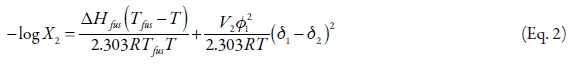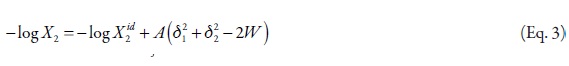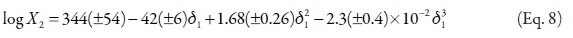Publicado
Extended Hildebrand Solubility Approach applied to Nimodipine in PEG 400 + ethanol mixtures
Método extendido de Hildebrand aplicado a la solubilidad de nimodipina en mezclas PEG 400 + etanol
Palabras clave:
Nimodipine, PEG 400 ethanol, Extended Hildebrand Solubility Approach, binary mixtures, solubility parameter (en)nimodipina, PEG 400 etanol, Método Extendido de Solubilidad de Hildebrand, mezclas binarias, parámetro de solubilidad (es)
Descargas
Artículo de investigación científica
Extended Hildebrand Solubility Approach applied to Nimodipine in PEG 400 + ethanol mixtures
Método extendido de Hildebrand aplicado a la solubilidad de nimodipina en mezclas PEG 400 + etanol
Jorge L. Gómez1, Gerson A. Rodríguez2, Diana M. Cristancho2, Daniel R. Delgado2, Carolina P. Mora3, Alicia Yurquina4, Fleming Martínez2*
1 Laboratorios Procaps, Barranquilla, Colombia.
2 Grupo de Investigaciones Farmacéutico-Fisicoquímicas, Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia, A. A. 14490, Bogotá, D. C., Colombia.
* Address for correspondence: E-mail: fmartinezr@unal.edu.co.
3 Grupo de Investigaciones Fisicoquímicas Farmacéuticas, Departamento de Ciencias Químicas, Facultad de Ciencias Naturales, Universidad Icesi, Santiago de Cali, Colombia.
4 Instituto de Química Inorgánica, Facultad de Bioquímica, Química y Farmacia, Universidad Nacional de Tucumán, San Miguel de Tucumán, Argentina.
Recibido para evaluación: 15 de febrero de 2013.
Aceptado para publicación: 7 de mayo de 2013.
SUMMARY
Nimodipine (NMD) is a drug used as cerebral vasodilator whose physicochemical properties in solution have not been studied completely. In this work the Extended Hildebrand Solubility Approach (EHSA) was applied to evaluate the equilibrium solubility of NMD in some polyethylene glycol 400 + ethanol mixtures at 298.15 K. An acceptable correlative capacity of EHSA was found using a regular polynomial model in order three (overall deviation lower than 1.3%), when the W interaction parameter is related to the solubility parameter of the mixtures. Moreover, the mean deviation obtained in the estimated solubility with respect to experimental solubility was lower than the one obtained directly by means of an empiric regression in order three of the logarithm experimental solubility as a function of the mixtures' solubility parameters (1.7%).
Key words:Nimodipine, PEG 400 + ethanol, Extended Hildebrand Solubility Approach, binary mixtures, solubility parameter.
RESUMEN
La nimodipina (NMD) es un agente usado como vasodilatador cerebral y cuyas propiedades fisicoquímicas en solución aún no han sido totalmente estudiadas. En la presente investigación se aplicó el Método Extendido de Solubilidad de Hildebrand (MESH) al estudio de la solubilidad de NMD en algunas mezclas binarias PEG 400 + etanol a 298,15 K. Se obtuvo una capacidad predictiva aceptable del MESH (desviación general inferior al 1,3%) al utilizar un modelo polinómico regular de tercer orden, relacionando el parámetro de interacción W con el parámetro de solubilidad de las mezclas solventes. De esta forma, las desviaciones obtenidas en la solubilidad estimada fueron de magnitud inferior a las obtenidas al calcular esta propiedad directamente, utilizando una regresión empírica regular del mismo orden, de la solubilidad experimental del fármaco en función del parámetro de solubilidad de las mezclas disolventes, en la cual se obtuvo una desviación promedio del 1,7%.
Palabras clave:nimodipina, PEG 400 + etanol, Método Extendido de Solubilidad de Hildebrand, mezclas binarias, parámetro de solubilidad.
INTRODUCTION
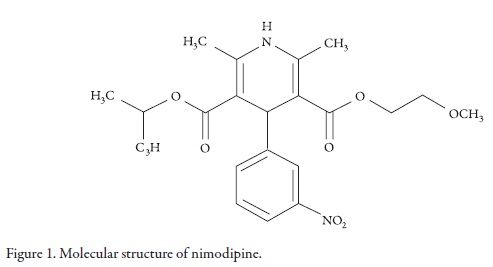
Nimodipine (NMD, Figure 1, molar mass 418.44 g mol-1) is a calcium channel blocker that prevents the calcium-dependent smooth muscle contraction and therefore, the subsequent vasoconstriction. NMD exhibits greater effects on cerebral circulation than on peripheral circulation, and for this reason, it is specially used as an adjunct to improve the neurologic outcome following subarachnoid hemorrhage from ruptured intracranial aneurysm [1].
On the other hand, NMD aqueous solubility is too low to allow the design of homogeneous liquid dosage forms with solvents strictly aqueous [2]. For this reason some disperse and coarse dosage systems have been investigated and developed, which include liposomes [3], solid dispersions [4, 5], cyclodextrin inclusion complexes [6-8], hydrogels and hydrophilic matrices [9, 10], and some emulsions [11]. Nevertheless, in every one of these formulations this drug is dispersed only in a coarse way instead of a molecular one as a real solution. In the Colombian market this drug is presented as tablets, injectable and soft gelatin capsules [12].
Although NMD is used in therapeutics, the physicochemical information about its solubility is not abundant. In this way, it is important to note that cosolvency is the best technique used in pharmacy for increasing drugs solubility to develop homogeneous liquid dosage forms [13]. On the other hand, it is clear that predictive methods of physicochemical properties of drugs are very important for industrial pharmacists because they allow optimizing design processes [14].
For this reason, this work presents a physicochemical study about the solubility prediction of NMD in binary mixtures conformed by polyethylene glycol 400 (PEG 400) and ethanol. The study was done based on the Extended Hildebrand Solubility Approach (EHSA) [15]. Up to the best of our knowledge no report about the application of EHSA method to this drug has been presented in the literature. It is relevant to keep in mind that EHSA method has been widely used to study the solubility of a lot of pharmaceutical compounds [16-38]. On the other hand, PEG 400 is, after ethanol and propylene glycol, the cosolvent more employed to develop liquid pharmaceutical dosage forms [39]. Moreover, PEG 400 is also employed to regulate the evaporation in liquid products [40].
THEORETICAL
The real solubility (X2) of a solid solute in a liquid solution is calculated adequately by means of the expression,
where, ΔHfus is the fusion enthalpy of the solute, R is the gas constant, Tfus is the melting point of the solute, T is the absolute temperature of the solution, and log γ2 is the non-ideality term. It is important to keep in mind that ideal solubility is calculated by means of Equation 1 without consider the second term of the right side. The γ2 term is the activity coefficient of the solute determined experimentally. One method of calculating γ2 is the referent to regular solutions obtained from,
where, V2 is the partial molar volume of the solute, φ1 is the volume fraction of the solvent in the saturated solution, and δ1 and δ2 are the solubility parameters of solvent and solute, respectively. Pharmaceutical dissolutions deviate of predicted by the regular solutions theory. On this way, Martin et al. developed the EHSA method (16-22). If the A term (defined as V2Φ21 ) is introduced in the equation 3, the real solubility of drugs can be calculated from the expression,
where, the W term is equal to 2Kδ1δ2 (where, K is the Walker parameter). The W factor can be calculated from experimental data by means of,
where, γ2 is the activity coefficient of the solute in the saturated solution, and it is calculated as, X2id/X2. The experimental values of the W parameter can be correlated by means of regression analysis by using regular polynomials as a function of δ1, as follows,
These empiric models can be used to estimate the drug solubility by means of back-calculation resolving this property from the specific W value obtained in the respective polynomial regression. A lot of examples of the application of the previous equations have been reported in the literature [15-38].
EXPERIMENTAL
Reagents
Nimodipine (1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 2-methoxyethyl 1-methylethyl ester; CAS No. 66085-59-4; Haohua Ind. & Co., China), polyethylene glycol 400 (Dow Chemical Co., USA), and absolute ethanol A.R. (Merck, Germany), used in this research, were in agreement with the quality requirements of the American Pharmacopeia, USP [41].
Solvent mixtures preparation
Both solvents were dried by using molecular sieve (Merck, 3 Å) before preparing the mixtures. All PEG 400 + ethanol mixtures were prepared by mass, using an Ohaus Pioneer TM PA214 analytical balance with sensitivity of ± 0.1 mg, in quantities of 50 g. The mass fractions of PEG 400 of the nine binary mixtures prepared varied by 0.10 from 0.10 to 0.90.
Solubility determinations
An excess of NMD was added to approximately 10 g of each solvent mixture or neat solvents, in stoppered dark glass flasks. The flasks with the solid-liquid mixtures were placed in re-circulating thermostatic baths (Neslab RTE 10 Digital One Thermo Electron Company) kept at 298.15 ± 0.05 K for at least 7 days to reach the equilibrium. After this time the supernatant solutions were filtered at isothermal conditions (Millipore Corp. Swinnex®-13) to ensure that they were free of particulate matter before sampling for composition analysis. NMD concentrations were determined by UV-spectrophotometric analysis at 236 nm after alcoholic dilution. All the solubility experiments were run at least in triplicates. In order to transform mole fractions to molar concentrations (mol dm-3), the density of the saturated solutions was determined with a digital density meter (DMA 45 Anton Paar) connected to the same re-circulating thermostatic baths [42].
Calorimetric study
Melting point and enthalpy of fusion of NMD were determined by DSC studies (DSC 823E Mettler Toledo). Thermal analyses were performed at a heating rate of 10 K min-1 in a dynamic nitrogen atmosphere (60 cm3 min-1). Nearly 1.5 mg of drug was used. The equipment was calibrated using Indium as standard [43].
Estimation of the volumetric contributions
Apparent specific volume (φVspc ) were calculated according to equation 6, where, m2 and m1 are the masses of solute and solvent in the saturated solution, respectively, VE1 is the specific volume of the solvent, and ρsoln is the solution density [44].
The NMD apparent molar volume is calculated by multiplying the φVspc value and the molar mass of the solute.
RESULTS AND DISCUSSION
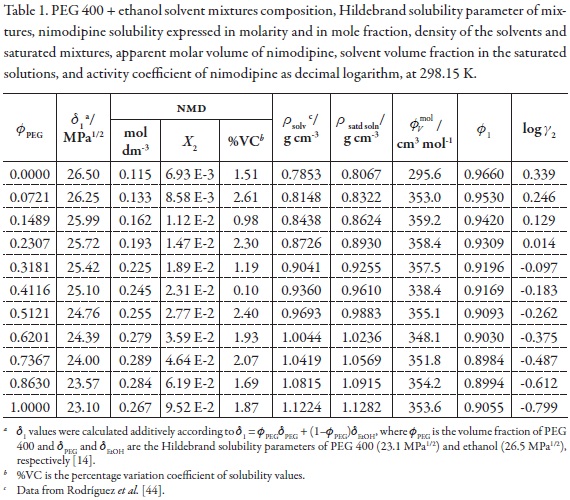
The information about polarity and volumetric behavior of PEG 400 + ethanol mixtures as a function of the composition is shown in Table 1 [45]. On the other hand, the calorimetric values of fusion for NMD were as follows, Tfus = 397.2 K and ΔHfus = 41.68 kJ mol-1. From these values the calculated ideal solubility for this drug calculated by using equation 7 was 1.513x10–2 in mole fraction.
Table 1 also summarizes the NMD solubility expressed in molarity and mole fraction, the density of the saturated mixtures, the apparent molar volume of NMD, and the solvent volume fraction in the saturated solutions at 298.15 K. It is interesting to note that when molarity is considered the maximum drug solubility is obtained in the mixture with δ1 = 24.00 MPa1/2 (0.7367 in volume fraction of PEG 400) but if the mole fraction scale is considered the maximum is obtained in neat PEG 400 (with δ1 = 23.10 MPa1/2). This result is a consequence of the definition of each concentration scale [15].
Nevertheless, in this case the useful scale is mole fraction.
From density values of cosolvent mixtures and saturated solutions, in addition to NMD solubility, the solvent volume fraction (φ1) and apparent molar volume of the solute (φvmol ) in the saturated mixtures, were calculated. These values are also presented in Table 1.
Ultimately, the activity coefficients of NMD as decimal logarithms are also presented in Table 1. These values were calculated from experimental solubility values and ideal solubility at 298.15 K (X2 = 1.513x10–2). In the vast majority of cases, γ2 values were lower than unit (negative logarithmic values) because in those systems (PEG 400-rich mixtures) the experimental solubilities are greater than the ideal one.
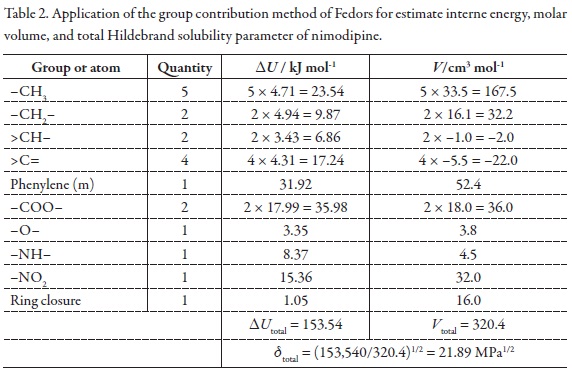
Because the maximum mole fraction solubility of NMD was obtained in neat PEG 400 instead of a mixture Table 2 summarizes the results of the application of the group contribution method of Fedors for estimate interne energy, molar volume, and total Hildebrand solubility parameter of nimodipine, δtotal = 21.89 MPa1/2 [46]. According to this result, it is clear that this drug is less polar than PEG 400, which is the less polar solvent employed in this study.
Figure 2 shows the experimental drug solubility and the calculated solubility by using the regular solutions model (Equation 2) as a function of the solubility parameter of solvent mixtures. It is interesting to note that the experimental solubility is greater than the calculated solubility by using the regular solution model in every one of the mixtures evaluated. This result could be attributed to the fact that this semiempirical model does not consider specific interactions between solvent and solute, and all the involved compounds present polar groups that could interact by hydrogen bonding. In particular PEG 400 could interact as Lewis base due to its ether oxygen atoms with the amine groups of NMD (Figure 1). Moreover, other possible interactions between ethanol and this drug could be present due to their carboxyl and nitro groups (Figure 1).
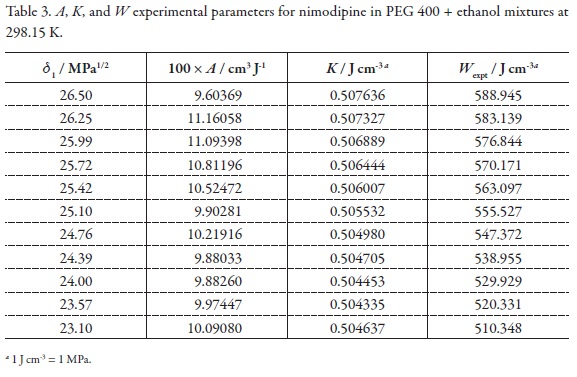
In order to calculate the W parameter the calculated δtotal value (21.89 MPa1/2, Table 2) was used [46]. On the other hand, the parameters A, K, and W are presented in Table 3. Figure 3 shows that the variation of the W parameter with respect to the solubility parameter of solvent mixtures, presents deviation from linear behavior, just as it is expectable because the W term implies the summation of two quadratic terms (δ12 and δ22 ) and one non-constant-quotient involving a logarithmic term (-log γ2/A) as it is shown in Equation 4.
W values were adjusted to regular polynomials in orders from 2 to 5 (Equation 5). Nevertheless linear model was also evaluated with comparative purposes.Table 4 summarizes the coefficients obtained in all the regular polynomials from degrees one to five, whereas the W values calculated by using the respective polynomials are presented in Table 5. It is well clear that these values depend on the model used in the W back-calculation. Similar behaviors have been reported in the literature for this drug and for several other compounds in different solvent mixtures [16-38].
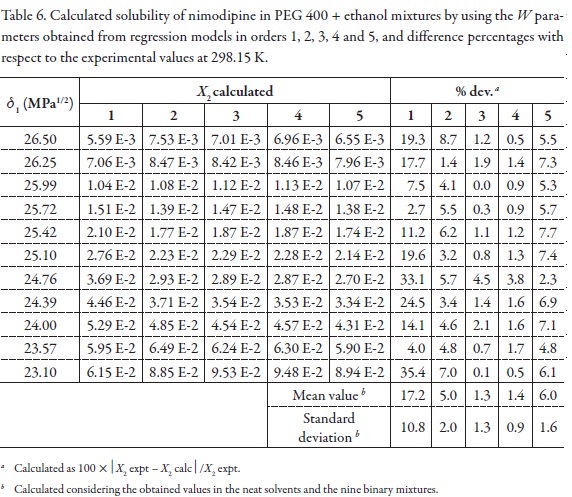
Table 6 summarizes the solubility values obtained by using the W values obtained by back-calculation from the polynomial models (Table 3) and presented in Table 4. In the same way as it was made previously [30-32, 34, 36-38] and because we are searching the best adjust, the first criterion used to define the polynomial order of W term as function of δ1 was the fitting standard uncertainties obtained, whose values were as follows, 0.4926, 0.1333, 0.0464, 0.0468, and 0.0496 (Table 4), for orders one to five, respectively. So, the best one is obtained by using a polynomial in order 3. As another comparison criterion, Table 6 also summarizes the percentages of difference between NMD experimental solubility and those calculated by using EHSA.
At the beginning, it is found that as more complex the polynomial used is, better the agreement found between experimental and calculated solubility is, reaching the maximum concordance using a polynomial in order three. This result is different way to those reported in the literature for other pharmaceutical compounds where the concordance always increases with the polynomial degree [16-38]. Most important increment in concordance is obtained passing from order 1 to order 2, but the more significant increment is also obtained from order 2 to order 3. Therefore, in the following comparisons the polynomial model in order 3 is used.
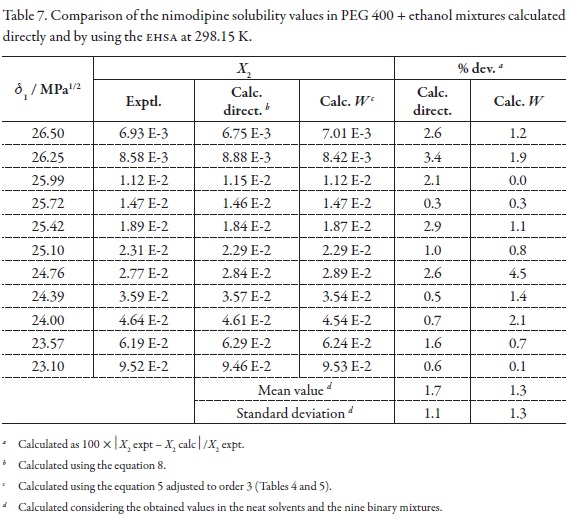
As has been exposed previously, an important consideration about the usefulness of the EHSA method is the one referent to justify the complex calculations involving any other variables, instead of the simple empiric regression of the experimental solubility as a function of the solvent mixtures' solubility parameters (Table 1, Figure 4). For this reason, in the Table 7 the experimental solubilities are confronted to those calculated directly by using a regular polynomial in order 3 of logX2 as a function of δ1 values (Equation 8, with adjusted determination coefficient r2 = 0.9989 and fitting standard uncertainty = 0.0108) and also to those calculated involving the W parameters obtained from Equation 5 adjusted to order 3 (Table 4 and 5). The respective difference percentages are also presented in Table 7.
Based on mean deviation percentages presented in Table 7 (1.7% and 1.3% for direct calculation and EHSA method, respectively) it follows that a small difference is found between the values obtained by using both methods. The present results would be showing a significant usefulness of EHSA method for practical purposes.
On the other hand, it is very interesting to note that this drug exhibit negative and positive deviations with respect to ideal log-linear additive model (dotted line in Figure 4). Nevertheless the molecular reasons for this behavior are unclear due to the lack of information about structural effects in these binary mixtures [47, 48].
CONCLUSION
In this investigation the EHSA method has been adequately used to study the solubility of NMD in PEG 400 + ethanol mixtures by using experimental values of molar volume and calculated Hildebrand solubility parameter of this drug. In particular, a good predictive character has been found by using a regular polynomial in order three of the interaction parameter W as a function of the solubility parameter of solvent mixtures free of solute. This result is different to that reported for other pharmaceutical compounds in terms of polynomial order and fitting obtained. Nevertheless, the predictive character is just slightly better than the one obtained by direct correlation between solubility and mixtures composition.
ACKNOWLEDGEMENTS
The authors thank to the Department of Pharmacy of the Universidad Nacional de Colombia for providing the equipment and installations used in this investigation. We also thank the Laboratorio Procaps de Colombia for facilitating us the DSC equipment.REFERENCIAS
1. A. Scriabine, T. Schuurman, J. Traber, Pharmacological basis for the use of nimodipine in central nervous system disorders, FASEB J., 3(7), 1799-1806 (1989).
2. S. Budavari, M.J. O'Neil, A. Smith, P.E. Heckelman, J.R. Obenchain Jr., J.A.R. Gallipeau, M.A. D'Arecea, "The Merck index, an encyclopedia of chemicals, drugs, and biologicals", 13th edition, Merck & Co., Inc., Whitehouse Station, NJ, 2001.
3. Z. Wang, Y. Deng, X. Zhang, T. Wang, F. Wu, Development and pharmacokinetics of nimodipine-loaded liposomes, J. Pharm. Pharmacol., 58(9), 1289-1294 (2006).
4. N.A. Urbanetz, Stabilization of solid dispersions of nimodipine and polyethylene glycol 2000, Eur. J. Pharm. Sci., 28(1-2), 67-76 (2006).
5. G.Z. Papageorgiou, D. Bikiaris, E. Karavas, S. Politis, A. Docoslis, Y. Park, A. Stergiou, E. Georgarakis, Effect of physical state and particle size distribution on dissolution enhancement of nimodipine/PEG solid dispersions prepared by melt mixing and solvent evaporation, AAPS J., 8(4), E623-E631 (2006).
6. F. Kopecky, B. Kopecká, P. Kaclík, Solubility study of nimodipine inclusion complexation with a- and ß-cyclodextrin and some substituted cyclodextrins, J. Incl. Phenom. Macrocycl. Chem., 39(3-4), 215-217 (2001).
7. A. Patil, S. Majahar, G. Irfani, Floating tablets of nimodipine and its inclusion complex with ß-cyclodextrin for controlled release, Int. J. PharmTech. Res., 3(2), 619-625 (2011).
8. J.S. Patil, N.R. Pandya, S.C. Marapur, S.S. Shiralashetti, Influence of method of preparation on physicochemical properties and in-vitro drug release profile of nimodipine-cyclodextrin inclusion complexes: A comparative study, Int. J. Pharm. Pharmaceut. Sci., 2(1), 71-81 (2010).
9. Y. Wang, Y. Lapitsky, C.E. Kang, M.S. Shoichet, Accelerated release of a sparingly soluble drug from an injectable hyaluronan-methylcellulose hydrogel, J. Control. Rel., 140(3), 218-223 (2009).
10. T. Steingräber, T. Schtoltz, P. Oening-Rodrigues, Avaliação da influência de adjuvantes não-poliméricos solúveis na liberação do nimodipino a partir de formulações matriciais de liberação prolongada, Rev. Colomb. Cienc. Quím. Farm., 37(2), 122-132 (2008).
11. Q. Zhang, X. Jiang, W. Jiang, W. Lu, L. Su, Z. Shi, Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain, Int. J. Pharm., 275(1-2), 85-96 (2004).
12. L.R. Lépori, A. Cohen, H.E. Castagneto, S. Benítez, E.C. Peyrás, "P.R. Vademecum", 4th ed., Licitelco S.A., Bogotá, 2005.
13. S.H. Yalkowsky, T.J. Roseman, Solubilization of drugs by cosolvents, in: "Techniques of solubilization of drugs", Edited by S.H. Yalkowsky, Marcel Dekker, Inc., New York, 1981, pp. 91-134.
14. A. Jouyban, "Handbook of solubility data for pharmaceuticals", CRC Press, Taylor & Francis Group, Boca Raton, FL, 2010, pp. 30-58.
15. A. Martin, P. Bustamante, A.H.C. Chun, "Physical pharmacy: Physical chemical principles in the pharmaceutical sciences", 4th ed., Lea & Febiger, Philadelphia, 1993.
16. A. Martin, J. Newburger, A. Adjei, Extended Hildebrand solubility approach: Solubility of theophylline in polar binary solvents, J. Pharm. Sci., 69(5), 659-661 (1980).
17. A. Martin, J. Carstensen, Extended solubility approach: Solubility parameters for crystalline solid compounds, J. Pharm. Sci., 70(2), 170-172 (1981).
18. P.L. Wu, A. Martin, Extended Hildebrand solubility approach: p-hydroxybenzoic acid in mixtures of dioxane and water, J. Pharm. Sci., 72(6), 587-592 (1981).
19. A. Martin, N.A. Paruta, A. Adjei, Extended Hildebrand solubility approach: Methylxanthines in mixed solvents, J. Pharm. Sci., 70(10), 1115-1120 (1981).
20. A. Martin, M.J. Miralles, Extended Hildebrand solubility approach: Solubility of tolbutamide, acetohexamide, and sulfisomidine in binary solvent mixtures, J. Pharm. Sci., 71(4), 439-442 (1982).
21. A. Martin, P.L. Wu, A. Adjei, M. Mehdizadeh, K.C. James, C. Metzler, Extended Hildebrand solubility approach: Testosterone and testosterone propionate in binary solvents, J. Pharm. Sci., 71(12), 1334-1340 (1982).
22. A. Martin, P.L. Wu, T. Velásquez, Extended Hildebrand solubility approach: Sulfonamides in binary and ternary solvents, J. Pharm. Sci., 74(3), 277-282 (1985).
23. A. Jouyban-Gharamaleki, W.E. Acree Jr., Comments concerning: Solubility prediction of caffeine in aqueous N,N-dimethylformamide mixtures using the extended Hildebrand solubility approach, Int. J. Pharm., 177(1), 127-128 (1999).
24. F. Martínez, Aplicación del método extendido de Hildebrand al estudio de la solubilidad del acetaminofén en mezclas etanol-propilenoglicol, Acta Farm. Bonaerense, 24(2), 215-224 (2005).
25. F. Martínez, Utilidad del método extendido de Hildebrand en el estudio de la solubilidad del acetaminofén en mezclas agua-propilenoglicol, Rev. Acad. Colomb. Cienc., 29(112), 429-438 (2005).
26. D.P. Pacheco, Y.J. Manrique, E.F. Vargas, H.J. Barbosa, F. Martínez, Validez del método extendido de Hildebrand en la predicción de las solubilidades de ibuprofén y naproxén en mezclas propilenoglicol-etanol, Rev. Colomb. Quím., 36(1), 55-72 (2007).
27. D.M. Aragón, D.P. Pacheco, M.A. Ruidíaz, A.D. Sosnik, F. Martínez, Método extendido de Hildebrand en la predicción de la solubilidad de naproxeno en mezclas cosolventes etanol + agua, Vitae, Rev. Fac. Quím. Farm., 15(1), 113-122 (2008).
28. M.A. Ruidíaz, F. Martínez, Método extendido de Hildebrand en la estimación de la solubilidad de la indometacina en mezclas acetato de etilo + etanol, Rev. Colomb. Quím., 38(2), 235-247 (2009).
29. P.B. Rathi, Prediction of satranidazole solubility in water-polyethylene glycol 400 mixtures using extended Hildebrand solubility approach, Iranian J. Pharm. Sci., 7(1), 17-24 (2010).
30. M. Gantiva, F. Martínez, Método extendido de Hildebrand en la predicción de la solubilidad del ketoprofeno en mezclas cosolventes etanol + agua, Quim. Nova, 33(2), 370-376 (2010).
31. S.J. Rodríguez, D.M. Cristancho, P.C. Neita, E.F. Vargas, F. Martínez, Extended Hildebrand solubility approach in the solubility estimation of the sunscreen ethylhexyl triazone in ethyl acetate + ethanol mixtures, Lat. Am. J. Pharm., 29(7), 1113-1119 (2010).
32. M.A. Ruidíaz, D.R. Delgado, C.P. Mora, A. Yurquina, F. Martínez, Estimation of the indomethacin solubility in ethanol + water mixtures by the extended Hildebrand solubility approach, Rev. Colomb. Cienc. Quím. Farm., 39(1), 79-95 (2010).
33. P.B. Rathi, V.K. Mourya, Extended Hildebrand solubility approach: Satranidazole in mixtures of dioxane and water, Indian J. Pharm. Sci., 73(3), 315-319 (2011).
34. M.A. Ruidíaz, D.R. Delgado, F. Martínez, Extended Hildebrand solubility approach to correlate the indomethacin solubility in 1,4-dioxane + water mixtures, Quim. Nova, 34(9), 1569-1574 (2011).
35. M. Kharwade, G. Achyuta, C.V.S. Subrahmanyam, P.R. Sathesh-Babu, Solubility behavior of lornoxicam in binary solvents of pharmaceutical interest, J. Solution Chem., 41(8), 1364-1374 (2012).
36. A.R. Holguín, D.R. Delgado, F. Martínez, Indomethacin solubility in propylene glycol + water mixtures according to the extended Hildebrand solubility approach, Lat. Am. J. Pharm., 31(5), 720-726 (2012).
37. E.A. Ahumada, D.R. Delgado, F. Martínez, Acetaminophen solubility in polyethylene glycol 400 + water mixtures according to the extended Hildebrand solubility approach, Rev. Colomb. Quím., in press.
38. R.G. Sotomayor, A.R. Holguín, D.M. Cristancho, D. Delgado, F. Martínez, Extended Hildebrand solubility approach applied to the piroxicam solubility in ethanol + water mixtures, J. Mol. Liquids, 180(1), 34-38 (2013).
39. J.I. Rubino, Cosolvents and cosolvency, in: "Encyclopedia of Pharmaceutical Technology", edited by J. Swarbrick, J.C. Boylan, Marcel Dekker, Inc., New York, 1988, vol. 3, pp. 375-398.
40. M.E. Aulton, "Pharmaceutics, the science of dosage forms design", 2nd ed., Churchill Livingstone, London, 2002.
41. United States Pharmacopeia/National Formulary, 30th ed., The United States Pharmacopeial Convention, Inc., Rockville, MD, 2007.
42. D.R. Delgado, F. Martínez, M.A.A. Fakhree, A. Jouyban, Volumetric properties of the glycerol formal + water cosolvent system and correlation with the Jouyban-Acree model, Phys. Chem. Liq., 50(3), 284-301 (2012).
43. S.A. McCauley, H.G. Brittain, Thermal methods of analysis, in: "Physical characterization of pharmaceutical solids", edited by H.G. Brittain, Marcel Dekker, Inc., New York, 1995, pp. 223-251.
44. J.A. Jiménez, F. Martínez, Thermodynamic study of the solubility of acetaminophen in propylene glycol + water cosolvent mixtures, J. Braz. Chem. Soc., 17(1), 125-134 (2006).
45. G.A. Rodríguez, A.R. Holguín, F. Martínez, M. Khoubnasabjafari, A. Jouyban, Volumetric properties of (PEG 400 + water) and (PEG 400 + ethanol) mixtures at several temperatures and correlation with the Jouyban-Acree model, Rev. Colomb. Cienc. Quím. Farm., 41(2), 187-202 (2012).
46. R.F. Fedors, A method for estimating both the solubility parameters and molar volumes of liquids, Polym. Eng. Sci., 14(2), 147-154 (1974).
47. Y. Marcus, "The properties of solvents", John Wiley & Sons, Ltd., Chichester, 1998.
48. Y. Marcus, "Solvent mixtures: Properties and selective solvation", Marcel Dekker, Inc., New York, 2002.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2013 Revista Colombiana de Ciencias Químico-Farmacéuticas

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.
El Departamento de Farmacia de la Facultad de Ciencias de la Universidad Nacional de Colombia autoriza la fotocopia de artículos y textos para fines de uso académico o interno de las instituciones citando la fuente. Las ideas emitidas por los autores son responsabilidad expresa de estos y no de la revista.
Todo el contenido de esta revista, excepto dónde está identificado, está bajo una Licencia Creative Commons de Atribución 4.0 aprobada en Colombia. Consulte la normativa en: http://co.creativecommons.org/?page_id=13