otero. (2023). Pachira aquatica (Malvaceae): An unconventional food plant with food, technological, and nutritional potential to be explored. Food Research International, 164, p.112354. https://doi.org/10.1016/j.foodres.2022.112354.
4. Alexsandra Pereira Rodrigues, Glaucia Maria Pastore. (2021). A review of the nutritional composition and current applications of monguba (Pachira aquatica Aubl.) plant. Journal of Food Composition and Analysis, 99, p.103878. https://doi.org/10.1016/j.jfca.2021.103878.
5. Leider José Castro Torres, Rubén Darío Blanco Fuentes, Miguel Mariano Espitia Camacho, Carlos Cardona Ayala, Hermes Araméndiz Tatis. (2021). PARÁMETROS GENÉTICOS DE CARACTERÍSTICAS BIOMÉTRICAS DEL FRUTO Y SEMILLA EN Caesalpinia ebano (FABACEAE). Acta Biológica Colombiana, 26(3), p.327. https://doi.org/10.15446/abc.v26n3.85718.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2017 Revista Facultad Nacional de Agronomía Medellín

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.






















