Primary Merkel cell carcinoma of the breast in a man with skin graft: a case report
Carcinoma de células de Merkel primario de la mama en un hombre con injerto cutáneo. Reporte de caso
DOI:
https://doi.org/10.15446/revfacmed.v71n1.93988Palabras clave:
Carcinoma Merkel Cell, Merkel Cells, Merkel cell polyomavirus, Breast, Breast Diseases (en)Carcinoma de Células de Merkel, Células de Merkel, Poliomavirus de Células de Merkel, Mama, Enfermedades de la Mama (es)
Descargas
Introduction: Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine skin cancer that usually appears as a flesh-colored or bluish-red nodule on the face, head, or neck. MCC is primarily found in older adults.
Case presentation: An 85-year-old white man visited the breast care service of the Servicio de Mastología del Instituto Nacional de Oncología y Radiobiología (National Institute of Oncology and Radiobiology, or INOR by its acronym in Spanish) in Havana, Cuba, due to an increase in volume, color change, and a burning sensation in the right breast. The patient had suffered thermal trauma to the right hemithorax 18 years before the consultation, which was treated with a skin graft of the thigh. Imaging studies (ultrasound, mammography, magnetic resonance imaging, and computed tomography) showed a nodule with imaging features suggestive of malignancy. CCM diagnosis was confirmed by core needle biopsy, reporting positivity of CD-56, CK-7, and Ki-67 markers.
Conclusions. Typically, MCC presents as a rapidly growing, firm skin nodule in sun-exposed areas, contrary to the present case, in which the lesion appeared on grafted skin on the right hemithorax. Recognizing imaging findings suggestive of this neoplasm is of great importance for its diagnosis in unusual areas of the body, such as the breast.
Introducción. El carcinoma de células de Merkel (CCM) es un cáncer neuroendocrino de la piel agresivo y muy poco frecuente que, por lo general, aparece como un nódulo de color carne o rojo azulado en la cara, cabeza o cuello. El CCM ocurre principalmente en adultos mayores.
Presentación del caso. Hombre de 85 años, blanco, que asistió al Servicio de Mastología del Instituto Nacional de Oncología y Radiobiología (INOR), en La Habana, Cuba, por aumento de volumen, cambio de color y temperatura en la mama derecha. El paciente había sufrido trauma térmico en el hemitórax derecho 18 años antes de la consulta, el cual fue tratado mediante injerto cutáneo del muslo. En los estudios de imagen (ultrasonido, mamografía, resonancia magnética y tomografía computarizada) se observó un nódulo con características imagenológicas sugestivas de malignidad. El diagnóstico de CCM se confirmó mediante biopsia por punción con aguja gruesa, donde se reportó positividad de marcadores CD-56, CK-7 y Ki-67.
Conclusiones. Característicamente, el CCM se presenta como nódulos cutáneos firmes de rápido crecimiento en las áreas expuestas al sol, a diferencia del presente caso, en el que la lesión apareció en la piel injertada en el hemitórax derecho. Reconocer hallazgos imagenológicos sugestivos de esta neoplasia es de gran importancia para el diagnóstico en zonas inusuales del cuerpo como la mama.
Case report
Primary Merkel cell carcinoma of the breast in a man with skin graft: a case report
Carcinoma de células de Merkel primario de la mama en un hombre con injerto cutáneo. Reporte de caso
Yoandry Calderón-Montero1 Naibel Quevedo-Ramírez1
Naibel Quevedo-Ramírez1 María de la Caridad Campos-Bernardo1
María de la Caridad Campos-Bernardo1 Raydel Pérez-Castillo2
Raydel Pérez-Castillo2
1 Instituto Nacional de Oncología y Radiobiología - Radiology Service - Havana - Cuba.
2 Instituto de Medicina del Deporte - Havana - Cuba.
Open access
Received: 02/03/2021
Accepted: 28/02/2022
Corresponding author: Naibel Quevedo-Ramírez. Servicio de Radiología, Instituto Nacional de Oncología y Radiobiología. La Habana. Cuba. Email: naiquevedoramírez@gmail.com.
Keywords: Carcinoma Merkel Cell; Merkel Cells; Merkel cell polyomavirus; Breast; Breast Diseases (MeSH).
Palabras clave: Carcinoma de células de Merkel; Células de Merkel; Poliomavirus de células de Merkel; Mama; Enfermedades de la mama (DeCS).
How to cite: Calderón-Montero Y, Quevedo-Ramírez N, Campos-Bernardo MC, Pérez-Castillo R. Primary Merkel cell carcinoma of the breast in a man with skin graft: a case report. Rev. Fac. Med. 2023;71(1):e93988. English. doi: https://doi.org/10.15446/revfacmed.v71n1.93988.
Cómo citar: Calderón-Montero Y, Quevedo-Ramírez N, Campos-Bernardo MC, Pérez-Castillo R. [Carcinoma de células de Merkel primario de la mama en un hombre con injerto cutáneo: reporte de caso]. Rev. Fac. Med. 2023;71(1):e93988. English. doi: https://doi.org/10.15446/revfacmed.v71n1.93988.
Copyright: Copyright: ©2023 Universidad Nacional de Colombia. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, as long as the original author and source are credited.
Abstract
Introduction: Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine skin cancer that usually appears as a flesh-colored or bluish-red nodule on the face, head, or neck. MCC is primarily found in older adults.
Case presentation: An 85-year-old white man visited the breast care service of the Servicio de Mastología del Instituto Nacional de Oncología y Radiobiología (National Institute of Oncology and Radiobiology, or INOR by its acronym in Spanish) in Havana, Cuba, due to an increase in volume, color change, and a burning sensation in the right breast. The patient had suffered thermal trauma to the right hemithorax 18 years before the consultation, which was treated with a skin graft of the thigh. Imaging studies (ultrasound, mammography, magnetic resonance imaging, and computed tomography) showed a nodule with imaging features suggestive of malignancy. CCM diagnosis was confirmed by core needle biopsy, reporting positivity of CD-56, CK-7, and Ki-67 markers.
Conclusions. Typically, MCC presents as a rapidly growing, firm skin nodule in sun-exposed areas, contrary to the present case, in which the lesion appeared on grafted skin on the right hemithorax. Recognizing imaging findings suggestive of this neoplasm is of great importance for its diagnosis in unusual areas of the body, such as the breast.
Resumen
Introducción. El carcinoma de células de Merkel (CCM) es un cáncer neuroendocrino de la piel agresivo y muy poco frecuente que, por lo general, aparece como un nódulo de color carne o rojo azulado en la cara, cabeza o cuello. El CCM ocurre principalmente en adultos mayores.
Presentación del caso. Hombre de 85 años, blanco, que asistió al Servicio de Mastología del Instituto Nacional de Oncología y Radiobiología (INOR), en La Habana, Cuba, por aumento de volumen, cambio de color y temperatura en la mama derecha. El paciente había sufrido trauma térmico en el hemitórax derecho 18 años antes de la consulta, el cual fue tratado mediante injerto cutáneo del muslo. En los estudios de imagen (ultrasonido, mamografía, resonancia magnética y tomografía computarizada) se observó un nódulo con características imagenológicas sugestivas de malignidad. El diagnóstico de CCM se confirmó mediante biopsia por punción con aguja gruesa, donde se reportó positividad de marcadores CD-56, CK-7 y Ki-67.
Conclusiones. Característicamente, el CCM se presenta como nódulos cutáneos firmes de rápido crecimiento en las áreas expuestas al sol, a diferencia del presente caso, en el que la lesión apareció en la piel injertada en el hemitórax derecho. Reconocer hallazgos imagenológicos sugestivos de esta neoplasia es de gran importancia para el diagnóstico en zonas inusuales del cuerpo como la mama.
Introduction
Merkel cell carcinoma (MCC), also known as neuroendocrine carcinoma of the skin, is a rare and aggressive neuroendocrine neoplasm of the skin1-3 that was first described in 1972 by Toker as a trabecular cancer of the skin.4 Despite being 40 times less common than malignant melanoma, MCC has twice its fatality rate.5-7
The incidence rate of MCC varies between 0.13 and 1.6 cases per 100 000 inhabitants; moreover, in the United States and Europe it has increased in recent years, with an average of 0.18-0.41 cases per 100 000 inhabitants.4,5,8 Australia is the country with the highest number of MCC cases, with an annual rate of 1.6 cases per 100 000 inhabitants,8-10 whereas in Cuba there are few reports of this type of neoplasm.
Risk factors for MCC include exposure to ultraviolet light, immunosuppression, advanced age, and polyomavirus infection.11,12 These neoplasms originate in the skin in 97% of cases, are very rare in mucous membranes (such as the larynx), and usually present as rapidly growing, asymptomatic subcutaneous nodules.13 MCCs are usually located on the head (face), extremities (both upper and lower) and, to a lesser extent, the trunk.14 In addition, palpable regional metastatic adenopathies are frequent. Distant metastases usually occur in the lungs, liver, bones, and brain.4,12,15 In the thorax, MCC may occur as a primary or secondary tumor.16
The breast is an uncommon site of metastases for virtually any type of tumor, including carcinomas, sarcomas, and hematolymphoid neoplasms,17-19 and although metastases generally recapitulate the histologic features of the primary tumor, in this location, they are a diagnostic challenge given their rare occurrence. MCC in the breast, which presents as an exceptionally rare subcutaneous mass,20 can be a primary or secondary tumor,2 and a cautious interpretation of medical records, and imaging, histopathologic and immunohistochemical findings is essential for its diagnosis.5,8
MCC diagnosis and staging using imaging techniques is achieved through computed tomography, magnetic resonance imaging, and positron emission tomography.2 Histopathologically, MCC is composed of small round blue cells with fine granular chromatin and high nuclear to cytoplasmic ratio, high mitotic rate, and occasional necrosis.21,22
The following report presents the case of an elderly man with a subcutaneous nodule in the right breast diagnosed as MCC, a location in which a skin graft had been transplanted years earlier.
Case presentation
On March 4, 2020, an 85-year-old white man went to the breast care service of the Instituto Nacional de Oncología y Radiobiología (National Institute of Oncology and Radiobiology or INOR by its acronym in Spanish) in Havana, Cuba, due to a change in color and an increase in volume and temperature in the right breast. The patient had a history of thermal trauma to the right hemithorax (18 years prior to consultation), which was treated with a skin graft from his right thigh.
Physical examination revealed the following findings: skin changes in the right breast and ipsilateral axillary region with increased volume, as well as a hard, firm and large palpable mass adhering to deep planes. No alterations were found in the breast or left axillary region. After the clinical evaluation, outpatient treatment was prescribed, and breast ultrasound and digital mammography were requested, which were performed the following day (05/03/20).
Ultrasound showed that the left breast had no alterations, but the right breast had a fatty breast pattern, an extensive solid nodular area located in the upper outer quadrant and axillary extension, microlobulated margins associated with skin thickening (6mm), and signs of lymphedema of the subcutaneous cellular tissue. This examination established that the right breast lesion was classified as BI-RADS 5; in addition, the right axilla showed lymph nodes classified as BRN-4 according to Rostagno’s classification, which corresponds to lymph nodes >3.5mm in size with increased thickness and/or focal thickening of the cortex (Figure 1).

Figure 1. Patient’s breast ultrasound.
Source: Image obtained while conducting the study.
Mammography also showed that the left breast had no alterations, and that the right breast had a fatty breast pattern, increased size with global diffuse distortion of the glandular architecture, skin thickening (6mm), and trabecular thickening of the subcutaneous cellular tissue. It was also noteworthy that there was an oval nodule with indeterminate margins, approximately 110x84mm, without calcifications and located in the upper outer quadrant towards the axillary extension, as well as lymph nodes with a metastatic appearance in the right axilla. Based on this examination, the right breast lesion was also classified as BI-RADS 5 (Figure 2).
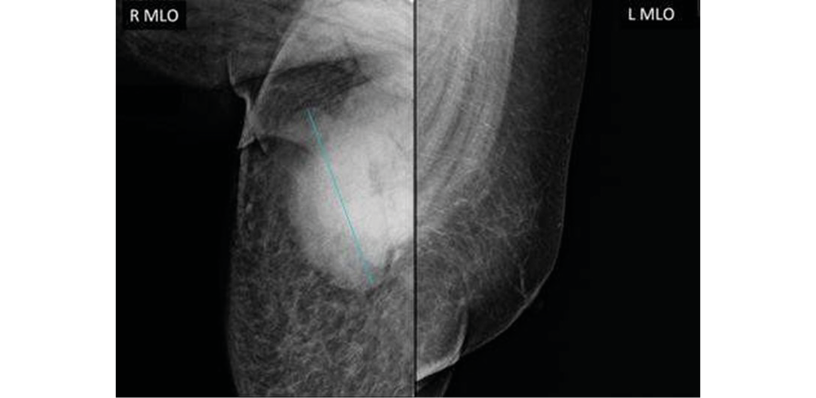
Figure 2. Patient’s mammogram.
Source: Image obtained while conducting the study.
On March 18, 2020, the patient was reassessed during a follow-up consultation by the breast care service, and in view of the ultrasound and mammography findings, confirmatory studies (ultrasound-guided automated TRU-CUT needle biopsy) and extension studies (computed axial tomography, magnetic resonance imaging, and blood analysis panel) were performed. Thus, the following day, a simple computed axial tomography of the thorax and abdomen with coronal reconstruction was performed, which showed the following findings (Figure 3):
- Hypodense solid mass with irregular borders in the upper outer quadrant of the right breast towards the axillary extension, without calcifications and without infiltration of the pectoralis major muscle.
- Metastatic lymph nodes in the right axilla.
- No metastases in the pleura, lungs and solid organs of the abdomen; no secondary bone lesions were identified.

Figure 3. Patient’s computed axial tomography.
Source: Image obtained while conducting the study.
On March 28, 2020, the patient underwent a chest MRI that showed an irregular nodule measuring 9.6x7.4cm, of hyperintense appearance in STIR sequence, with non-circumscribed margins located in the upper outer quadrant of the right breast, extending to the tail of the breast (Figure 4A), and of heterogeneous features in T2-weighted sequences in axial slice (Figure 4B), which did not invade the pectoralis major muscle or the chest wall. Diffuse distortion of the architecture of the right breast was evident in all sequences (BI-RADS 5).
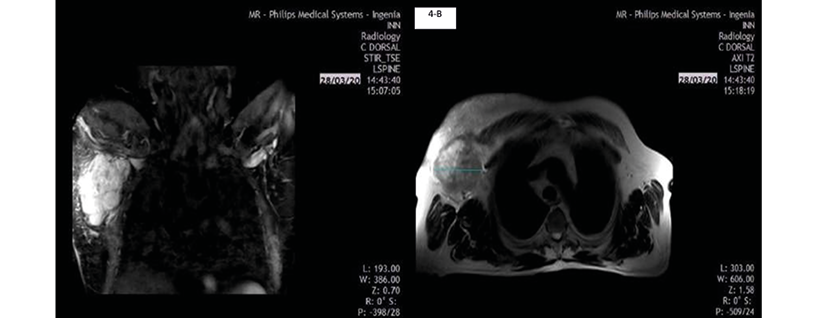
4-A
4-B
Figure 4. Patient’s MRI of the thorax.
Source: Image obtained while conducting the study.
On March 30, 2020, the results of the ultrasound-guided core needle biopsy of the breast were received (Figure 5). The sample obtained in this procedure was stained with hematoxylin and eosin (HE) and immunohistochemistry. HE staining showed poorly differentiated malignant proliferation with diffuse infiltrative growth pattern alternating with areas of necrosis; uniform medium-sized cells, scant cytoplasm, and nuclei with fine granular chromatin; and atypical mitoses and apoptosis. The immunohistochemistry report showed the following neuroendocrine markers: cytoplasmic CD-56 positive in tumor cells, nuclear CK-7 positive in some tumor cells, and nuclear Ki-67 intensely positive, which translates into a high proliferation rate and poor prognosis.

Figure 5. Images of histological studies using different staining techniques.
HE: hematoxylin and eosin.
Source: Images obtained while conducting the study.
Based on the findings, the patient was diagnosed with primary MCC and reevaluated by the breast care service. The patient also received follow-up by the oncology service in accordance with the treatment protocol established by the INOR.
Discussion
This study describes the clinical characteristics and the diagnostic, imaging and histopathologic findings in a male patient with MCC of the right breast, whose risk factors were age (85 years), skin color, and a history of a burn in the anterior region of the right hemithorax that required skin graft. The latter fact, together with the location of the neoplasm (breast), represent the unique qualities of the case.
As stated by Villa-Blanco & Nabhan13 in a descriptive clinical study that included 3 adult patients treated in a hospital in Cuenca (Spain), the mean age at the time of MCC diagnosis is 76.2 years in men and 73.6 in women, with rare cases in individuals younger than 50 years of age; no cases have been reported in children.
Primary breast MCC is much more common than breast metastasis. However, metastatic tumors in this area often pose diagnostic challenges when they present as solitary masses because they do not have distinct clinical features;16,20 in the present case, the history of skin graft as a result of thermal trauma is noteworthy. It should be kept in mind that, rarely, axillary adenopathy may herald a non-breast metastasis with the same caveats.17
In 2019, Korsa et al.16 published the case of a 79-year-old Croatian woman with a history of plaque psoriasis treated with methotrexate, who presented with a palpable, painless tumor in the upper outer quadrant of the breast associated with another 2-cm superficial retroareolar mass located below the discrete skin erythema. In that case, breast ultrasound, magnetic resonance imaging and computed tomography allowed the visualization of both lesions, which was also the case in the patient reported in the present study. Similarly, following core needle biopsy and histopathologic analysis, the presence of small, round, blue cells with nested architecture and high mitosis was demonstrated, findings consistent with a diagnosis of MCC.
Likewise, Chou et al.,23 in a case report of a 77-year-old woman with MCC, found a clear association of chronic arsenism with two different primary cutaneous carcinomas. In that patient, breast ultrasound and mammography confirmed the presence of a subcutaneous mass and a suspicious axillary node; in addition, as in the present case and the one described by Korsa et al.,16 histopathology revealed small round poorly differentiated blue cells with a high nuclear ratio compatible with MCC. Similarly, Albright et al.,20 in a case report of an 86-year-old woman with MCC, stated that, in addition to the imaging tests performed (mammography and OctreoScan), sentinel lymph node biopsy was also required to confirm the diagnosis.
As demonstrated by Fernández-Figueras et al.24 in a study of 72 tumor cases comparing the immunohistochemical expression of the p27Kip1, p45Skp2 and Ki67 prognostic markers in MCC, small cell neuroendocrine carcinoma of the lung, and urinary bladder and cutaneous squamous cell carcinoma, the Ki-67 protein is highly expressed in the majority of MCC cases. However, their value, rather than diagnostic, is prognostic/biological as they are associated with increased proliferation/aggressiveness.
Tondare et al.5 reported the case of a 55-year-old man with MCC in the breast, which was associated with lymphocytic leukemia; in this patient CD-56 and Ki-67 markers were positive and CK-7 was negative. In turn, Ansai25 stated in a review that CD-117 can help distinguish sweat or sebaceous gland tumors from other non-Merkel cell epithelial tumors.
Histologically, the differential diagnosis of MCC includes metastatic high-grade neuroendocrine carcinomas, lymphomas, and mesenchymal neoplasms.26 Thus, authors such as Llombart et al.2 and Brooks et al.8 have reported the usefulness of immunohistochemical markers in the diagnosis and prognosis of MCC based on neuroendocrine markers for epithelial cytokeratins.
Furthermore, some studies have established that an important differential diagnosis of MCC is primary neuroendocrine neoplasia of the breast, which must demonstrate neuroendocrine marker positivity in more than 50% of the cells. This marker also normally expresses CK-7, estrogen receptors, and progestin receptors, usually associated with ductal or lobular carcinoma.21,23,27
Treatment of primary MCC includes surgery and/or radiation; chemotherapy is usually recommended for advanced cases since this type of neoplasm is highly radiosensitive and within a few months becomes chemoresistant and prone to recurrence. Outcomes in patients with metastases are poor, as evidenced in the patient reported here, with a historical 5-year survival of 13.5%.3
Based on similarities to small cell carcinoma, schedules with platinum and etoposide or cyclophosphamide, doxorubicin, and vincristine are most commonly used for first-line chemotherapy in cases of MCC.28 In general, chemotherapy schemes combining carboplatin, cisplatin and etoposide, cyclophosphamide with vincristine, doxorubicin, prednisone, bleomycin, or 5-fluorouracil have been used in the treatment of MCC.29
In patients with localized disease, surgical excision with a 1-2cm margin and adjuvant radiotherapy to the primary site are recommended.30 Patients with a positive node biopsy result should undergo a complete lymph node dissection, which in some cases should be followed by radiotherapy, while those with a negative result should undergo sentinel lymph node biopsy.
Nodal basin radiation administered at the same time as radiation to the primary site is also a treatment option for patients with MCC, especially when dealing with large, locally inoperable tumors, with narrow excision margins, tumor involvement that cannot be improved with additional surgery, and involved regional nodes, mainly after sentinel lymph node dissection. In cases of primary MCC, it is recommended to initiate radiotherapy after surgical excision.31
In a retrospective analysis that included data from 6 908 patients with MCC available in the National Cancer Data Base, Bhatia et al.32 found that, after adjustment for the other variables, patients undergoing surgery plus adjuvant radiotherapy had statistically significantly improved overall survival compared to patients undergoing surgery alone among those with localized MCC (stage I: HR=0.71; 95%CI: 0.64-0.80; p<0.001, stage II: HR=0.77; 95%CI: 0.66-89; p<0.001).
A few years ago, patients with metastatic MCC were treated with the previously described conventional chemotherapy models seeking a palliative effect. Currently, although there are no randomized controlled trials comparing chemotherapy with immunotherapy, there are promising clinical trials that allow us to recommend immunotherapy as first-line treatment in this type of neoplasm.33,34 Avelumab was approved in 2017 by the Food and Drug Agency and subsequently by the European Medicines Agency for treatment of metastatic MCC. Other antibodies such as pembrolizumab (approved in the United States but not in Europe) and nivolumab are currently under study.35,36
Given the low frequency of primary MCC in the thorax, it is crucial for its diagnosis and subsequent treatment to rule out metastases or primary breast cancer from another type of neuroendocrine tumor given the implications for future treatment. In this sense, the complete analysis of clinical, radiological, histopathological, and immunohistochemical findings is decisive in appropriate decision making.
Conclusion
Typically, MCC presents as a rapidly growing, firm skin nodule in sun-exposed areas, contrary to the present case, in which the lesion appeared on the skin graft on the right hemithorax. Recognizing imaging findings suggestive of this neoplasm is of great importance for diagnosis in unusual areas of the body such as the breast.
Ethical considerations
For the preparation of this case report, the patient’s legal consent and approval by the Research Ethics Committee of the Instituto Nacional de Oncología y Radiobiología was obtained, as stated in the minutes issued on March 10, 2021.
Conflicts of interest
None stated by the authors.
Funding
None stated by the authors.
Acknowledgments
None stated by the authors.
References
1.Palencia R, Sandhu A, Webb S, Blaikie T, Bharmal M. Systematic literature review of current treatments for stage I-III Merkel cell carcinoma. Future Oncol. 2021;17(34):4813-22. https://doi.org/j6w6.
2.Llombart B, Kindem S, Chust M. Merkel Cell Carcinoma: An Update of Key Imaging Techniques, Prognostic Factors, Treatment, and Follow-up. Actas Dermosifiliogr 2017;108(2):98-107. https://doi.org/j6w7.
3.Stachyra K, Dudzisz-Śledź M, Bylina E, Szumera-Ciećkiewicz A, Spałek MJ, Bartnik E, et al. Merkel Cell Carcinoma from Molecular Pathology to Novel Therapies. Int J Mol Sci. 2021;22(12):6305. https://doi.org/gkkh2f.
4.Keller N, Haemmerle B, Schmid S. A rare case of Merkel cell carcinoma with ovarian metastasis. Gynecol Oncol Rep. 2019;28:76-8. https://doi.org/j6w8.
5.Tondare A, Sahay A, Joshi S, Bagal B, Shet T. A rare case of aggressive, Merkel Cell Carcinoma of the male breast co-existing with chronic lymphocytic leukemia. Breast J. 2020;26(7):1389-91. https://doi.org/j6w9.
6.Madu MF, van Veenendaal LM, van de Wiel B, Tesselaar MET, van Akkooi ACJ. Concurrent Merkel Cell Carcinoma and Melanoma in Individual Patients Presents a Treatment Challenge: A Case Series. Clinical Skin Cancer. 2017;2(1-2):69-72. https://doi.org/j6xb.
7.Kotelnikova EF, Laus M, Croce A. Evidence and Considerations on Treatment of Small Size Merkel Cell Head and Neck Carcinoma. Int Arch Otorhinolaryngol. 2020;24(4):e487-91. https://doi.org/j6xc.
8.Brooks RG, Jiménez-Galainena JJ, Torriani-Mendoza GP. Carcinoma de células de Merkel. Presentación de un caso. Rev Haban Cienc Méd. 2013;13(1):55-60.
9.Sims JR, Grotz TE, Pockaj BA, Joseph RW, Foote RL, Otley CC, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: The Mayo Clinic experience of 150 patients. Surg Oncol. 2018;27(1):11-7. https://doi.org/gc7sc7.
10.García-Zamora E, Vela-Ganuza M, Martín-Alcalde J, Miñano-Medrano R, Pinedo-Moraleda F, López-Estebaranz JL. Carcinoma de células de Merkel: estudio descriptivo de 11 casos. Actas Dermosifiliogr- 2021;112(1):63-8. https://doi.org/j6xf.
11.Ishikawa M, Yamamoto T. Malignant melanoma after treatment for Merkel cell carcinoma. An Bras Dermatol. 2020;95(5):662-4. https://doi.org/j6xg.
12.Schadendorf D, Lebbé C, zur Hausen A, Avril MF, Hariharan S, Bharmal M, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53-69. https://doi.org/f9hf7q.
13.Villa-Blanco JM, Nabhan S. Carcinoma de células de Merkel. Estudio de 3 casos. Rev Chil Cir. 2016;68(6):456-61. https://doi.org/j69j.
14.Kuwamoto S. Recent advances in the biology or Merkel cell carcinoma. Hum Pathol. 2011;42(8):1063-77. https://doi.org/dn23kn.
15.Llombart B, Requena C, Cruz J. Update on Merkel Cell Carcinoma: Epidemiology, Etiopathogenesis, Clinical Features, Diagnosis, and Staging. Actas Dermosifiliogr. 2017;108(2):108-19. https://doi.org/j688.
16.Korsa L, Kovacevic L, Barsic-Ostojic S, Prutki M, Marusic Z. In-transit breast metastasis as the primary clinical presentation of Merkel cell carcinoma. Breast J. 2020;26(5):1033-4. https://doi.org/j689.
17.Troxell ML. Merkel cell carcinoma, melanoma, metastatic mimics of breast cancer. Semin Diagn Pathol. 2017;34(5):479-95. https://doi.org/gpzws7.
18.Chen HK, Li CF, Uen YH, Tian YF, Chen MJ, Yan CS, et al. Huge Dermatofibrosarcoma Protuberans Mimicking a Breast Malignant Tumor with Abscess. J Cancer Res Pract. 2014;1(3):267-72. https://doi.org/j69c.
19.Ranade M, Shah A, Desai SB, Rekhi B. A curious case of Ewing sarcoma with epithelial differentiation, presenting as a breast mass. Breast J 2020;26(11):2244-5. https://doi.org/j69d.
20.Albright EL, Keeney ME, Bashir A, Weigel RJ. Poorly differentiated neuroendocrine carcinoma of the breast with Merkel cell features. Breast J. 2018;24(4):644-7. https://doi.org/gc2j9m.
21.Nambudiri VE, Vivero M, Watson AJ, Thakuria M, Ng A, Russell S, et al. Merkel Cell Carcinoma Presenting as Subcutaneous Breast Masses: An Uncommon Presentation of a Rare Neuroendocrine Neoplasm. Breast J. 2016;22(1):113-5. https://doi.org/j69g.
22.Vázquez-Doval J, Llombart-Cussac B, Pérez-Bustillo A, Paradela-de la Morena SP, Fuente-González MJ, Fernández-Figueras MT, et al. Diagnosis and Treatment of Merkel Cell Carcinoma in Specialized Dermatology Units: A Clinical Practice Guideline of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr. 2019;110(6):460-8. https://doi.org/j69h.
23.Chou TC, Tsai KB, Wu CY, Hong CH, Lee CH. Presence of the Merkel cell polyomavirus in Merkel cell carcinoma combined with squamous cell carcinoma in a patient with chronic arsenism. Clin Exp Dermatol. 2016;41(8):902-5. https://doi.org/f9cdd2.
24.Fernández-Figueras MT, Puig L, Musulen E, Gilaberte M, Ferrándiz C, Lerma E, et al. Prognostic significance of p27Kip1, p45Skp2 and Ki67 expression profiles in Merkel cell carcinoma, extracutaneous small cell carcinoma, and cutaneous squamous cell carcinoma. Histopathology. 2005;46(6):614-21. https://doi.org/bwh92h.
25.Ansai SI. Topics in histopathology of sweat gland and sebaceous neoplasms. J Dermatol. 2017;44(3):315-26. https://doi.org/f9z3j4.
26.Sallman MA, Li JY, Swaby M, Chon SY. Cutaneous scalp metastasis of cholangiocarcinoma in hepatitis C. JAAD Case Rep. 2020;6(5):468-70. https://doi.org/j7ct.
27.Rodriguez N, Volmaro K, Martinez M, Pets E, Pignata L, Machado J, et al. Carcinoma de células de Merkel: Características clínico patológicas de una neoplasia agresiva de infrecuente presentación. Rev Fac Cien Med Univ Nac Cordoba. 2018;(Suppl JIC XIX):43-4.
28.Tai PT, Yu E, Winquist E, Hammond A, Stitt L, Tonita J, et al. Chemotherapy in Neuroendocrine/Merkel Cell Carcinoma of the Skin: Case Series and Review of 204 Cases. J Clin Oncol. 2000;18(12):2493-9. https://doi.org/j7cv.
29.Desch L, Kunstfeld R. Merkel Cell Carcinoma: Chemotherapy and Emerging New Therapeutic Options. J Skin Cancer. 2013; 2013:327150. https://doi.org/gbcmj4.
30.Naseri S, Steiniche T, Ladekarl M, Bønnelykke-Behrndtz ML, Hölmich LR, Langer SW, et al. Management Recommendations for Merkel Cell Carcinoma—A Danish Perspective. Cancers (Basel). 2020;12(3):554. https://doi.org/j7cw.
31.Petrelli F, Ghidini A, Torchio M, Prinzi N, Trevisan F, Dallera P, et al. Adjuvant radiotherapy for Merkel cell carcinoma: A systematic review and meta-analysis. Radiother Oncol. 2019;134:211-9. https://doi.org/gfzt99.
32.Bhatia S, Storer BE, Iyer JG, Moshiri A, Parvathaneni U, Byrd D, et al. Adjuvant Radiation Therapy and Chemotherapy in Merkel Cell Carcinoma: Survival Analyses of 6908 Cases From the National Cancer Data Base. J Natl Cancer Inst. 2016;108(9):djw042. https://doi.org/j7cx.
33.Cornejo C, Miller CJ. Merkel Cell Carcinoma. Dermatol Clin. 2019;37(3):269-77. https://doi.org/j7cz.
34.Robinson CG, Tan D, Yu SS. Recent advances in Merkel cell carcinoma. F1000Res. 2019;8:1995. https://doi.org/j7c2.
35.Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Brohl AS, et al. Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. J Clin Oncol. 2019;37(9):693-702. https://doi.org/j7c3.
36.Walocko FM, Scheier BY, Harms PW, Fecher LA, Lao CD. Metastatic Merkel cell carcinoma response to nivolumab. J Immunother Cancer. 2016;4:79. https://doi.org/f9dj9t.
Referencias
Palencia R, Sandhu A, Webb S, Blaikie T, Bharmal M. Systematic literature review of current treatments for stage I-III Merkel cell carcinoma. Future Oncol. 2021;17(34):4813-22. https://doi.org/j6w6.
Llombart B, Kindem S, Chust M. Merkel Cell Carcinoma: An Update of Key Imaging Techniques, Prognostic Factors, Treatment, and Follow-up. Actas Dermosifiliogr 2017;108(2):98-107. https://doi.org/j6w7.
Stachyra K, Dudzisz-Śledź M, Bylina E, Szumera-Ciećkiewicz A, Spałek MJ, Bartnik E, et al. Merkel Cell Carcinoma from Molecular Pathology to Novel Therapies. Int J Mol Sci. 2021;22(12):6305. https://doi.org/gkkh2f.
Keller N, Haemmerle B, Schmid S. A rare case of Merkel cell carcinoma with ovarian metastasis. Gynecol Oncol Rep. 2019;28:76-8. https://doi.org/j6w8.
Tondare A, Sahay A, Joshi S, Bagal B, Shet T. A rare case of aggressive, Merkel Cell Carcinoma of the male breast co-existing with chronic lymphocytic leukemia. Breast J. 2020;26(7):1389-91. https://doi.org/j6w9.
Madu MF, van Veenendaal LM, van de Wiel B, Tesselaar MET, van Akkooi ACJ. Concurrent Merkel Cell Carcinoma and Melanoma in Individual Patients Presents a Treatment Challenge: A Case Series. Clinical Skin Cancer. 2017;2(1-2):69-72. https://doi.org/j6xb.
Kotelnikova EF, Laus M, Croce A. Evidence and Considerations on Treatment of Small Size Merkel Cell Head and Neck Carcinoma. Int Arch Otorhinolaryngol. 2020;24(4):e487-91. https://doi.org/j6xc.
Brooks RG, Jiménez-Galainena JJ, Torriani-Mendoza GP. Carcinoma de células de Merkel. Presentación de un caso. Rev Haban Cienc Méd. 2013;13(1):55-60.
Sims JR, Grotz TE, Pockaj BA, Joseph RW, Foote RL, Otley CC, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: The Mayo Clinic experience of 150 patients. Surg Oncol. 2018;27(1):11-7. https://doi.org/gc7sc7.
García-Zamora E, Vela-Ganuza M, Martín-Alcalde J, Miñano-Medrano R, Pinedo-Moraleda F, López-Estebaranz JL. Carcinoma de células de Merkel: estudio descriptivo de 11 casos. Actas Dermosifiliogr- 2021;112(1):63-8. https://doi.org/j6xf.
Ishikawa M, Yamamoto T. Malignant melanoma after treatment for Merkel cell carcinoma. An Bras Dermatol. 2020;95(5):662-4. https://doi.org/j6xg.
Schadendorf D, Lebbé C, zur Hausen A, Avril MF, Hariharan S, Bharmal M, et al. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53-69. https://doi.org/f9hf7q.
Villa-Blanco JM, Nabhan S. Carcinoma de células de Merkel. Estudio de 3 casos. Rev Chil Cir. 2016;68(6):456-61. https://doi.org/j69j.
Kuwamoto S. Recent advances in the biology or Merkel cell carcinoma. Hum Pathol. 2011;42(8):1063-77. https://doi.org/dn23kn.
Llombart B, Requena C, Cruz J. Update on Merkel Cell Carcinoma: Epidemiology, Etiopathogenesis, Clinical Features, Diagnosis, and Staging. Actas Dermosifiliogr. 2017;108(2):108-19. https://doi.org/j688.
Korsa L, Kovacevic L, Barsic-Ostojic S, Prutki M, Marusic Z. In-transit breast metastasis as the primary clinical presentation of Merkel cell carcinoma. Breast J. 2020;26(5):1033-4. https://doi.org/j689.
Troxell ML. Merkel cell carcinoma, melanoma, metastatic mimics of breast cancer. Semin Diagn Pathol. 2017;34(5):479-95. https://doi.org/gpzws7.
Chen HK, Li CF, Uen YH, Tian YF, Chen MJ, Yan CS, et al. Huge Dermatofibrosarcoma Protuberans Mimicking a Breast Malignant Tumor with Abscess. J Cancer Res Pract. 2014;1(3):267-72. https://doi.org/j69c.
Ranade M, Shah A, Desai SB, Rekhi B. A curious case of Ewing sarcoma with epithelial differentiation, presenting as a breast mass. Breast J 2020;26(11):2244-5. https://doi.org/j69d.
Albright EL, Keeney ME, Bashir A, Weigel RJ. Poorly differentiated neuroendocrine carcinoma of the breast with Merkel cell features. Breast J. 2018;24(4):644-7. https://doi.org/gc2j9m.
Nambudiri VE, Vivero M, Watson AJ, Thakuria M, Ng A, Russell S, et al. Merkel Cell Carcinoma Presenting as Subcutaneous Breast Masses: An Uncommon Presentation of a Rare Neuroendocrine Neoplasm. Breast J. 2016;22(1):113-5. https://doi.org/j69g.
Vázquez-Doval J, Llombart-Cussac B, Pérez-Bustillo A, Paradela-de la Morena SP, Fuente-González MJ, Fernández-Figueras MT, et al. Diagnosis and Treatment of Merkel Cell Carcinoma in Specialized Dermatology Units: A Clinical Practice Guideline of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr. 2019;110(6):460-8. https://doi.org/j69h.
Chou TC, Tsai KB, Wu CY, Hong CH, Lee CH. Presence of the Merkel cell polyomavirus in Merkel cell carcinoma combined with squamous cell carcinoma in a patient with chronic arsenism. Clin Exp Dermatol. 2016;41(8):902-5. https://doi.org/f9cdd2.
Fernández-Figueras MT, Puig L, Musulen E, Gilaberte M, Ferrándiz C, Lerma E, et al. Prognostic significance of p27Kip1, p45Skp2 and Ki67 expression profiles in Merkel cell carcinoma, extracutaneous small cell carcinoma, and cutaneous squamous cell carcinoma. Histopathology. 2005;46(6):614-21. https://doi.org/bwh92h.
Ansai SI. Topics in histopathology of sweat gland and sebaceous neoplasms. J Dermatol. 2017;44(3):315-26. https://doi.org/f9z3j4.
Sallman MA, Li JY, Swaby M, Chon SY. Cutaneous scalp metastasis of cholangiocarcinoma in hepatitis C. JAAD Case Rep. 2020;6(5):468-70. https://doi.org/j7ct.
Rodriguez N, Volmaro K, Martinez M, Pets E, Pignata L, Machado J, et al. Carcinoma de células de Merkel: Características clínico patológicas de una neoplasia agresiva de infrecuente presentación. Rev Fac Cien Med Univ Nac Cordoba. 2018;(Suppl JIC XIX):43-4.
Tai PT, Yu E, Winquist E, Hammond A, Stitt L, Tonita J, et al. Chemotherapy in Neuroendocrine/Merkel Cell Carcinoma of the Skin: Case Series and Review of 204 Cases. J Clin Oncol. 2000;18(12):2493-9. https://doi.org/j7cv.
Desch L, Kunstfeld R. Merkel Cell Carcinoma: Chemotherapy and Emerging New Therapeutic Options. J Skin Cancer. 2013; 2013:327150. https://doi.org/gbcmj4.
Naseri S, Steiniche T, Ladekarl M, Bønnelykke-Behrndtz ML, Hölmich LR, Langer SW, et al. Management Recommendations for Merkel Cell Carcinoma—A Danish Perspective. Cancers (Basel). 2020;12(3):554. https://doi.org/j7cw.
Petrelli F, Ghidini A, Torchio M, Prinzi N, Trevisan F, Dallera P, et al. Adjuvant radiotherapy for Merkel cell carcinoma: A systematic review and meta-analysis. Radiother Oncol. 2019;134:211-9. https://doi.org/gfzt99.
Bhatia S, Storer BE, Iyer JG, Moshiri A, Parvathaneni U, Byrd D, et al. Adjuvant Radiation Therapy and Chemotherapy in Merkel Cell Carcinoma: Survival Analyses of 6908 Cases From the National Cancer Data Base. J Natl Cancer Inst. 2016;108(9):djw042. https://doi.org/j7cx.
Cornejo C, Miller CJ. Merkel Cell Carcinoma. Dermatol Clin. 2019;37(3):269-77. https://doi.org/j7cz.
Robinson CG, Tan D, Yu SS. Recent advances in Merkel cell carcinoma. F1000Res. 2019;8:1995. https://doi.org/j7c2.
Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Brohl AS, et al. Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. J Clin Oncol. 2019;37(9):693-702. https://doi.org/j7c3.
Walocko FM, Scheier BY, Harms PW, Fecher LA, Lao CD. Metastatic Merkel cell carcinoma response to nivolumab. J Immunother Cancer. 2016;4:79. https://doi.org/f9dj9t.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2022 Revista de la Facultad de Medicina

Esta obra está bajo una licencia Creative Commons Reconocimiento 3.0 Unported.
Derechos de autor
Los autores deben aceptar transferir a la Revista de la Facultad de Medicina los derechos de autor de los artículos publicados. La editorial tiene el derecho del uso, reproducción, transmisión, distribución y publicación en cualquier forma o medio. Los autores no podrán permitir o autorizar el uso de la contribución sin el consentimiento escrito de la revista. Estos archivos están disponibles en https://goo.gl/EfWPdX y https://goo.gl/6zztk4 y deben cargarse en el paso 4 del envío OJS (archivos complementarios).
La carta de cesión de derechos de autor y la de responsabilidad de autoría deben ser entregadas junto con el original.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
- Los autores/as conservarán sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación esta revista.
- Los autores/as podrán adoptar otros acuerdos de licencia no exclusiva de distribución de la versión de la obra publicada (p. ej.: depositarla en un archivo telemático institucional o publicarla en un volumen monográfico) siempre que se indique la publicación inicial en esta revista.
- Se permite y recomienda a los autores/as difundir su obra a través de Internet (p. ej.: en archivos telemáticos institucionales o en su página web) antes y durante el proceso de envío, lo cual puede producir intercambios interesantes y aumentar las citas de la obra publicada. (Véase El efecto del acceso abierto).





















