Insecticidal activity of the ethanolic extract from Croton species against Plutella xylostella L. (Lepidoptera: Plutellidae)
Actividad insecticida de extractos etanólicos de especies de Croton contra Plutella xylostella L. (Lepidoptera: Plutellidae)
DOI:
https://doi.org/10.15446/rfna.v71n2.62881Palabras clave:
Croton spp., Botanical insecticide, Diamondback moth (en)Croton spp., Insecticida botánico, Polilla del repollo (es)
Descargas
The objective of this work was to study the effect of ethanolic extracts from different parts of Crotonspecies on the diamondback moth (Plutella xylostella L.). Extracts from the leaves of Croton rhamnifoliusH.B.K., Croton jacobinensis Baill., Croton sellowii Baill. and Croton micans Muell. C. rhamnifolius had the most lethal effect (LC50 = 14.95 μg mL-1), followed by C. rhamnifolius (stem), C. jacobinensis (stem),C. jacobinensis (leaf), C. sellowii (leaf) and C. sellowii (stem) with LC50 values of 42.40, 116.21, 183.85, 801.36 and 1252 μg mL-1, respectively. Plutella xylostella larvae fed kale disks with all extracts, exceptC. sellowii (stem), exhibited prolonged larval duration. None of the extracts affected the duration of the pupal stage of the moth.
El objetivo de este trabajo fue estudiar el efecto de los extractos etanólicos de especies de Croton en la polilla del repollo (Plutella xylostella L.). Croton rhamnifolius H.B.K., Croton jacobinensis Baill., Croton sellowii Baill. y Croton micans Muell. C. rhamnifolius (hojas) tuvieron el efecto más letal con una CL50de 14,95 μg mL-1, seguido por C. rhamnifolius (tallos), C. jacobinensis (tallos), C. jacobinensis (hojas),C. sellowii (hojas) y C. sellowii (tallos) con valores de CL50 de 42,40; 116,21; 183,85; 801,36 y 1252 μg mL-1, respectivamente. Las larvas de Plutella xylostella se alimentaron con discos de col y todos los extractos, excepto C. sellowii (tallos), mostraron una duración larval prolongada. Por otra parte, ninguno de los extractos afectó la duración de la etapa de pupas de P. xylostella.
Recibido: 23 de febrero de 2017; Aceptado: 12 de noviembre de 2017
ABSTRACT
The objective of this work was to study the effect of ethanolic extracts from different parts of Croton species on the diamondback moth (Plutella xylostella L.). Extracts from the leaves of Croton rhamnifolius H.B.K., Croton jacobinensis Baill., Croton sellowii Baill. and Croton micans Muell. C. rhamnifolius had the most lethal effect (LC50 = 14.95 µg mL-1), followed by C. rhamnifolius (stem), C. jacobinensis (stem), C. jacobinensis (leaf), C. sellowii (leaf) and C. sellowii (stem) with LC50 values of 42.40, 116.21, 183.85, 801.36 and 1252 µg mL-1, respectively. Plutella xylostella larvae fed kale disks with all extracts, except C. sellowii (stem), exhibited prolonged larval duration. None of the extracts affected the duration of the pupal stage of the moth.
Keywords:
Croton spp., Botanical insecticide, Diamondback moth.RESUMEN
El objetivo de este trabajo fue estudiar el efecto de los extractos etanólicos de especies de Croton en la polilla del repollo (Plutella xylostella L.). Croton rhamnifolius H.B.K., Croton jacobinensis Baill., Croton sellowii Baill. y Croton micans Muell. C. rhamnifolius (hojas) tuvieron el efecto más letal con una CL50 de 14,95 µg mL-1, seguido por C. rhamnifolius (tallos), C. jacobinensis (tallos), C. jacobinensis (hojas), C. sellowii (hojas) y C. sellowii (tallos) con valores de CL50 de 42,40; 116,21; 183,85; 801,36 y 1252 µg mL-1, respectivamente. Las larvas de Plutella xylostella se alimentaron con discos de col y todos los extractos, excepto C. sellowii (tallos), mostraron una duración larval prolongada. Por otra parte, ninguno de los extractos afectó la duración de la etapa de pupas de P. xylostella.
Palabras clave:
Croton spp., Insecticida botánico, Polilla del repollo.The use of synthetic insecticides is the principal method for controlling Plutella xylostella L. (Lepidoptera: Plutellidae) and high doses are often required to minimize the damage caused by this insect. However, populations of this pest exhibit resistance to active ingredients in these insecticides as a result of selective pressure (Gong et al., 2014). Therefore, natural insecticides have been increasingly studied for their effects on the suppression of pest populations, as such substances enable the low-cost control of arthropods, easy management and potential for the discovery of novel insecticidal molecules (Furlong et al., 2013). However, the susceptibility of insects to extracted plant allelochemicals depends on the extraction method, insect species, plant organ and plant species (War et al., 2012).
The mortality rates of Myzus persicae Sulzer and second instar P. xylostella larvae by extracts from the leaves of Prosopis juliflora Swartz was respectively 90 and 28% with the ethanolic extract and 6 and 10% with the aqueous extract (Stein and Klingauf, 1990). Aqueous extracts from Aspidosperma pyrifolium Mart, Azadirachta indica A. Juss and a commercial formulation made from A. indica negatively affected the larval viability of P. xylostella (Torres et al., 2001).
Species from the genus Croton belong to the family Euphorbiaceae and are found in swamps in the Caatinga biome of the state of Pernambuco, Brazil. The species are shrubs or undershrubs that can reach a height of two meters. These plants are often employed in folk remedies as depuratives and agents for relieving pain, vomiting and bloody diarrhea (Braga, 1976). According to the communities around the collection sites, Croton rhamnifolius H.B.K., C. jacobinensis Baill., C. sellowii Baill. and C. micans Muell. are considered to be useful for gastric ailments, stomach pain and attenuating fever.
Croton species are known for the production and accumulation of terpenoids, especially monoterpenes, sesquiterpenes and diterpenes, which are generally found in all parts of the plant and with considerable structural diversity, including many bioactive molecules with effects against arthropods (Filho et al., 2013; Kuo et al., 2013).
As part of a systematic study on the insecticidal potential of the medicinal flora growing in the state of Pernambuco (Bandeira et al., 2013; Pereira et al., 2009), the objective of this work was to evaluate the potential of ethanolic extracts prepared from the stems and leaves of C. rhamnifolius, C. jacobinensis, C. sellowii and C. micans. on the development and survival of P. xylostella. This is the first report of the insecticidal action of organic extracts from species of Croton.
MATERIALS AND METHODS
Plant material
The leaves and stems of the plants investigated were collected during the morning hours. C. jacobinensis Baill. (08°08'39"S 36°22'17"W; altitude: 1800 m), C. micans Muell. (08°08'17"S 36°21'52"W; altitude: 900 m), C. rhamnifolius H.B.K (08°08'13"S 36°21'47"W; altitude: 795 m) and C. sellowii Baill (08°21'07"S 34°56'34"W; altitude: 300 m) were selected for the bioassays. The former three species of Croton were collected from highland forests in the municipality of Brejo da Madre de Deus and the latter species was collected from a fragment of the Atlantic forest in the municipality of Cabo de Santo Agostinho, state of Pernambuco, Brazil. The plants were identified by botanist Dr. Maria de Fátima Araújo Lucena, researcher from the Universidade Federal de Pernambuco. Voucher specimens of each species were registered under the numbers 45553 (C. jacobinensis), 48218 (C. micans), 48217 (C. rhamnifolius) and 45622 (C. sellowii) and maintained at the “Vasconcelos Sobrinho" Herbarium of the Botany Department, Universidade Federal Rural de Pernambuco (UFRPE), Brazil.
Organic extract preparations
For the preparation of the extracts, leaves and stems were washed and oven-dried at 40 °C for 48 h. Portions of each species were ground in a mill and weighed separately. Stems and leaves were placed in separate flasks and ethanol was added until covering all the plant material. Maceration was performed at 24-h intervals for 72 h to ensure the complete extraction of all active substances. The extract was filtered and evaporated at low pressure to minimize possible degradation of the chemical constituents at high temperatures. After removal of the solvent, the crude ethanol extracts of the plants were obtained. Yield was expressed as a percentage value (g per 100 g of dry weight plant material).
The concentrations used were prepared from stock solutions of the different ethanolic extracts. A one-gram aliquot of ethanol extract was suspended in 19.9 mL of distilled water and 0.1 mL of Tween 80 as dispersant. The solution was agitated until the complete dissolution of the extract and filtered through a Whatman No. 1 paper filter for the obtainment of the stock solution (50 mg mL-1) for the preparation of the different concentrations used in the bioassays. Dilutions of the aqueous stock solution were used for the immersion solution (50 mL) at the desired concentrations, ranging from 5.0 µg mL-1 to 4000 µg mL-1.
Insect and general procedures of experiments
Rearing of P. xylostella was performed from pupae maintained at the UFRPE Laboratory of Insect Biology. Newly-emerged adults were sexed and placed in plastic cages with a sponge soaked in water to maintain proper humidity. A disk of filter paper (∅ 8 cm) and a leaf disk of kale (Brassica oleracea L. var. acephala) were placed on the sponge to stimulate oviposition. The adults were fed a 10% honey solution provided in a polyurethane foam recipient attached to a circular hole at the top of the cage.
Bioassay
Kale leaf disks with the eggs were transferred to Petri dishes daily and kept until the hatching of the P. xylostella. Disks containing the larvae were kept in rectangular plastic containers with organic kale leaves, which were used as food. The larvae remained in these containers and the kale leaves were changed daily until the larvae reached the pupal stage.
Pupae were collected in test tubes covered with PVC film that enabled the circulation of air and kept at room temperature until the emergence of the adults, which were transferred to cages. Kale leaf disks measuring 8 cm in diameter were sprayed with 2 mL of a water-alcohol solution and different concentrations of crude ethanolic extracts from the stems and leaves of the Croton species in a Potter spray tower calibrated to a pressure of 10 psi. Kale leaf disks sprayed with the water-alcohol solution without the extracts were used as control. After spraying, the disks were placed on filter paper at room temperature to remove excess moisture, subsequently transferred to Petri dishes and placed on filter paper disks. The tests were conducted at a temperature of 30 ± 1 °C, 70 ± 10% relative humidity and a 12-h photoperiod. Preliminary tests were performed to determine extract concentration ranges that caused insect mortality from near zero to near 100%. More precise response ranges for average lethal concentrations (LC50) were obtained from this wide range.
The method for evaluating the larval and pupal viability after exposure to different concentrations of crude ethanolic extracts was outlined by Torres et al. (2001) and Boiça-Junior et al. (2005). Treated leaf disks were placed in Petri dishes containing filter paper moistened with distilled water. Ten newly-hatched P. xylostella larvae were confined in each dish. The number of individuals dead was determined 72 h after the confinement of the larvae and treated kale disks were replaced with untreated leaves. Other assessments were performed every 24 h and leaf disks were changed every 48 h until the larvae reached the pupal stage. The pupae from each treatment were isolated on ELISA plates and the viability assessment was performed by daily monitoring for the emergence of adults.
Statistical analyses
The experimental design was completely randomized, consisting of 47 treatments (concentrations of the extracts from the stems and leaves of Croton species) with 10 replicates, each replicate containing 10 newly-hatched P. xylostella larvae. Extract concentration-larval mortality curves were calculated using the Statistical Analysis Software (SAS Institute, 2002). Data were also analyzed with the Probit model with the aid of the Polo-PC software program (LeOra Software, 1987) for the determination of the LC50 (lethal concentration for 50% population mortality) with 95 percent confidence levels for all experiments. Some concentrations were suppressed to better fit the model for the calculation of LC50. Mortality data for extracts were fitted to the Probit model (χ2 test; P>0.05). The results were submitted to a regression analysis to detect whether the extracts influenced larval and pupal duration (SAS Institute, 2002).
RESULTS AND DISCUSSION
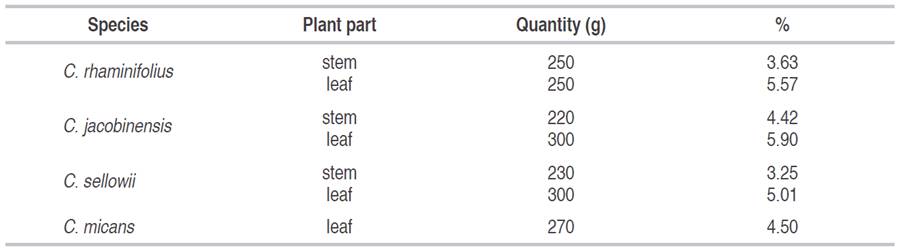
The yield of the extracts varied depending on the part of the plant and species studied. However, yields were slightly higher for the leaf extracts in comparison to the stem extracts, regardless of the species investigated (Table 1).
Table 1: Percentage of crude ethanolic extracts from stems and leaves of species of Croton.
All extracts tested were toxic to P. xylostella larvae. Toxicity also varied depending on the part of the plant and species. The leaf extract from C. rhamnifolius demonstrated the greatest toxicity, as demonstrated by the the lowest concentration required for 50% population mortality (LC50 = 14.95 µg mL-1), followed by extracts from C. rhamnifolius (stem), C. jacobinensis (stem), C. jacobinensis (leaf), C. sellowii (leaf), C. sellowii (stem), with LC50 values of 42.4, 116.21, 183.85, 801.36 and 1252 µg mL-1, respectively. The leaf extract from C. micans did not demonstrate Probit distribution due to the heterogeneity of the data (Table 2).
Table 2: Toxicity of ethanolic extracts from species of Croton to first-instar P. xylostella larvae fed on leaves of B. oleracea var. acephala.
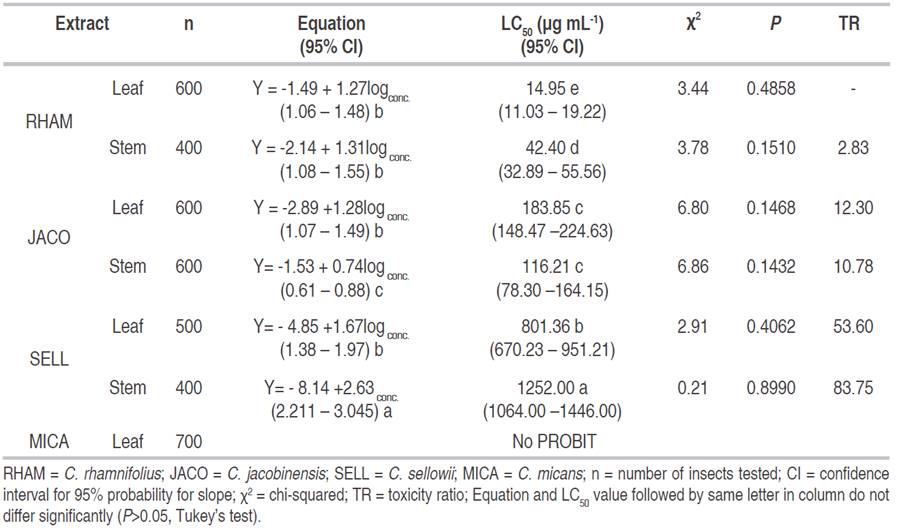
The lethal concentrations of C. rhamnifolius (leaf), C. rhamnifolius (stem), C. jacobinensis (leaf) and C. sellowii (leaf) were similar. Extracts from the stems of C. jacobinensis and C. sellowii differed between each other as well as the other treatments.
The steepest slope of the mortality curve for P. xylostella occurred with the extract from C. sellowii (leaf), signifying that small variations the concentration of this extract can cause large variations in its mortality potential. The toxicity ratio between the leaf extract from C. rhamnifolius and extracts from C. rhamnifolius (stem), C. jacobinensis (leaf), C. jacobinensis (stem), C. sellowii (leaf) and C. sellowii (stem) was 2.83, 12.30, 10.78, 53.60 and 83.75, respectively (Table 2).
Comparing the present results with those reported in the literature for the same pest involving extracts prepared with different solvents, the C. rhamnifolius extract is much more toxic. For example, Boiça Junior et al. (2005) used greater amounts of Enterolobium contortisillidium (fruit), Nicotiana tabacum (leaf), Sapindus saponaria (fruit) and Trichilia pallida (twigs) to achieve a 100% mortality rate of P. xylostella larvae fed on kale (B. oleracea var. acephala) treated with 10% aqueous extracts. Torres et al. (2001) report the same result with 10% aqueous extracts from the seeds and bark of Azadirachta indica A. Juss. and Aspidosperma pyrifolium Mart.. Li et al. (2008) found that the acetone fraction of the chloroform extract from Xanhtium sibiricum promoted a 91.67% larval mortality rate at a concentration of 50 µg mL-1, whereas the ethanolic extract from C. rhamnifolius in the present investigation had an LC50 of 14.95 µg mL-1. Rani et al. (1999) achieved a 100% mortality rate of P. xylostella larvae with an ethanol extract from the twigs of Melia azaderach at a concentration greater of 7.5%, which is higher than that used in the present study.
On the other hand, using chemical constituents isolated from active extracts, Yang et al. (2008) reported insecticidal action against P. xylostella larvae for two active chemical components from the fruit of Ginkgo biloba L., with estimated LC50 values for bilobol (LC50 = 2.0613 g L-1) and ginkgo acid (LC50 = 4.6002 g L-1) that were respectively 3.3-fold and 7.3-fold greater than estimated LC50 for the leaf extract from C. rhamnifolius, which achieved the best result in the present study. Moreover, the ethanolic extract from Zanthoxylum armatum (Kumar et al., 2016) was slightly more toxic than the ethanolic leaf extract from C. rhamnifolius. However, in comparison to oil from the fruit of Azadirachta indica (Kolani et al., 2016), the leaf extract from C. rhamnifolius was about 3.9-fold more toxic.
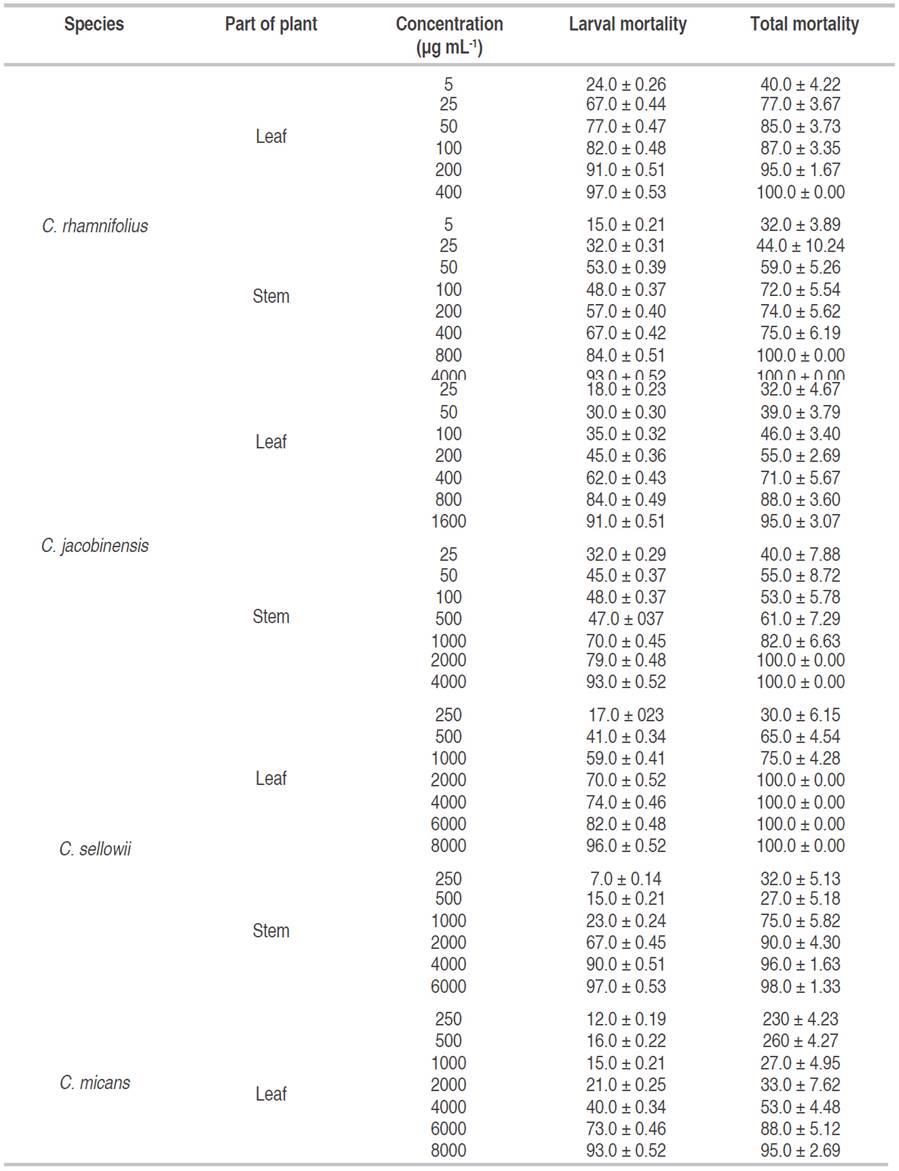
With regard to the viability of P. xylostella larvae and pupae exposed to Croton extracts, only very high concentrations of the stem extracts from C. jacobinensis and C. rhamnifolius and the leaf extracts from C. sellowii and C. rhamnifolius caused 100% mortality (Table 3).
Table 3: Larval and pupal viability based on larval mortality and total mortality (larvae and pupae of Plutella xylostella larvae (Lepidoptera: Plutellidae) fed leaf disks of B. oleracea var. acephala treated with different concentrations of ethanolic extracts from species of Croton.
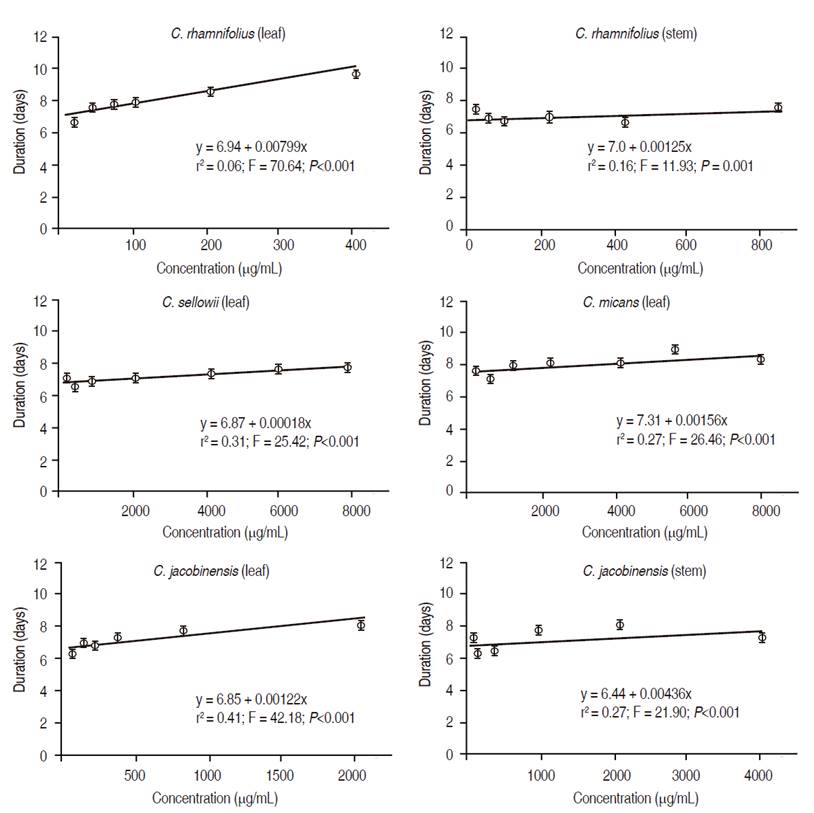
P. xylostella larvae fed on B. oleracea var. acephala disks treated with leaf extracts from C. jacobinensis, C. rhamnifolius, C. sellowii and C. micans and stem extracts from C. jacobinensis and C. rhamnifolius exhibited prolonged duration of the larval stage (Figure 1). For the stem extract from C. sellowii, the Y value of the equation averaged 7.2 ± 0.06 days, demonstrating no significant effect.
Figure 1: Effect of extracts from species of Croton on duration of larval stage of Plutella xylostella.
Based on these data, we can infer that the leaf extract of C. rhamnifolius was the most toxic to P. xylostella. This result may be associated with its higher concentrations of deterrents, as found in Croton jatrophoides, which contains limonoids with anti-feeding properties for Pectinophora gossypiella Sauders (Lepidoptera: Gelechiidae) and Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) (Nihei et al., 2006). Moreover, species of Croton contain higher concentrations of terpenoids in leaves and roots, which probably resulted in the higher P. xylostella mortality found for the leaf extract from C. rhamnifolius (Randau et al., 2004).
Extract activity is associated with the part of the plant from which the extraction is taken (Trindade et al., 2000), as demonstrated by the greater toxicity of the leaf extracts from C. rhamnifolius and C. sellowii compared to the stem extracts of these plants. Ethanolic extracts from the stems, bark, roots and fruit of A. pyrifolium exhibited differences in P. xylostella mortality, which is similar to the findings for C. rhamnifolius in the present study (Trindade et al., 2008).
All extracts, except the leaf extract from C. rhamnifolius, prolonged the larval phase (Figure 2). In practical terms, this result is quite important, as it increases the average time for each generation of the insect, consequently increasing the exposure to natural enemies. In contrast, no extract affected the duration of the pupal phase, although all extracts affected pupal viability (Table 3).
Figure 2: Effect of extracts from species of Croton on duration of pupal stage of Plutella xylostella.
Extract activity on P. xylostella reflects the diversity of secondary metabolites produced by plants of the genus Croton (Marino et al., 2008). On other hand, insecticidal activity is associated with the solvent, which can make an extract more efficient depending on the insect tested (Moreira et al., 2007).
The longer cycle of P. xylostella with ethanol extracts from the leaves of C. jacobinensis, C. rhamnifolius, C. sellowii, C. micans and stems of C. jacobinensis and C. rhamnifolius suggests that these plants have inhibitory properties that affect the development of this pest. The present findings suggest that Croton plants have substances with an insecticidal effect against P. xylostella. Thus, investigation of these resources could provide new substances for the control of the diamondback moth. All ethanolic extracts from the species of Croton evaluated were toxic to P. xylostella in the larval phase, with greatest toxicity found for the ethanolic leaf extract from Croton rhamnifolius. On the other hand, none of the extracts affected the duration of the pupal stage, which indicates an effect on food intake in P. xylostella.
CONCLUSIONS
The results demonstrate that the search for insecticidal properties in Croton plants is promising with regard to the discovery of new vegetal species for the control of agricultural pests. With the exception of the raw extract from the stems of C. micans, all extracts were active and caused 100% mortality of larvae and pupas at the highest concentrations. The greatest toxicity to larvae was found with the leaf extract from C. rhamnifolius, which caused 100% mortality at a concentration of 400 μg mL-1 one day after treatment, with an estimated LC50 of 14.95 µg mL-1. However, considering the angular coefficients (ß) of the equations obtained regarding the toxicity of the different raw extracts tested, the greatest slope of the curve occurred for the leaf extract from C. sellowii, which means that small variations in the amount of this extract can cause a considerable change in its insecticidal potential. The present results reveal the insecticidal potential of the crude ethanolic extract from the leaves of C. rhamnifolius. Bio-monitored fractioning of this extract to isolate and identify the active ingredient and its possible mechanism of action is under way.
ACKNOWLEDGMENTS
The authors are grateful to the Brazilian fostering agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq [National Council for Scientific and Technological Development]) for awarding a productivity grant (Nº 312277/2013-0) and financial support (CNPq - universal 477778/2013-5; CT-AGRO 403162/2013-0) and Fundação de Amparo a Ciência e Tecnologia de Pernambuco (FACEPE [State of Pernambuco Science and Technology Assistance Foundation], Nº A PQ-0476-1.06/14).
REFERENCES
Referencias
Bandeira GN, da Camara CAG, de Moraes MM, Barros R, Muhammad S, Akhtar Y. 2013. Insecticidal activity of Muntingia calabura extracts against larvae and pupae of diamondback, Plutella xylostella (Lepidoptera, Plutellidae). Journal of King Saud University 25(1): 83-89. doi: 10.1016/j.jksus.2012.08.002
Boiça-Júnior AL, Medeiros CAM, Torres AL, Chagas Filho NR. 2005. Efeito de extratos aquosos de plantas no desenvolvimento de Plutella xylostella (L.) (Lepidoptera: Plutellidae) em couve. Arquivos do Instituto Biológico 72(1): 45-50.
Braga RA. 1976. Plantas do Nordeste, especialmente do Ceará. Terceira edição. ESAM, Fortaleza. 540 p.
Filho FAS, da Silva Junior JN, Braz-Filho R, de Simone CA, Silveira ER, Lima MAS. 2013. Crotofolane- and Casbane-Type Diterpenes from Croton argyrophyllus. Helvetica Chimica Acta 96(6): 1146-1154. doi: 10.1002/hlca.201200347
Furlong MJ, Wright DJ, Dosdall LM. 2013. Diamondback moth ecology and management: problems, progress and prospects. Annual Review of Entomology 58: 517-541. doi: 10.1146/annurev- ento-120811-153605
Gong W, Yan H, Gao L, Guo Y, Xue CB. 2014. Chlorantraniliprole resistance in the diamondback moth (Lepidoptera: Plutellidae). Journal of Economic Entomology 107(2): 806-814.
Kolani L, Sanda K, Agboka K, Mawussi G, Koba K, Djouaka R. 2016. Investigation of Insecticidal Activity of Blend of Essential Oil of Cymbopogon schoenanthus and neem oil on Plutella xylostella (Lepidoptera: Plutellidae). Journal of Essential Oil-Bearing Plants 19(6): 1478-1486. doi: 10.1080/09720660X.2016.1221742
Kumar R, Sharma S, Sharma S, Kumari A, Kumar D, Nadda G, Padwad Y, Ogra RK, Kumar N. 2016. Chemical composition, cytotoxicity and insecticidal activities of. Acorus calamus accessions from the western Himalayas. Industrial Crops and Products 94: 520- 527. doi: 10.1016/j.indcrop.2016.09.024
Kuo PC, Yang ML, Hwang TL, Lai YY, Li YC, Thang TD, Wu TS. 2013. Anti-inammatory diterpenoids from Croton tonkinensis. Journal of Natural Products 76(2): 230-236. doi: 10.1021/np300699f
LeOrA Software. 1987. POLO-PC: a user’s guide to Probit or Logit analysis. LeOra Software, Berkeley, CA.
Li M, Gao X, Gao Z, Zhao W, Sun Z. 2008. Insecticidal activity of extracts from forty-eithg plants inclunding Xanthium sibiricum Patrin. Huanjing Xuebao 17(1): 33-37.
Marino S, Gala F, Zollo F, Vitalini S, Fico G, Visioli F, Iorizzi M. 2008. Identi cation of minor secondary metabolites from the latex of Croton lechleri (Muell-Arg) and evaluation of their antioxidant activity. Molecules 13(6): 1219-1229. doi: 10.3390/molecules13061219
Moreira MD, Picanco MC, Barbosa LCD, Guedes RNC, Campos MR, Silva GA, Martins JC. 2007. Plant compounds insecticide activity against Coleoptera pests of stored products. Pesquisa Agropecuária Brasileira 42(7): 909-915. doi: 10.1590/S0100-204X2007000700001
Nihei K, Asaka Y, Mine Y, Yamada Y, Iigo M, Yanagisawa T, Kubo I. 2006. Musidunin and musiduol, insect antifeedants from Croton jatrophoides. Journal of Natural Products 69(6): 975-977. doi: 10.1021/ np060068d
Pereira ACRL, Oliveira JV, Gondim Junior MGC, Câmara CAG. 2009. Influência do período de armazenamento do caupi (Vigna unguiculata (L.) Walp.), tratado com óleos essenciais e xos, no controle de Callosobruchus maculatus (Fabricius, 1775) (Coleoptera, Chrysomelidae, Bruchinae). Revista Ciência e Agrotecnologia 33(1): 319-325. doi: 10.1590/S1413- 70542009000100044
Randau KP, Florêncio DC, Ferreira CP, Xavier HS. 2004. Estudo farmacognóstico de C. rhamnifolius H.B.K e C. rhaminifolioides Pax & Hoffms (Euphorbiaceae). Revista Brasileira de Farmacognosia 14(2): 89-96. doi: 10.1590/S0102-695X2004000200001
Rani M, Suhag P, Kumar R, Singh R, Kalidhar SB. 1999. Chemical components and biological ef cacy of Melia azedarach Stems. Journal of Medicinal and Aromatic Plant Sciences 21(4): 1043-1047.
SAS Institute. (2002). SAS user’s guide: Statistics, version 9.0, 7th ed. SAS Institute, Cary, NC.
Stein U and Klingauf F. 1990. Insecticidal effect of plant extracts from tropical and subtropical species. Traditional methods are good as long as they are effective. Journal of Applied of Entomology 110(2): 160-166. doi: 10.1111/j.1439-0418.1990. tb00109.x
Torres AL, Barros R, Oliveira JV. 2001. Efeitos de extratos aquosos de plantas no desenvolvimento de Plutella xylostella (L.) (Lepidoptera: Plutellidae). Neotropical Entomology 30(1): 151-156. doi: 10.1590/S1519-566X2001000100022
Trindade RCP, Marques IMR, Xavier HS, Oliveira JV. 2000. Extrato metanólico da amêndoa da semente de nim e a mortalidade de ovos e lagartas da traça-do-tomateiro. Scientia Agrícola 57(3): 407-413. doi: 10.1590/S0103-90162000000300006
Trindade RCP, Silva PP, Araújo-Júnior JX, Lima IS, Paula JE, Sant’ana AEG. 2008. Mortality of Plutella xylostella larvae treated with Aspidosperma pyrifolium ethanol extracts. Pesquisa Agropecuária Brasileira 43(12): 1813-1816. doi: 10.1590/S0100- 204X2008001200024
Yang Z, Deng Y, Hou M, Yu Y, Kong Z. 2008. Insecticidal ingredients of Ginkgo biloba L. Sarcotesta. Ziran Kexueban 26(6): 68-71.
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC. 2012. Mechanisms of plant defense against insect herbivores. Plant Signaling & Behavior 7(10): 1306- 1320. doi:10.4161/psb.21663.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Nour‐El Houda Hafsi, Kaouther Hamaidia, Noureddine Soltani. (2022). Chemical screening, insecticidal and reprotoxic activities of Tecoma stans ethanolic leaf extract against the vector mosquito Culex pipiens. Physiological Entomology, 47(3), p.176. https://doi.org/10.1111/phen.12386.
2. Abla Déla Mondédji, Pierre Silvie, Wolali Seth Nyamador, Pierre Martin, Lakpo Koku Agboyi, Komina Amévoin, Guillaume Koffivi Ketoh, Isabelle Adolé Glitho. (2021). Cabbage Production in West Africa and IPM with a Focus on Plant-Based Extracts and a Complementary Worldwide Vision. Plants, 10(3), p.529. https://doi.org/10.3390/plants10030529.
3. Wilson R. Tavares, Maria do Carmo Barreto, Ana M. L. Seca. (2021). Aqueous and Ethanolic Plant Extracts as Bio-Insecticides—Establishing a Bridge between Raw Scientific Data and Practical Reality. Plants, 10(5), p.920. https://doi.org/10.3390/plants10050920.
4. Nelson Mpumi, Kelvin M. Mtei, Revocatus L. Machunda, Patrick A. Ndakidemi, Nguya K. Maniania. (2021). Efficacy of Aqueous Extracts from Syzygium aromaticum, Tephrosia vogelii, and Croton dichogamus against Myzus persicae on Brassica oleracea in Northern Tanzania. Psyche: A Journal of Entomology, 2021, p.1. https://doi.org/10.1155/2021/2525328.
5. Joseane de Jesus Oliveira, Eliana M. dos Passos, Suely M. Alves, Victor H.V. Sarmento, Thiago R. Bjerk, Juliana C. Cardoso, Cristina Blanco-Llamero, Eliana B. Souto, Patrícia Severino, Marcelo da Costa Mendonça. (2024). Microemulsion of essential oil of Citrus aurantium var. dulcis for control of Aleurocanthus woglumi and evaluation of selectivity against Aschersonia aleyrodis and Ceraeochrysa cornuta. Crop Protection, 178, p.106586. https://doi.org/10.1016/j.cropro.2024.106586.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2018 Revista Facultad Nacional de Agronomía Medellín

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
La Revista permite al autor(es) mantener los derechos de explotación (copyright) de sus artículos sin restricciones. El(os) autor(es) acepta(n) la distribución de sus artículos en la web y en soporte papel (300 ejemplares por número), bajo acceso abierto a nivel local, regional e internacional; la inclusión y difusión del texto completo, a través del Portal de Revistas y Repositorio Institucional de la Universidad Nacional de Colombia; y en todas las bases de datos especializadas que la Revista considere pertinentes para su indexación, con el fin de proporcionarle visibilidad y posicionamiento al artículo. Todos los artículos deben cumplir la legislación colombiana e internacional, relacionada con derechos de autor.
Compromisos del autor
El autor(es) se compromete(n) a ceder los derechos de impresión y reimpresión del material publicado a la Revista Facultad Nacional de Agronomía Medellín y cualquier cita a los artículos editados en la Revista se deberá hacer si se adiciona el crédito respectivo. En caso de duplicación del contenido de la Revista o su publicación parcial o total en otro idioma, se deberá contar con el permiso escrito del Director.
Responsabilidad de los contenidos
La Facultad de Ciencias Agrarias y la Revista no se responsabilizan o solidarizan, necesariamente, con los conceptos emitidos en los artículos publicados, cuya responsabilidad será en su totalidad del autor o los autores.