Growth of irrigated and fertilized pequi trees in the Cerrado of Goiás, Brazil
Crecimiento del pequíe irrigado y fertilizado en el Cerrado de Goiás, Brasil
DOI:
https://doi.org/10.15446/rfna.v71n2.67674Palabras clave:
Caryocar brasiliense Camb, Water stress, Native fruits from Cerrado, Localized irrigation, Sap flow (en)Caryocar brasiliense Camb, Estrés hídrico, Frutos nativos del Cerrado, Riego localizado, Flujo de savia (es)
Descargas
Recibido: 11 de septiembre de 2017; Aceptado: 7 de febrero de 2018
ABSTRACT
The objective of this study is to evaluate the growth of pequi trees in function of irrigation and organic fertilization. A total of 96 pequi plants (6-8 years of age), spaced 5 x 5 m, were used in a randomized blocks experiment with six plots and for repetitions. Four treatments were evaluated: IF = Irrigation with fertilization, IWF = Irrigation without fertilization, WIF = Without irrigation with fertilization, and WIWF = Without irrigation and without fertilization, in subdivided plots. The irrigation system was microsprinklers. The volume of water applied was estimated by the evapotranspiration of pequi trees. Cover fertilizations were performed in Nov/2014 using composting material and Yorim, and in Nov/2015 using tanned cattle manure and chicken litter. The growth was monthly evaluated by the height and the stem perimeter, and the canopy was evaluated in a 7.1 years old orchard. Leaf temperature and transpiration were evaluated by sap flow in irrigated and non-irrigated trees. The pequi tree did not respond to treatments in terms of height and stem perimeter. Irrigation provided trees with larger canopy areas. When irrigated, the leaf temperature of pequi trees remained below average air temperatures, independently of dry or rainy periods. Without irrigation, plants presented water stress during the dry season. Under rainy conditions, the mean transpiration of the pequi tree was 24.09 L d-1 per plant. When irrigated, it was on average 42.29 L d-1 per plant.
Keywords:
Caryocar brasiliense, Camb, Water stress, Native fruits from Cerrado, Localized irrigation, Sap flow.RESUMEN
El objetivo de este estudio fue evaluar el crecimiento de árboles de pequi en función de la irrigación y la fertilización orgánica, en un experimento de bloques al azar con seis parcelas y cuatro repeticiones. Se utilizaron 16 plantas por parcela espaciadas 5 x 5 m. Se evaluaron cuatro tratamientos: IF = Riego con fertilización, IWF = Riego sin fertilización, WIF = Sin riego con fertilización, y WIWF = Sin riego y sin fertilización, en parcelas subdivididas. El sistema de riego utilizado fue microaspersión. El volumen de agua aplicada se estimó en función de la evapotranspiración de los árboles de pequi. Las dosis de fertilización se aplicaron en noviembre de 2014 utilizando material de compostaje y Yorim; y en noviembre de 2015 se utilizó estiércol de ganado y estiércol de pollo. Cada mes se evaluaron la altura y el perímetro del tallo; el dosel fue evaluado en un huerto de 7,1 años. La temperatura de la hoja y la transpiración fueron evaluadas por el flujo de savia en árboles irrigados y no irrigados. El pequi no respondió a los tratamientos en términos de altura y perímetro del tallo. El riego proporcionó árboles con áreas más grandes de copa. Cuando se regaron, la temperatura de las hojas de los árboles de pequi se mantuvo por debajo de las temperaturas promedio del aire, independientemente de los períodos secos o lluviosos. Sin riego, las plantas presentaron estrés hídrico durante la estación seca. Bajo condiciones de lluvia, la transpiración media del árbol de pequi fue de 24,09 L d-1 por planta. Cuando se regaba, era en promedio de 42,29 L d-1 por planta.
Palabras clave:
Caryocar brasiliense, Camb, Estrés hídrico, Frutos nativos del Cerrado, Riego localizado, Flujo de savia.Among the fruit trees native from the Cerrado, the pequi tree (Caryocar brasiliense Camb.) can be emphasized due to a possibility of using fruits, seeds, leaves and peels in the food, energy and pharmaceutic industry (Pinto et al., 2016). However, the industrial use of the fruit is still limited. Adequate management techniques and a long period of juvenility, which begins its production between eight and ten years of age, are issues to the expansion of commercial cultivation (Silva, 2005). As a consequence, commercialization takes place at local fairs and road margins only during the harvest period (October to February). The production is restricted to the regional demand.
For commercial fruit production, producers have adopted techniques such as irrigation and fertilization, which ensures the quality and the productivity of their orchards, as well as the possibility of producing during the off-season (Silva et al., 2016). An alternative that has been diffused in fertilizing soils for food production is organic fertilization. The technique may provide improvements in the quality of production and soil without causing damage to the environment (Corrêa et al., 2013). Organic fertilizer can be produced by using residues of plants, such as leaves, roots and barks, and residues of animals, such as manures, bones, feathers and viscera, generated by agricultural farms (Nunes, 2009). This is an alternative to the disposal of such waste and a low-cost fertilizer source.
In terms of the ability of native species to respond to a great availability of nutrients in the soil, studies conducted in this area (Haridasan, 2000) indicated a positive response to fertilization. However, few studies quantify the increase in plant growth or characterize all the changes caused by fertilization. Comparing the pequi tree with other fruit trees grown in the Cerrado region, it is believed that, under good soil fertility conditions and absence of water deficit, pequi may develop better, begin the reproductive period earlier, produce during the whole year and provide fruits with better physical-chemical and nutritional qualities (Neto et al., 2015).
In view of the above, the objective of this study is to evaluate the response of growth of pequi trees to irrigation and organic fertilization.
MATERIALS AND METHODS
Description of the experiment
The study was conducted from January 2015 to December 2016 in an experimental pequi orchard (Caryocar brasiliense C.) established in January 2009. Planting details can be obtained in Alves et al. (2015). 120 pequi seedlings were transplanted, in a 5 x 5 m spacing, into a 3000 m² experimental area at the School of Agriculture of the Federal University of Goiás (EA/UFG) (16°35’12"S, 49°21’14"W, altitude: 730 m), Goiânia, Goiás (GO) state. The soil of the area was classified as a dystrophic Red Latosol with a loam-clayey texture (Table 1). The climate of the region, according to the Köppen-Geiger classification, is Aw: hot and semi-humid with a well-defined dry season. The average annual air temperature is 22.9°C. The dry season is concentrated from May to September, and the rainy season lasts from October to April, with an annual average of 1520 mm of rainfalls.
Table 1: Chemical and physical attributes of the dystrophic red latosol of the experimental pequi orchard area (Caryocar brasiliense Camb.).

The experimental design was randomized blocks with 6 plots, analyzed by subdivided plots. The plots consisted of two irrigation levels (with and without irrigation) and the subplots consisted of two fertilization levels (with and without fertilization). Each block contained sixteen plants, eight of which were irrigated and eight were not irrigated. Each subplot was composed of eight plants: four plants grown with and four plants grown without fertilization. The treatments were defined as:
-
Irrigated and fertilized (IF): Application of irrigation using a water depth calculated to restore 100% of the evapotranspiration (ETc) during the whole dry season, and organic fertilization with a dose corresponding to the general recommendations made for other fruit plants cultivated in the region (Crisostomo and Naumov, 2009).
-
Irrigated and without fertilization (IWF): Irrigated under the same conditions as IF, but without fertilization.
-
Without irrigation and fertilized (WIF): fertilized under the same conditions of IF, but without irrigation.
-
Without irrigation and without fertilization (WIWF): Cultivation under the natural growth conditions in the Cerrado of Goiás. Thus, water and fertilizer were not supplied to plants.
Irrigation and soil water content
The irrigation treatments (IF and IWF) were irrigated by microsprinklers containing one microsprinkler per plant (working pressure (WP): 10 mca, flow rate (q) = 43 L h-1, wet ray (r) = 2 m), weekly in 2015 and biweekly in 2016. Irrigation intervals were adopted seeking to deepen the volume of water in the soil applied by irrigation. Irrigation water management was carried out by atmospheric monitoring. The meteorological variables were obtained by the automatic agrometeorological station (HOBO) of the EA/UFG located 350 m from the experimental orchard. ETo was estimated by the Penman-Monteith model (Allen et al., 1998), and the volume of water applied by irrigation (microsprinklers) was calculated by adopting a crop coefficient (Kc) of 0.9 because it is representative of aged fruit trees cultivated in a spacing similar to that of the pequi orchard (Doorembos and Kassam, 1977; Doorembos and Pruit, 1979). As only 50% of the useful area of each plant was wet, the ETo was corrected for the calculation of water volume supplied per plant in each irrigation using the correction coefficient Kloc = 0.85 (Faci and Fereres, 1980). The irrigation criterion was no water restriction, replacing 100% of the maximum evapotranspiration estimated for the orchard.
To monitor the soil water content, two ECH2O sensor batteries (model: EC-5, brand: Decagon) were installed in the canopy projection (0.50 m from the stem) of two pequi plants, one representative of the irrigation treatment and the other of the dry land. The sensors were calibrated according to the manufacturer’s recommendation (Decagon) and installed at the depths 0.50, 1.00, 1.50, 2.00 and 2.50 m connected to an Em50 Datalogger. The recordings of the readings were performed every one hour from Sep/2015 to Dec/2016.
In order to adjust the water retention curve in the soil, 15 undisturbed soil samples were collected at the same installation depths of the EC-5 sensor (0.50, 1.00, 1.50, 2.00 and 2.50 m). The samples were saturated, taken to a centrifuge and subjected to rotations corresponding to the tensions -6, -8, -10, -33, -60, -100 and -1500 kPa. Data were adjusted to the Van Genuchten (1980) model using the SWRC software (Dourado et al., 1990). The tensions of -10 and -1500 kPa were considered for water content at field capacity and permanent wilting point, respectively.
Fertilization
Fertilizations were carried out in November. In 2014, the fertilization consisted of the application of 2.5 kg of compost material and 1.0 kg of Yoorin; in 2015, 5.0 kg of cattle manure and 2.5 kg of chicken litter were applied (Tables 2 and 3). The applications were performed within a 0.50 m radius of the plant stem.
Table 2: Nutritional composition of the compost and the Yoorin used for the fertilization of the experimental pequi orchard.

Table 3: Nutritional composition of the cattle manure and chicken litter used for the fertilization of the experimental pequi.

After 30 days of application of the organic fertilizer, in December 2015, an analysis of macro and micronutrients was conducted (Malavolta et al., 1997; Embrapa, 1997) to perform a nutritional evaluation of pequi trees.
Growing of the pequi trees
Variations in stem perimeter were monitored monthly between Jan/2015 and Dec/2016. A tape measure was used to measure the stem perimeter at 0.10 m from the soil surface. Monthly, plant height was also measured using an electronic forest clinometer from the soil surface to the highest branch of the plant.
The canopy area was measured in Aug/2015. For this, all the trees of the orchard were individually photographed, placing some object with a known height next to the tree for comparison purposes. The photographs were processed in the software AutoCad 2015 for scale correction using the photographed object as a reference and canopy delimitation using the outline of the tree in the photograph. Then, the area of the object formed by the delimited canopy was obtained.
Growth data were submitted to analysis of variance. The treatments were compared by Snedecor F test and, when necessary, by Tukey test at a 5% level of significance.
Water stress
As a sign of water stress, leaf temperature and sap flow were evaluated in pequi trees. Leaf temperature was monitored biweekly from Nov/2015 to Aug/2016, when the orchard aged 6.9-7.7 years, using a digital infrared laser thermometer (Fluke, model: 59 max+). The readings were always performed in the morning, between 08:00 and 10:00, by sampling three irrigated plants and three other plants in an unirrigated area. Leaf temperature values indicated by the thermometer were compared to the mean air temperature provided by the EA/UFG agrometeorological station at the time of the evaluation (Trentin et al., 2011).
Sap flow was monitored between Oct/2015 and May/2016 using Granier heat dissipation probes (HDPs) (1987). In Oct/2015, seven pairs of HDPs were installed: three in pequi plants in the irrigation treatment, three in unirrigated areas, and one unheated HDP to record the natural thermal difference in unirrigated plants. The measurements of thermal differences were performed every 30 seconds and the averages of the readings were stored every 15 minutes in a data acquisition storage system ("datalogger" CR 21X Campbell SCi.). The estimation of sap flow by HDP readings was performed using the equation proposed by Granier (1987).
RESULTS AND DISCUSSION
Irrigation and soil water content
For the dry period of 2015, the volumes of water replenished by irrigation were 84.50, 105.63, 126.75, 126.75 and 137.31 L d-1 per plant from June to October, respectively. In 2016, 88.73, 105.63, 73.94, 95.06 and 95.06 L d-1 of water per plant were replenished from May to October, respectively (Figure 1).
Figure 1: Volume of irrigation water provided by microsprinklers in irrigation treatments (IF and IWF) to pequi trees, between the age of 6.4 and 7.8 years and reference evapotranspiration (ET0) for June/2015 to Oct/2016.
On average, the volume of water the orchard received by irrigation was 116.95 L d-1 per plant in 2015 and 92.24 L d-1 per plant in 2016, corresponding to a water depth of 9.36 and 7.38 mm, respectively. During this period, the contribution of rainfalls to the water volume received by the orchard was 0.55 mm (2015) and 1.48 mm (2016), while evapotranspiration (ETo) presented values of 5.67 and 5.22 mm d-1, respectively. Thus, the balance between the water volume received by the orchard and evapotranspiration during the same period presented a negative balance for non-irrigated plants.
On the other hand, irrigations performed between 7 and 15 days were sufficient to keep only the 0.5 m surface layer wet, while deeper layers received only rain water (Figure 6).
At the depth of 0.50 m (in the canopy projection of the irrigated plant), the soil water content was replaced by irrigation, and the contribution of rainfalls ranged between θcc and the water content considered critical for the crop (θcritical). Between April and May 2016, the water content was close to the θcritical (f = 0.7). Between Aug/16 and Sep/16, a biweekly irrigation period, the same behavior was observed. However, the contribution of rainfalls in Oct/16 was sufficient to raise the soil water content above θcritical. At the depth 1.00 m, irrespective of irrigation, the water volume remained below the θpmp. At 2.00 m, such volume ranged above and below the θpmp, while at 2.50 m, although not at θpmp, soil water content remained below θcritical during the evaluation period.
In the non-irrigated area, at a 0.50 m depth, soil water content remained below the θpmp until Oct/15. Thereafter, it presented increasing values, remaining above the θcritical, then decreasing again until Apr/16, when it remained below the θcritical. This was due to rainfalls. With the beginning of rains in Oct/16, the soil water volume at the 0.50 m depth presented the same increasing tendency. At the 1.00 m depth, there was an analogous behavior. However, the contribution of rainfalls was sufficient to raise the soil water content little above θpmp between February and April and November and December 2016. At 1.50 and 2.50 m, the soil water content, although below θpmp in Sep/2015 with the onset of rains in October of that year, remained above the θcritical between December 2015 and April 2016 at the depth 1.50 m and between February and April 2016 at the depth 2.50 m.
Fertilization
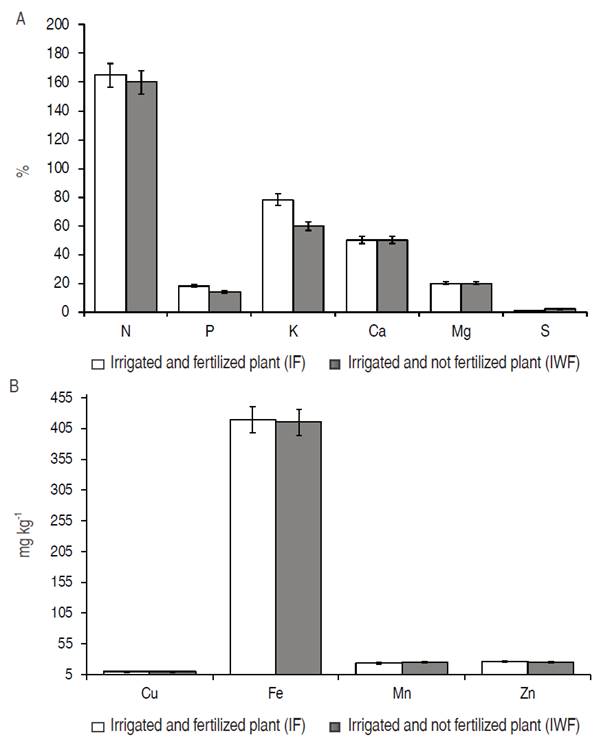
The organic fertilization provided a high K content in pequi leaves. Regarding other nutrients, no significant differences were observed (Figure 2). In fertilized plants, the K content was 78%. In plants without fertilization, it was 60%.
Figure 2: A. Macronutrients content; B. Micronutrients content in the plant tissue of pequi plants (Caryocar brasiliense Camb.) cultivated using irrigation and organic fertilization (IF) and irrigation without organic fertilization (IWF).
Growing of the pequi trees
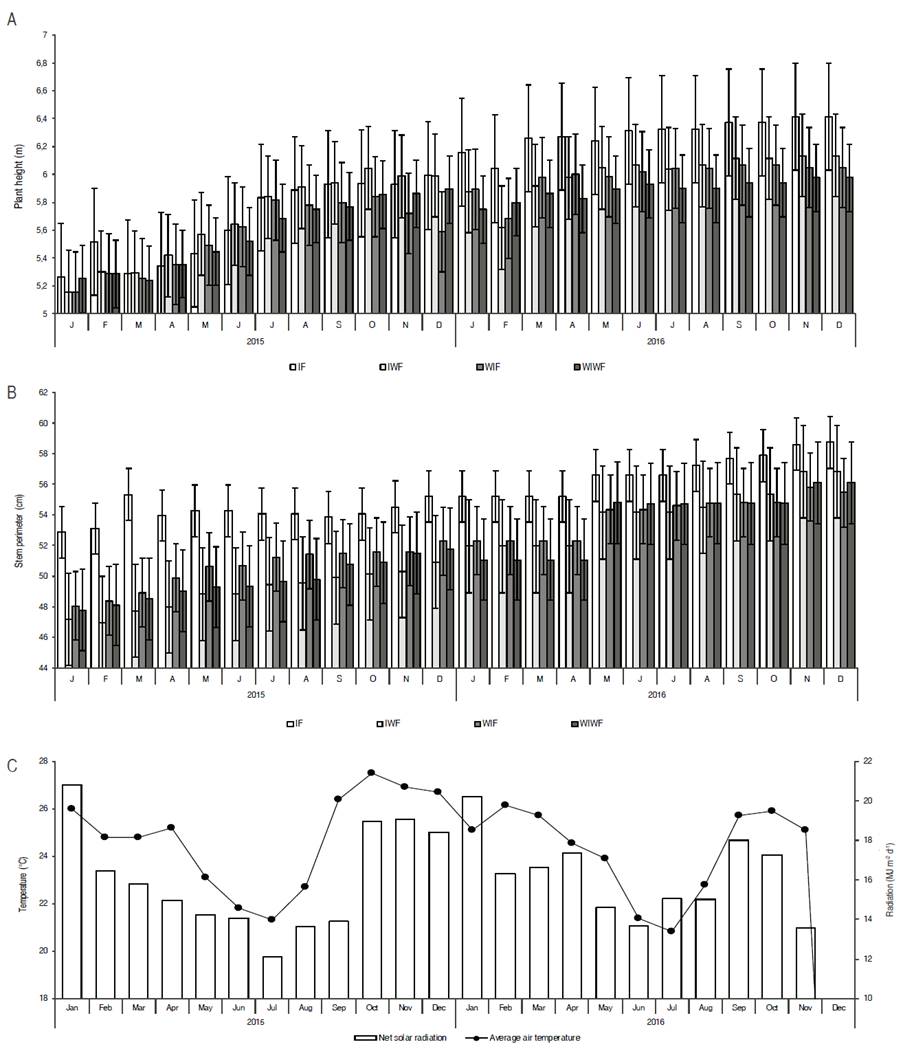
The results obtained showed that there was no significant interaction (P>≥0.5) between irrigation and fertilization management for the variables plant height and stem perimeter, but that there was a significant effect of irrigation on canopy area (P<0.5). At 24 months of evaluation, plant height ranged from 5.26 to 6.41 m (IF), 5.16 to 6.13 m (IWF), 5.16 to 6.04 m (WIF) and 5.25 to 5.98 m (WIWF). The stem perimeter varied from 52.88 to 58.74 cm, 47.17 to 56.82 cm, 48.06 to 55.47 cm and 47.78 to 56.11 cm in the treatments IF, IWF, WIF and WIWF, respectively (Figure 3).
Figure 3: A. Plant height; B. Stem perimeter of pequi trees grown in an experimental orchard, between the ages of 6 and 7.9 years; C. Average air temperature and mean monthly solar radiation.
There was a greater stem perimeter in irrigated and fertilized plants (IF) compared to other treatments during the first half of 2015 (Figure 3B). However, during the second half of the year, plants submitted to the other treatments were similar regarding stem perimeter. This is due to a higher availability of energy (average air temperature and radiation) during this period (Figure 3C), in addition to a greater availability of soil water due to the rainfalls beginning on September and lasting up to May (Figure 3). Thus, the plant did not respond to irrigation and organic fertilization in relation to height and stem perimeter. Previous studies also found no significant effects of fertilization and irrigation on plant growth (Alves et al., 2013, 2015; Miranda et al., 2016). An explanation for such results is that the pequi tree, as a native species from the Cerrado, has drought tolerance mechanisms, such as osmotic adjustment and deep root systems, which maintain its slow growth even during dry season and dry conditions (Sternberg et al., 2004; Palhares et al., 2010).
In relation to canopy area, values of 13.28, 10.84, 8.34 and 10.17 m² were obtained for the treatments IF, IWF, WIF and WIWF, respectively (Figure 4). Ferreira et al. (2015) reports that there is a positive and significant correlation between the number of fruits and the canopy projection area of pequi trees.
Figure 4: Average air and leaf temperature of pequi trees, irrigated and not irrigated, cultivated in an experimental orchard, between the ages of 6.10 and 7.6 years.
The authors also evaluated other dendometric characteristics, such as height and diameter, but the canopy area explained the best plant productivity. In this sense, the effects of irrigation on the growth of the canopy area of pequi trees (Figure 4) deserves continued studies, given that it may make the implementation and the productivity of commercial pequi orchards feasible.
Water stress
Leaf temperature evaluations indicated that treatment plants without irrigation presented water deficit (Figure 5). During the rainy season (Nov/2015-Mar/2016), there was no evidence of water stress. However, from April, a transition period between the dry and the rainy seasons in the Cerrado of Goiás, there is stress in plants without irrigation. In that month, the leaf temperature was up to 5.90 °C higher than the average air temperature. Even in the irrigated treatment, the interruption of irrigation during the rainy season made the plant show a tendency to water stress (leaf temperature equivalent to average air temperature) during that same period, which did not occur due to the restart of irrigation in May.
Figure 5: Average air and leaf temperature of pequi trees, irrigated and not irrigated, cultivated in an experimental orchard, between the ages of 6.10 and 7.6 years.
Even the supply of water from rainfalls (Figure 6) was not sufficient to avoid water stress of non-irrigated plants during the first dry months (May-June 2016) (Figure 6). In June and July, the thermal gradient between average leaf and air temperatures was 1.20 and 1.50 °C, respectively. Under irrigation, the mean thermal gradient for the evaluation period was -2.00 °C.
Figure 6: Soil water content of irrigated and not irrigated soils in the experimental pequi orchard.
Although the evaluation of leaf temperature as an indicative for water stress (Khatum et al., 2015; Brunini and Turco, 2016) is rather widespread, little is discussed about the value of the gradient in which physiological changes occur, especially because this is an information that depends directly on plant sensitivity to water deficit (Taiz and Zeiger, 2004). For the conditions of this study, it was possible to observe water stress in pequi trees in gradients of temperature above 1.20 °C. However, the adaptation of the plant to the edaphoclimatic conditions of the Cerrado of Goiás allowed its growth even under water stress.
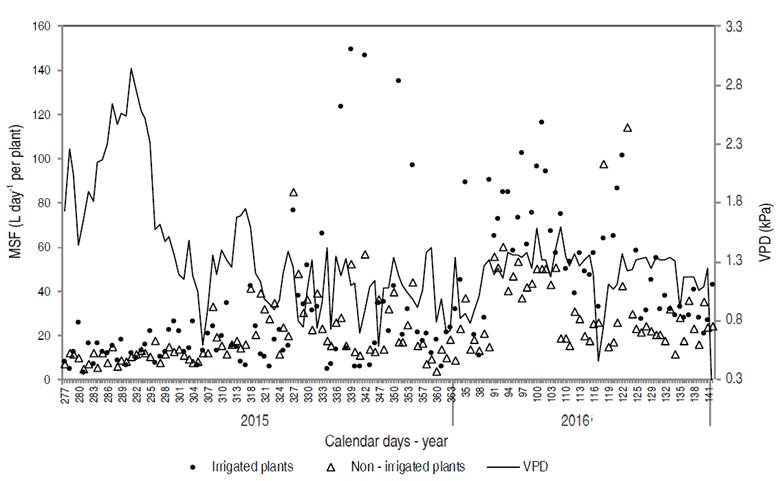
During the rainy season (calendar days 277 to 121), the mean sap flow was 39.30 and 23.29 L d-1 per plant for irrigated and non-irrigated pequi trees, respectively. With the beginning of the dry season, the flow was 64.26 L d-1 per plant for irrigated plants, whereas this value was 29.06 L d-1 per plant for non-irrigated plants (Figure 7). The occurrence of a higher sap flow during drought is due to a shorter evaluation period under this condition (19 days) associated to the transition period between the end of rainfalls and the beginning of the drought period, ensuring the availability of soil water.
Figure 7: Vapor pressure deficit (VPD) and mean sap flow (MSF) of irrigated and non-irrigated pequi trees cultivated in an experimental orchard between the ages of 6.6 and 7.4 years.
The raw sap flow is associated with plant transpiration. In order to carry sap, there is a need for water stress in the xylem (the main water transport cell in vascular plants) established by transpiration (Pimentel, 2004). Considering the whole evaluation period (calendar days 277 to 142), the mean orchard transpiration was 42.79 and 24.09 L d-1 per plant for irrigated plants and plants cultivated without irrigation, respectively. Thus, if irrigated, pequi plants transpire approximately 56% more than under natural conditions in the Cerrado biome.
The effects of soil water content, although at the superficial layer and with a higher content of leaf K due to fertilization, on leaf temperature and sap flow (Figures 6 and 7) of pequi trees suggest that, under irrigation, the pequi tree presents more absorbent roots on the soil surface, which provides the plant with a greater absorption of water and nutrients. Well-supplied potassium plants have a high water use efficiency, while potassium-deficient plants have a low photosynthetic performance due to an irregular stomatal opening, reducing the intake of CO2 (Silva et al., 2016). In guava trees, the appropriate level of K ranges from 1.7 to 2.0 dag kg-1 (Natale et al., 1994). Although guava was initially considered as rustic as and morphologically similar to pequi trees, they are possibly physiologically different plants with different nutritional requirements, since the fertilized and irrigated pequi tree tolerated water stress.
CONCLUSIONS
Between 6 and 8 years of age, pequi trees do not respond to irrigation and organic fertilization in terms of growth in height and stem perimeter. On the other hand, irrigation provides trees with larger canopies.
REFERENCES
Referencias
Allen RG, Pereira LS, Raes D and Smith M. 1998. Crop evapotranspiration – Guidelines for computing crop water requirements - FAO Irrigation and Drainage Paper 56. FAO 300(9): 109.
Alves Júnior J, Taveira MR, Casaroli D, Evangelista, AWP, Vellame LM and Mozena WL. 2015. Respostas do pequizeiro à irrigação e adubação orgânica. Global Science and Technology 8(1): 47-60.
Alves Júnior J, Taveira MR, Evangelista AWP, Casaroli D and Barbosa LHA. 2013. Crescimento de plantas jovens de pequizeiro irrigadas na região do Cerrado. Revista Agrotecnologia 4(1): 58-73.
Brunini RG and Turco JEP. 2016. Water stress indices for the sugarcane crop on different irrigated surfaces. Revista Brasileira de Engenharia Agrícola e Ambiental 20(10): 925-929. doi: 10.1590/1807-1929
Corrêa MLP, Galvão JCC, Lemos JP, Miranda GV, Rodrigues OL, Conceição PM and Fontanetti A. 2013. Qualidade química do solo e características produtivas do capim-elefante submetido à adubação química e orgânica. Revista Brasileira de Agropecuária Sustentável 3(1): 99-104.
Crisostomo LA and Naumov A. 2009. Adubando para alta produtividade e qualidade: Fruteiras tropicais do Brasil. Fortaleza: Embrapa Agroindústria Tropical. 238 p. (IIP. Boletim 18).
Doorembos J and Kassan AH. 1977. Las necessidades de água de los cultivos. FAO. 144 p. (FAO. Boletin Irrigación y Drenage, 24).
Doorembos J and Kassan AH. 1979. Yield response to water. FAO. 193 p. (FAO. Irrigation Drainage Paper, 33).
Dourado Neto D, Jong Van Lier QD, Botrel TA and Libardi PL. 1990. Programa para confecção da curva de retenção de água no solo utilizando o modelo de Genuchten. Engenharia Rural 1(2): 92- 102.
EMBRAPA – Empresa Brasileira de Pesquisa Agropecuária. 1997. Centro Nacional de Pesquisa de Solos. Manual de Métodos de Análise de Solos. 2.ed. Embrapa, Rio de Janeiro. 212 p.
Faci JM and Fereres E. 1980. Responses of grain sorghum to variable water supply under two irrigation frequencies. Irrigation Science 1(3): 149-159.
Ferreira GA, Naves RE, Chaves LJ, Veloso VR and Barboza ER. 2015. Produção de frutos de populações naturais de pequizeiro no estado de Goiás. Revista Brasileira de Fruticultura 37(1): 121- 129. doi: 10.1590/0100-2945-404/13
Granier A. 1987. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree physiology 3(4): 309-320. doi: 10.1093/treephys/3.4.309
Haridasan M. 2000. Nutrição mineral das plantas nativas do Cerrado. Revista Brasileira de Fisiologia Vegetal 12(1): 54-64.
Khatum S, Ahmed JU and Mohi-ud-din M. 2015. Variation of wheat cultivars in their relationship between seed reserve utilization and leaf temperature under elevated temperature. Irrigation Science 18(2): 97-101. doi: 10.1007/s12892-014-0117-y
Malavolta E, Vitti GC and Oliveira SA. 1997. Avaliação do estado nutricional das plantas: princípios e aplicações. 2. ed. Potafos, Piracicaba. 319 p.
Miranda RF, Alves Júnior J, Xavier GL, Casaroli D, Evangelista AWP and Mesquita M. 2016. Crescimento do pequizeiro em resposta a irrigação e adubação. Revista Cultura Agronômica 25(4): 351-360.
Natale W, Coutinho ELM, Boaretto AE and Banzatto DA. 1994. Influência da época de amostragem na composição química das folhas de goiabeira (Psidium guajava L.). Revista de Agricultura 69(3): 247-255.
Neto JD, Sousa AA, Azevedo CAV, Fernandes PD, Lima VLA. 2015. Caracterização morfoagronomica de frutos de goiabeira submetida a diferentes lâminas de água e adubação nitrogenada. Revista Caatinga 28(3): 174-183. doi: 10.1590/1983- 21252015
Nunes MUC. 2009. Compostagem de resíduos para produção de adubo orgânico na pequena propriedade. Embrapa Tabuleiros Costeiros. Aracaju, Circular Técnica. 130 p.
Palhares D, Franco AC and Zaidan LBP. 2010. Respostas fotossintéticas de plantas de cerrado nas estações seca e chuvosa. Revista Brasileira de Biociências 8(2): 213-220.
Pimentel C. 2004. A relação da planta com a água. Edur. Rio de Janeiro. 191 p.
Pinto LCL, Morais LMO, Guimarães AQ, Almada ED, Barbosa PM, Drumond MA. 2016. Traditional knowledge and uses of the Caryocar brasiliense Cambess. (Pequi) by “quilombolas” of Minas Gerais, Brazil: subsidies for sustainable management. Brazilian Journal of Biology 76(2): 511-519. doi: 10.1590/1519-6984.22914
Silva JTA, Silva IP and Simão FR. 2016. Produção e nutrição de limoeiro ‘Tahiti’ em função da adubação com nitrogênio e potássio em cinco safras. Pesquisa Agropecuária Brasileira 51(4): 357-363. doi: 10.1590/S0100-204X2016000400008
Silva Júnior MC. 2005. Guia de Campo: 100 árvores do cerrado. Editora Rede de Sementes do Cerrado. Brasília. 278 p.
Sternberg LSL, Bucci S, Franco AC, Goldstein G, Hoffman WA, Meinzer FC, Moreira MZ and Scholz F. 2004. Long range lateral root activity by neo-tropical savanna trees. Plant and Soil 1(270): 169- 178. doi: 10.1007/s11104-0041334-9
Taiz L and Zeiger E. 2004. Fisiologia vegetal. 3. ed. Artmed. Porto Alegre. 722 p.
Trentin R, Zolnier S, Ribeiro A and Steidle Neto AJ. 2011. Transpiração e temperatura foliar da cana-de-açúcar sob diferentes valores de potencial matricial. Engenharia Agrícola 31(6): 1085- 1095. doi: 10.1590/S0100-69162011000600006
Van Genuchten MT. 1980. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Science Society of America Journal 44(5): 892-898.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2018 Revista Facultad Nacional de Agronomía Medellín

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
La Revista permite al autor(es) mantener los derechos de explotación (copyright) de sus artículos sin restricciones. El(os) autor(es) acepta(n) la distribución de sus artículos en la web y en soporte papel (300 ejemplares por número), bajo acceso abierto a nivel local, regional e internacional; la inclusión y difusión del texto completo, a través del Portal de Revistas y Repositorio Institucional de la Universidad Nacional de Colombia; y en todas las bases de datos especializadas que la Revista considere pertinentes para su indexación, con el fin de proporcionarle visibilidad y posicionamiento al artículo. Todos los artículos deben cumplir la legislación colombiana e internacional, relacionada con derechos de autor.
Compromisos del autor
El autor(es) se compromete(n) a ceder los derechos de impresión y reimpresión del material publicado a la Revista Facultad Nacional de Agronomía Medellín y cualquier cita a los artículos editados en la Revista se deberá hacer si se adiciona el crédito respectivo. En caso de duplicación del contenido de la Revista o su publicación parcial o total en otro idioma, se deberá contar con el permiso escrito del Director.
Responsabilidad de los contenidos
La Facultad de Ciencias Agrarias y la Revista no se responsabilizan o solidarizan, necesariamente, con los conceptos emitidos en los artículos publicados, cuya responsabilidad será en su totalidad del autor o los autores.