Effect of cassava-starch coatings with ascorbic acidic and N-acetylcysteine on the quality of harton plantain (Musa paradisiaca)
Efecto de recubrimientos de almidón de yuca con ácido ascórbico y N-acetilcisteína en la calidad del plátano hartón (Musa paradisiaca)
DOI:
https://doi.org/10.15446/rfnam.v68n2.50985Keywords:
Browning, polyphenol oxidase, fruit, vegetables (en)Pardeamiento, polifenoloxidasa, frutas, vegetales (es)
El efecto de recubrimientos fue evaluado sobre la calidad de frutos de plátano Hartón (Musa paradisiaca) en la etapa de poscosecha. Se prepararon soluciones para tres tratamientos a partir de almidón de yuca (Manihot esculenta) 50 g L-1, con glicerol como plastificante 30 g L-1 y polietilen glicol-600® 6 g L-1, como agentes antipardeantes se adicionó ácido ascórbico (AA) 6 g L-1 y N-Acetil-Cisteína (NAC) 8 g L-1 y una mezcla de AA 6 g L-1 más NAC 8 g L-1. Los frutos se recubrieron por inmersión y se almacenaron a 18 ± 4°C y 85% de HR. Se determinaron las propiedades físico-químicas durante 32 días de poscosecha. Los recubrimientos aplicados disminuyeron la pérdida fisiológica de peso (%PP) y presentaron una mayor firmeza de la pulpa (FP), igualmente no se presentó diferencia significativa con un nivel de confianza del 95% en la concentración de sólidos solubles totales (SST), ni en la acidez e índice de madurez; el color de la epidermis fue medido instrumentalmente por el método CIE L*a*b*, se encontró para la coordenada L un valor medio de 70 para frutos recubiertos con la mezcla de AA 6 g L-1 más NAC 8 g L-1, mientras que para el control se encontró un valor de 57, además se encontraron menores valores para la coordenada a* e índice de pardeamiento para los frutos recubiertos. La actividad enzimática de la polifenoloxidasa se mostró decreciente con los días de poscosecha para todos los tratamientos, siendo menor para la mezcla de antioxidantes en un 27% con respecto al control. Se pudo concluir que el recubrimiento que contenía la mezcla de agentes antioxidantes presentó mejor efecto como alternativa de conservación para los frutos y prolongó el tiempo de vida útil del plátano hartón.
Effect of cassava-starch coatings with ascorbic acidic and N-acetylcysteine on the quality of harton plantain (Musa paradisiaca)
Efecto de recubrimientos de almidón de yuca con ácido ascórbico y N-acetilcisteína en la calidad del plátano hartón (Musa paradisiaca)
Carlos Julio Márquez Cardozo1; José Roberto Palacín Beltrán2 and Lorenzo Fuentes Berrio3
1 Associate Professor. Universidad Nacional de Colombia, Sede Medellín - Facultad de Ciencias Agrarias. A.A. 1779, Medellín, Colombia. <cjmarque@unal.edu.co>
2 Pharmaceutical Chemist. Universidad de Cartagena - Facultad de Ingeniería de Alimentos. Avenida del Consulado, Calle 30 No. 48-152, Catagena, Colombia. <jo.spa53@hotmail.com>
3 Professor. Universidad de Cartagena - Facultad de Ingeniería de Alimentos. Avenida del Consulado, Calle 30 No. 48-152, Catagena, Colombia. <lfuentesb@unicartagena.edu.co>
Received: June 16, 2014; Accepted: March 8, 2015
DOI: 10.15446/rfnam.v68n2.50985
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract. The effect of coatings was evaluated on the quality of harton plantain fruits (Musa paradisiaca) in the postharvest stage. Solutions for three treatments were prepared from 50 g L-1 cassava starch (Manihot esculenta) with 30 g L-1 glycerol as the plasticizer and 6 g L-1 polyethylene glycol-600®; for anti-browning agents, 6 g L-1 ascorbic acid (AA) and 8 g L-1 N-Acetyl-Cysteine (NAC) were added. The fruits were coated by immersion, stored at 18 ± 4°C and 85% RH. Physicochemical properties were determined at 32 days postharvest. The applied coatings decreased the physiological weight loss (%WL) and resulted in a higher pulp firmness (PF); no significant difference was seen with a confidence level of 95% in the concentration of total soluble solids (TSS), acidity or maturity index. The skin color was measured by the CIE L*a*b* method, with an average L value of 70 for the fruits coated with the 6 g L-1 AA and 8 g L-1 NAC mixture, while the control fruits had a value of 57. Also, lower a* coordinate values and browning indices were found for the epidermis of the coated fruits. The enzymatic activity of the polyphenol oxidase decreased with the number of postharvest days for all of the treatments, being lower for the fruits with the mixture of anti-browning agents by 27%, as compared to the control. It was concluded that the coating mixture containing the anti-browning agents ascorbic acid, 6 g L-1, and N-acetyl-cysteine, 8 g L-1, showed a better effect as an alternative for storing fruits and prolonging the shelf-life of harton plantain.
Key words: Browning, polyphenol oxidase, fruit, vegetables.
Resumen. El efecto de recubrimientos fue evaluado sobre la calidad de frutos de plátano Hartón (Musa paradisiaca) en la etapa de poscosecha. Se prepararon soluciones para tres tratamientos a partir de almidón de yuca (Manihot esculenta) 50 g L-1, con glicerol como plastificante 30 g L-1 y polietilen glicol-600® 6 g L-1, como agentes antipardeantes se adicionó ácido ascórbico (AA) 6 g L-1 y N-Acetil-Cisteína (NAC) 8 g L-1 y una mezcla de AA 6 g L-1 más NAC 8 g L-1. Los frutos se recubrieron por inmersión y se almacenaron a 18 ± 4°C y 85% de HR. Se determinaron las propiedades físico-químicas durante 32 días de poscosecha. Los recubrimientos aplicados disminuyeron la pérdida fisiológica de peso (%PP) y presentaron una mayor firmeza de la pulpa (FP), igualmente no se presentó diferencia significativa con un nivel de confianza del 95% en la concentración de sólidos solubles totales (SST), ni en la acidez e índice de madurez; el color de la epidermis fue medido instrumentalmente por el método CIE L*a*b*, se encontró para la coordenada L un valor medio de 70 para frutos recubiertos con la mezcla de AA 6 g L-1 más NAC 8 g L-1, mientras que para el control se encontró un valor de 57, además se encontraron menores valores para la coordenada a* e índice de pardeamiento para los frutos recubiertos. La actividad enzimática de la polifenoloxidasa se mostró decreciente con los días de poscosecha para todos los tratamientos, siendo menor para la mezcla de antioxidantes en un 27% con respecto al control. Se pudo concluir que el recubrimiento que contenía la mezcla de agentes antioxidantes presentó mejor efecto como alternativa de conservación para los frutos y prolongó el tiempo de vida útil del plátano hartón.
Palabras claves: Pardeamiento, polifenoloxidasa, frutas, vegetales.
Edible coatings are an environmentally friendly technology that is applied to many products to control moisture transfer, gas exchange or oxidation processes. One major advantage of using edible films and coatings is that several active ingredients can be incorporated into the polymer matrix and consumed with the food, thus enhancing safety or even nutritional and sensory attributes. Quality criteria for fruits and vegetables coated with edible films must be determined carefully and quality parameters must be monitored throughout the storage period, especially color change, firmness loss, ethanol fermentation, decay ratio and weight loss of edible-film coated fruits (Dhall, 2013). Banana fruits (Musa sp.) are palatable products worldwide. The plantain commonly grows in subtropical regions and countries, mainly produced in and exported from South America, the Caribbean and South-East Asia (Soltani and Omid, 2010). This edible fruit has obtained an important role, after rice, wheat, maize and potatoes, mainly in poorer tropical regions of Africa, Latin America and Asia, being a safe and nutritious source of subsistence (Pinto, 2010). The three major sectors that consume more than 80% of domestic production are, in order of importance, rural households, urban households and restaurants; less than 1% is consumed by industry and marketing and transport losses are estimated at 12%. The clone variety "or AAB Simmonds Dominico" or Musa paradisiaca AAB is also called "harton" or the cooking plantain (Lassois et al., 2009).
The banana production chain currently has agreements between producers, traders and industrialists. The primary link has seen better organization of producers, with the creation, recovery and grouping of organizations (Corpoica, 2004). However, economic losses from oxidative reactions are still continuing, which are generated in the fruits during handling and transport (Maqbool and Alderson, 2010). One way to identify ripening is by assessing certain chemical characteristics, such as: pH, acidity, fiber, starch, total soluble solids (TSS), and sugar, such characteristics are related to the degree of commercial acceptability of the product (Brummel and Harpster, 2001).
One of the main factors of damage for banana is caused by enzymatic browning due to polyphenol oxidase (Sheryl et al., 2010). Preserving bananas and prolonging the shelf-life are of great interest, transforming purposes in times of low production using different mechanisms. Edible coatings of polysaccharides are considered technologies that are particularly useful for delaying or controlling this important physiological process. These coatings lead to benefits by being biodegradable and adding antioxidant compounds for reducing enzymatic activity (Gol and Ramana, 2011; Maqbool and Alderson, 2010; Jafarizadeh et al., 2011).
The main effects of the edible coating components chitosan and glycerol (0-2%, w/w) on weight loss, firmness, total color difference, total soluble solids content (TSS) and titratable acidity (TA) of coated bananas were studied. In general, the chitosan concentration appeared to be the most significant (P<0.1) factor that influenced all of the variables except for TSS. The optimum concentrations of chitosan and glycerol were predicted to be 2.02% and 0.18%, respectively, for 10 days of storage at 26±2°C and 40-50% relative humidity (Malmiri et al., 2011). Other authors have found that coating with chitosan, more than gibberellic acid, has the potential to prolong the shelf-life and preserve quality attributes of banana (Neeta and Ramana, 2011).
The effect of different degrees of deacetylation (DD) of chitosan (70%, 80%) in various chitosan concentrations (1, 1.5, 2% w/w) in solution on weight loss and vitamin C loss were investigated in coated bananas. The effect of the presence of emulsifier triethanolamine (TEA) was also examined. Sensory analyses were conducted to monitor the changes in color, texture, and aroma. The results showed that the coated banana fruit demonstrated delayed ripening processes, as compared to the uncoated bananas. This was also confirmed by a reduction in weight loss, as well as in vitamin C, in comparison to the uncoated bananas (Mehdi et al., 2011).
Other researchers have studied the influence of treatments with edible cassava-starch coatings and ascorbic acid, citric acid, and calcium lactate dipping on quality parameters and the shelf-life of other fruits for 12 d at 5°C. It was concluded that the use of edible cassava-starch coatings as gas and water vapor barriers and anti-browning agents can extend the storage time and maintain the quality of the produce. Cassava starch and alginate coatings are alternatives for preserving minimally processed pineapples without changing the quality parameters of the fresh fruit (Bierhals and Hubinger, 2011).
Fruit color is an index of quality and freshness that has been related to the state of maturation. Musa paradisiaca and Musa cavendish fruits were stored at 20°C, increasing values of a* and b* changed the color from green to yellow (Salvador et al., 2007).
The objective of this research was to evaluate the effect of three solutions used as cassava-starch coatings (Maniot sculenta) with the addition of antioxidant agents on harton fruit quality (Musa paradisiaca) when stored for 32 days at 18 ± 4°C and 85% RH. Physicochemical properties and the color of the peel were evaluated and the polyphenol oxidase activity was measured in the postharvest days.
MATERIALS AND METHODS
Experimental design and statistical analysis. The experimental design of this study was a randomized factorial one, in order to appropriate the treatments of the three coverings 6 g L-1 AA, 8 g L-1 NAC and a mixture, in equal parts, of each of these with applications through immersion for 3 min, with a factor (storage temperature) at one level (18 ± 4°C) and another factor for the storage time at 9 levels: 0, 4, 8, 12, 16, 20, 24, 28 and 32 days. The assays were carried out in triplicate on 100 experimental units and the response variables were weight loss, firmness, total soluble solids (°Brix), pH, titratable acidity, maturity index, color, browning index, and enzymatic activity of polyphenol oxidase. For the statistical analysis, each of the considered variables were subjected to the mean and standard error, as well as ANAVA Analysis of variance. Statisti XL® statistical software was used to compare the multiple variables with a confidence level of 95% and 80% power; the Student t-test was applied to compare the differences between the 3 treatments and the control of response variables, peel color index (PCI), browning index and firmness.
Plant material. A total of 10 clusters was used for harton plantain harvested in three different months, collecting samples on the 1, 3 and 5 of each one for 100 units in each experiment (which was performed in triplicate), for a total of 300 fruits of the same chronological age of cutting and same ripening, completely green fruit visual color scale No.1, classified according to the Colombian Technical Standard (NTC 1190, 2000), export type, obtained in the Villa Victoria, Mampujan district of the municipality of María La Baja, Bolívar Department, located at 14 meters above sea level with an average temperature of 27.5°C, annual rainfall between 1500 - 2000 mm and 85% HR.
Reagents. N-acetyl-L-cysteine GNC®, 600 mg capsules (General Nutrition Corporation, dietary supplement, Pittsburg, PA 19222, USA), food grade ascorbic acid (Chemicals Atlantic Colombia), native cassava starch flour (extracted in the pilot food plant of the Engineering Faculty at the University of Cartagena), glycerol (99%, USP, and polyethylene glycol 600®), USP Atlantic Chemicals, were used. The chemicals solutions were: 0.1% (w/v) sodium hypochlorite, magnesium sulfate 0.5% (w/v) and calcium chloride 1% (w/v), as pre-treatment for improving the texture; additionally, distilled water was used for the different solutions.
Formulation and application of solutions. The solutions to coat the bananas were prepared with cassava starch obtained by dissolution of 50 g of native cassava starch in one liter of distilled water, with 6 g of glycerol as the plasticizers (30 polyethylene g 600®), homogenizing with a magnetic stirrer on a heating platform (Nuova II) for 40 min, controlling the temperature at 71 ± 3°C for 20 min. At 40°C, the pH was adjusted to 5.0 with a 1M NaOH solution using a digital potentiometer (Hanna Instruments Inc E200 HI 9126). The solution to coat the fruit with treatment 1 (tr1) was obtained by adding 6 g ascorbic acid (AA) based on 1 liter of a cassava-starch solution; to coat the fruit with treatment 2 (tr2), 1 liter of cassava-starch solution was prepared with 8 g of N-acetyl-L-cysteine (NAC); and to coat the fruit with treatment 3 (tr3), equal parts of an AA solution of 6 g L-1 were mixed with 8 g L-1 NAC. All of the treatments were packaged in polyvinyl chloride containers (PVC) that were previously sterilized and then stored at a room temperature of 30°C until use.
The application of the coatings was conducted by immersing one hundred randomly selected bananas, in each replication, in a magnesium sulfate solution at 0.5% w/v for 5 min to remove the latex exudation, then in a solution of sodium hypochlorite 0.1% w/v for 3 min (as a disinfectant) and subsequently in a solution of calcium chloride, CaCl2, 1% p/v (as a texture enhancer) for 5 min. The assays were divided into five lots of 20 units for applying the treatments, tr1, tr2 and tr3; then, they were immersed for 3 minutes. Two lots were used for the control sample: one covered with a cassava-starch based solution without antioxidants and the other was uncoated. The lots were deposited in trays where they were kept in a ventilated place; once the films of the fruits were dry, they were packed in corrugated carton boxes and transported to the University of Cartagena, where they were stored at a temperature of 18 ± 4°C and 85% RH to monitor the physicochemical characteristics with monitoring of the color every 4 days and the enzymatic activity during the postharvest stage.
Physic-chemical characterization. The percentage of weight loss (WL), every 4 days and postharvest, was evaluated using Equation 1 and an analytical, digital Ohaus balance. The results are expressed as percent of weight loss of the fruit (% WL).

Where: W0 is the initial weight and Wf is the final weight. The measurement of firmness was performed immediately afterwards in the whole fruit with the peel and a texturometer, EZ-S Shimadzu, using a standard cylindrical probe of 3 mm in diameter and penetrating 20 mm into 5 segments of the fruit; the results were expressed as the highest penetration force in Newton (N) (Dadzie and Orchard, 1997).
The total soluble solids content was expressed in °Brix, performing the measurement in a sample of the pulp in a refractometer (Atago CO Ltda, Itabashi-Ku, Tokyo, Japan; Scale of 0-32%), according to AOAC method 932.12 (1992). To calculate the correction, acidity (TA) and °Brix obtained in the experiments were used (Colombian Technical Standard NTC 4086, 1996), according to Equation 2.

Where: A is the proportion of malic acid and TSS are the total soluble solids.
The titratable acidity (TA) in the pulp was evaluated from 25 g of homogenized pulp with 100 mL of distilled water (Method A.O.A.C. 942.05/90, 1995), filtered to remove the crude tissue, and the extract of the pulp decanted to 250 mL in an Erlenmeyer. The pH was measured with a digital potentiometer, Hanna HI 9126, in 50 mL of the filtrate mixed with 0.1 N sodium hydroxide, using phenolphthalein as an endpoint indicator or pH of 8.2. It was expressed as a percentage of malic acid, which is the strongest acid presented in Musa, using Equation 3 (Hobson, 1993).
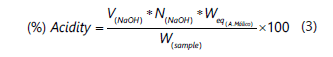
Where, V is volume in liters of sodium hydroxide (NaOH), N is the normality of the base used in the titler, Weq is the equivalent weight in grams of malic acid, and W is the weigh of the sample in grams.
The maturation rate was calculated from the ratio between the total soluble solids and the titratable acidity and was determined using Equation 4.
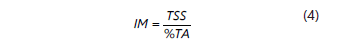
Where IM is the maturation index, TSS is the Total Soluble Solids expressed in º Brix and %TA is the percentage of Titratable Acidity expressed with malic acid. The pH was established by direct immersion of the electrode with a Schott CG840B pH meter (Márquez et al., 2011).
In all of the fruits, the peel color was evaluated with monitoring during the postharvest days at 18±4°C and 85% RH, taking photographs of 100 fruits (in triplicate) of the portion of the opposite sides of the equatorial part of the fruit with a Sony Cyber-Shot camera, T series, at a distance of 40 cm with white light illumination of the central region (Padrón, 2009), as determined with a Hunter Lab colorimeter, Miniscan model (Hunter Associates Laboratory, 1995®). The color values of luminosity were L* (0 means black and 100 means white), a* indicating the position of the rectangular coordinate between green (-a) and red (+a) and b* indicating the rectangular coordinate position between blue (-b) and yellow (+b), determined at six different points of the photographed area and averaging the values. The variation of the total coloration of the peel, Delta E (DE), was calculated using Equation 5 (Maskan, 2001), the chroma or color saturation (C) with Equation 6, the peel color index (PCI) with Equation 7, and the Browning Index (BI) or oxidationindex (OI) with Equation 8 during the storage period from day 0 until day 32, at an interval of 4 days.

Assay of the enzymatic activity of polyphenol oxidase (PPO). The enzymatic activity for PPO was performed spectrophotometrically at days 0, 20, 24 and 32, measuring the absorbance at 420 nm with 10 second intervals for a time of 240 seconds at 30°C, using a cell quartz from the reaction mixture, composed of 0.1 mL aliquots of the enzyme solution of the PPO of the peel, which was incubated at 4°C, plus 1.3 mL of 0.1 M phosphate buffer, pH 7, and 0.1 mL of a 0.005 M solution of dopamine as the substrate. The PPO activity values were obtained from the values of the inclinations of the linear portion of the absorbance curve vs time, conducted in triplicate. One unit of enzyme activity was defined as U equal to 0.001 Abs 420 nm g-1 of extract min-1 (Guerrero, 2009).
RESULTS AND DISCUSSION
The Figure 1 shows that the effect of the coatings, with respect to the percentage of physiological weight loss (WL), was not significant (P<0.05) in the treatments (tr) tr1 (AA), tr2 (NAC) and tr3 (AA + NAC). In the three treatments, the average weight loss values at day 32 of storage was 8.9% in the fruits with AA and NAC and, in the fruits with AA + NAC, it was 8.3%. The highest value occurred in the control samples of bananas covered without anti-browning agents and bananas uncovered after storage until day 20; at which time, the commercial and sensory quality losses for the control fruits were estimated, reporting values of 11.56% and 11.78%, respectively. This value is different from the one reported by (Beltrán et al., 2010) for harton fruits (Musa AAB Simmons) from Armenia, Quindio, 21%; however, other researchers have reported physiological weight losses for other banana cultivars (AAA pisang Berangan) of 10% during 28 days of storage when coated with a solution composed of 10% acacia gum and 1% of chitosan, stored at 13°C and 80% RH (Maqbool and Alderson, 2010).
Baldwin et al. (1996) stated that coatings prevented weight loss, probably due to the action of creating an antagonist semi-permeable barrier with oxygen to carbon dioxide, moisture, the movement of solutes, the reduction in the rate of respiration, loss of water and the rate of oxidation reactions. According to Lammertyn et al. (2003), weight loss is related to the transpiration rate, which refers to the water diffusion and other volatile substances from the fruit, through the epidermis, as a consequence of the nature of metabolism (respiration, perspiration), and is described by Fick's first law, which states that the flow of a gas through a barrier of tissue is proportional to the concentration gradient. Asilah et al. (2002) stated that decreasing the weight of fresh "Dominico" harton fruits varies with the climate, location and maturity state since fruits lose more weight in the dry season and change color from dark green to light green.
The effect of the coatings on fruit firmness was evidenced in the storage period by a significant difference (p<0.05) in the decrease in the penetration force in tissues between covered fruits and uncovered fruits. Figure 2 shows that more firmness was seen in the fruits with the tr1, tr2 and tr3 treatments, which were the maximum values, with better results in fruits with tr3 (AA + NAC), which had a firmness of 30.47 N by day 28; for day 32, the firmness decreased to 30 N in tr3. These results were higher than those reported by Arrieta et al. (2006) in bananas named "Papocho" during the postharvest stage at 8.2 kg cm-2 (0.805 N); the values reported by Barrera et al. (2010), who gave values less than 0.5 N in ripe fruits of plantain Dominico Harton (Musa AAB Simmonds) and the results reported by Beltrán et al. (2010), with lower values at 5 N in 14 days of storage of Harton. The process of softening of fruits can be explained by referencing the concept of metabolism of the cell wall of plants, as done by Brummell and Harpster (2001), who described the process of structural changes that are occur during differentiation and development in the organs of the fruit.
The decrease in the firmness strength value was due to the degradation of starch to form sugars. Besides this, it was caused by cell disruption and solubility of pectin substances. This characteristic depends on the effect that results from enzymes on the pectin and the starch, from the catalytic action of enzymes, where the pectate lyase is emphasized, which activates the breakdown of esterified pectin through the elimination of mechanism Beta, in the presence of Ca+2, polygalacturonase, which activates the hydrolysis of the α - (1→4) unions and bonds with the galacturonic acid residues through the pectinmethylesterase enzyme, which catalyzes the demethylation of the carboxylic group on the carbon-6 position of galacturonic acid residues in pectin molecules of high molecular mass (Brummel, 2001). Another factor is the movement of water from the skin to the pulp of the fruit due to the osmosis process (Azcón and Talon, 2008).
Total soluble solids. Carbohydrates soluble in fruits are major components of the total soluble solids (TSS), so these solutes are used as criteria to establish standards of maturation of some fruits and, therefore, their edible quality is better correlated with total soluble solids (Wills et al., 1984). Figure 3A shows the concentration of total soluble solids (TSS), expressed in °Brix in the pulp, in which the increase was observed in the control fruits with significant differences (P<0.05). The uncovered control reached a maximum at day 16, 17.48 °Brix; the covered control reached the maximum on day 24, 21.89°Brix. The treatments showed significant differences for the 24th day, showing different concentration levels, being higher on day 32 in the fruits with tr1, 12.68°Brix; in fruits with tr2, there were 11.76°Brix, while the fruits with tr3 had 10.6 °Brix. The results found in the TSS content of the fruits in the three treatments and the control of this study differed markedly from those reported by Barrera et al. (2010), who showed higher values at 30 °Brix in banana fruits with 5 days of storage at 28°C and 85% RH, evidencing that a storage temperature of 18°C is a favorable factor for preserving fruits. Gol and Ramana (2011), in banana fruits covered with chitosan at 1.5% plus gibberellic acid at 100 ppm with 10 days of storage at 34 ± 1°C, found a TSS concentration of 14.02 ± 0.03 °Brix. Some authors have correlated the highest concentration of TSS in typically climacteric fruits with the peak of CO2 production and increased sugars, explaining that the increase is due to the hydrolysis of starch and mainly glucose , fructose and sucrose accumulation, due to a metabolic activity that involves α and β amylase enzymes (Arrieta et al., 2006; Kader, 2002).
pH and titratable acidity. Figures 3B and 3C show the behavior of pH and acidity of the fruit, respectively, wherein significantly differences were observed between the three treatments and the control fruits; the treatments showed a very similar behavior at the beginning in the pH values, being higher in the uncovered control, where the value decreased from 5.5 ± 0.1 to 4.3 ± 0.48 and the treatments showed similarity until the end, noticing an increase to 6.61 ± 0.48 on day 32 of storage. In the progress of the acidity, significant differences between all of the samples were observed, being higher with the uncovered control, which reached the highest value (0.67% ± 0.17), and between treatment tr3 (AA + NAC), which showed the smallest increase (0.32% ± 0.04). Dadzie and Orchard (1997) reported that the titratable acidity in bananas depends on the cultivar; in some cases, there is an acidity increase during ripening, while in others there is not a significant change.
The results of this research, taking into account the pH, were similar to those reported by several authors in different studies: Barrera et al. (2010) in the ripening process of banana fruit stored for 5 days at 28°C and 85% HR, García et al. (1998) in raspberry (Fragaria ananassa) covered with starch and plasticizers, García et al. (2006) in four different varieties of plantains with AAA and without applying ethylene, stored at 22 ± 2ºC and 75 ± 10% RH, and Gol and Ramana (2011) in bananas covered with chitosan stored for 10 days at 34°C.
According to the authors Beaulieu and Gorny (2002) and Olivas (2005), low-level changes in pH values, titratable acidity and total soluble solids are related to a reduction in the respiration rate. Hobson (1993) considered that the acidity in fruits is due to the presence of ascorbic acid and malic acid until full physiological development, when it begins to decrease with fruit maturation. According to Arrieta et al., (2006), the chronological state of fruit harvest influences the TSS content, pH and acidity.
Maturity index (MI). Figure 3D shows the behavior of the maturity index, highly variable, which had significant differences (p<0.05) between the control fruits and the three treatments. The larger increases in this variable, from 20 ± 1.65 to 75.99 ± 2 on day 20, were seen in the uncovered control fruits and after 24 days in the uncovered control fruits with 58.35 ± 2.
In the treatments, the MI values reached 33.1 ± 0.75 in the fruits with tr1; in fruits with tr2, it reached 34.44 ± 0.75 and 32.65± 0.75 in fruits with tr3 at 32 days of storage. This behavior is explained because, at maturity, the concentration levels of TSS increases for the conversion of organic acids to sugars (Dadzie and Orchard, 1997).
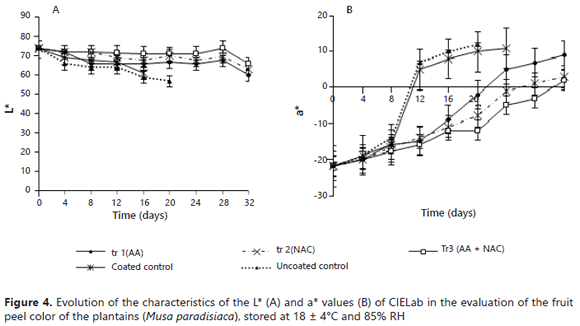
The color. Figures 4A and 4B show the influence of the coatings on the peel color of the fruits of banana treated with tr1, tr2 and tr3.
Statistically significant difference was found between control and treatments, for L*, storage on 20 days. The control, by day 24, showed a value for a* equal to 11, and the coated fruits (tr3) with storage at day 24 showed a value for a* equal to -5, indicating that the fruits had only green colored tips and the rest was yellow, which did not happen in the fruits with treatments because fruits with tr2 (NAC) and tr3 (AA + NAC), by day 32, reached maximum a* values of 3 and 2, respectively.The green color change to yellow in the peel is mainly due to the degradation of chlorophyll (Palmer, 1971). However, the tr1fruits, at day 32, showed a value of 10, indicating a completely yellow color with some degree of brown spots (Mendoza and Aguilera, 2004), but the pulp was firm, a characteristic that consumers prefer in fresh bananas. Due to the non-uniformity of the color space, the lighter the color is, the bigger the tolerance of L* is with an often smaller tolerance of a* and b* (Hunterlab, 2001).
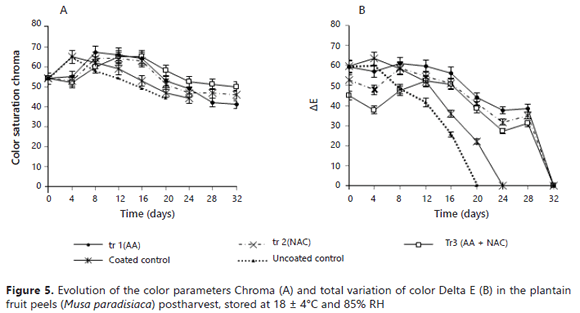
The Figure 5A and 5B shows the parameters of Chroma and total variation of color Delta E, respectively.
The Figure 6A and 6B shows the color index of the peel (CIP) and oxidation index or browning index (BI), respectively.
In browning tests between color saturation Chroma and Delta E at day 32, significant differences were not found. In the monitoring performed on the coated and uncoated fruits with the variables: Chroma (Figure 5A), Delta E (Figure 5B), CIP (Figure 6A) and OI (Figure 6B), a similar behavior was seen between the fruits from tr1, tr2 and tr3, which was different from the one seen in the control fruits. Although in all of the samples, both in the control and coated fruits, the ΔE values were similar at the end of each experiment (zero), significant differences (P <0.05) were observed in all of the samples on days 16, 20, 24 and 28 of the experiment because the values of ΔL*, Δa* and Δb* corresponded to zero, which indicated that on the last day of the experiment, the sample and standard values of each parameter appeared equal.
The maximum values calculated for CIP (Figure 6A) were found in uncoated fruits (4.7), which were taken as the characteristic color of entirely yellow fruits and minimum values for CIP were found in coated tr3 fruits (1.0), with shades of yellow-green tones that are accepted as fresh fruit.
The minimum value for the Oxidation Index or Browning Index OI (Figure 6B) was seen (94.3) in the fruits coated with tr3, whose values were within the discretion of the color of the peel under the name "yellow without severe browning". The maximum value of OI was (170) in the uncoated control fruits, thus indicating a high degree of browning, probably due to the enzymatic activity of the polyphenol oxidase (Kader, 2002).
Mendoza and Aguilera (2004), in the ripening of banana (Musa cavendisch), reported parameter values of L* equal to 69, a* equal to 11.1 and b* equal to 46.2, corresponding to fully yellow fruit with some brown spots on the ninth day of storage, designated as commercial acceptance by color.
Enzymatic activity of polyphenol oxidase - PPO. The Figure 7 shows the influence of the coatings on the enzymatic activity of the PPO.
The treatment with the lowest enzyme polyphenol oxidase activity had the mixture of anti-browning compounds AA + NAC, tr3, while the highest enzyme activity was found in the control and uncovered fruits and the coated fruits without anti-browning substances. The above behavior may have been due to the action of the anti-browning agents, especially AA for the capability of reducing quinones to diphenols, which obstruct the browning reaction, as well as sulfur containing amino acids such as N-acetyl-cysteine, which prevent browning by reaction with the quinones to form colorless compounds, thus combining these two substances and creating a synergistic action that results in an anti-browning effect on the fruits (Robards et al., 1999).
The concentration of anti-browning compounds could also be a factor that potentiates the effect on the enzyme activity of the PPO, probably due to the degradation of sugars during storage, while maintaining low levels of phenolic compounds, results that differ with those found in this experiment, since the fruits treated with 8 g L-1 N-acetylcysteine showed less activity than those treated with 6 g L-1 AA. Differences exist between the varieties of fruits and different stages of development; the enzyme activity may be higher in young fruits than in mature fruits although the cause of these differences may be due solely to the different results in the extraction and purification of the polyphenol oxidase enzyme (Robards et al., 1999).
Garcia et al. (2006) determined the enzyme kinetics in bananas and Michel Gross reported that the enzymatic activity of PPO decreases as maturity increases and that there is an emergence of various phenolic compounds, such as putrescine (NH2 - CH2 - 4NH2) and cadaverine (NH2 - CH2 - 5NH2), which are free or combined; this type of enzyme masks the kinetic to increase the maturation. Probably, porphyrins and polyphenols are conjugated to form a compound that is not easily recognized as PPO with a spectrophotometer.
CONCLUSIONS
The use of coatings based on cassave tarch with additions of 6 g L-1 ascorbic acid and 8 g L-1 N-acetyl-cysteine is an alternative for improving the quality of plantain fruits and increasing shelf life to 32 days postharvest.
The enzyme activity of the PPO was lowered in the postharvest for the coated fruits, compared with the control fruits. Also, the weight loss was lower and there was better firmness in the fruits treated with coatings, as compared to the control fruits.
REFERENCES
Arrieta, A., U. Baquero and J. Barrera. 2006. Physico-chemical characterization of the maturation process papocho banana (Musa ABB Simmonds). Agronomía Colombiana 24(1): 48-53.
Asilah, P., G. Giraldo., F. Celis, and J. Duarte. 2002. Physical and chemical changes during ripening of banana Dominico-Harton (Musa AAB Simmonds) in the central Colombian coffee region. En: Memories XV meeting of Banana Growers Association of Colombia. Cartagena de Indias. Colombia.
Azcón, J. and M. Talon. 2008. Fundamentos de fisiología vegetal. Second Edition. McGraw-Hill. Barcelona. 978 p.
Baldwin, E., M. Nisperos-Carriedo, X. Chen and R. Hagenmaier. 1996. Improving storage life of cut apple and potato with edible coating. Postharvest Biology and Technology 9: 151-163. https://doi.org/10.1016/s0925-5214(96)00044-0
Barrera, J., G. Arrazola and D. Cayon. 2010. Physicochemical and physiological maturation process Horn plantain (Musa AAB Simmonds) in two production systems characterization. Agronomia Colombiana 59(1): 20-29.
Beaulieu, J.C. and J. Gorny, 2002. Fresh cut Fruits. http://www.ba.ars.usda.gov/ hb66/146freshcutfruits.pdf (accessed 14.05.2012).
Beltrán, G., T. Velásquez and G. Giraldo. 2010. Physicochemical characterization of ripening Dominico-Harton plantain (Musa AAB Simmonds) Revista Investigaciones de la Universidad de Quindío 20: 166-170.
Bierhals, V.S. and M. Hubinger. 2011. Effect of cassava starch coating on quality and shelf life of fresh-cut pineapple (Ananas comosus L. Merril cv "Pérola"). Journal Food Science 76(1): E62-E72. https://doi.org/10.1111/j.1750-3841.2010.01951.x
Brummell, D.A. and M. Harpster. 2001. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Molecular Biology 47: 311-340. https://doi.org/10.1007/978-94-010-0668-2_18
Corpoica 2004. Banana (Musa spp) harvest and post-harvest in the agribusiness chain. Alliance: Sena Regional Meta. Produmedios 8: 3-40.
Dadzie, B.K. and J. Orchard. 1997. Routine assessment of postharvest banana and plantain hybrids: criteria and methods-Technical Guidelines and International Network for the Improvement of Banana and Plantain. 63 p.
Dhall, R.K. 2013. Advances in edible coatings for fresh fruits and vegetables: a review. Rev Food Science Nutrition 53(5): 435-450. https://doi.org/10.1080/10408398.2010.541568
García, M.A., M. Martino and N. Zaritzky. 1998. Plasticized starch-based coatings toimprove strawberry (Fragaria ananassa) quality and stability. Journal Agricultural Food Chemistry 46(9): 3758-3767. https://doi.org/10.1021/jf980014c
García, W., G. Giraldo, T. Hurtado and C. Mendivil. 2006. Kinetics of enzymatic Gros Michel banana polyphenoloxidase in different stages of maturation. Vitae, Journal of the Faculty of Pharmaceutical Chemistry. Universidad de Antioquia, Medellin - Colombia 13(2):13-19.
Gol, N.B. and M. Ramana. 2011. Banana Fruit Ripening as Influenced by Edible Coatings. International Journal of fruit Science 11: 119-135. https://doi.org/10.1080/15538362.2011.578512
Guerrero, E. 2009. Inhibition of enzymatic activity of polyphenoloxidase extracted from banana (Cavendish valery) using aqueous biphasic systems with isoespintanol and ascorbic acid. Thesis. Universidad Nacional de Colombia. Facultad de Ciencias Agrarias. Medellín. 91 p.
Hobson, G.E. 1993. Fruit ripening: Plant physiology and biochemistry. McGraw-Hill, Madrid. 478 p.
Hunterlab, 2001. www.info @ Hunterlab.com.
ICONTEC. 2000. NTC 1190, Instituto Colombiano de Normas Técnicas.
ICONTEC. 1996. NTC 4086, Instituto Colombiano de Normas Técnicas.
Jafarizadeh, M.H, A. Osman, T. Ping and R. Abdul. 2011. Effects of edible surface coatings (sodium carboxymethyl cellulose, sodium caseinate and glycerol) on storage quality of berangan banana (Musa sapientum cv. Berangan) using response surface methodology. Journal of Food Processing and Preservation 36(3): 252-261. https://doi.org/10.1111/j.1745-4549.2011.00583.x
Kader, A. 2002. Post-harvest technology of horticultural crops. Agriculture and Natural Resources. Davis, University of California. California. 535 p.
Lammertyn, J., N. Scheerlinck, P. Jancsók, B.E. Verlinden, B.M Nicolaï. 2003. A respiration diffusion model for 'Conference' pears. I. Model development and validation. Postharvest Biology and Technology 30(1): 29-42. https://doi.org/10.1016/s0925-5214(03)00061-9
Lassois, L., J. Busogoro and Jijakli. 2009. The banane: de son origine and commercialization. Biotechnology, Agronomy, Society and Environment 13(4): 575-586.
Malmiri, J.H., A. Osman, C. Tan and R. Abdul-Rahman, 2011. Development of an edible coating based on chitosan-glycerol to delay 'Berangan' banana (Musa sapientum cv. Berangan) ripening process. International Food Research Journal 18: 989-997.
Maqbool, M.A. and P. Alderson. 2010. Effect of cinnamon oil on incidence of anthracnose disease and post-harvest quality of bananas during storage. International Journal of Agricultural and Biological 12: 516-520.
Márquez, C.J., A. Jimenez, C. Osorio and J.R. Cartagena. 2011. Volatile compounds during the ripening of colombian soursop (Annona muricata L. cv. Elita). Vitae Journal of the Faculty of Pharmaceutical Chemistry. Universidad de Antioquia, Medellin - Colombia 18(3):2145-2660.
Maskan, M. 2001. Kinetics of colour change of kiwifruits during hot air and microwave drying. Journal of Food Engineering 48(2): 169-175. https://doi.org/10.1016/s0260-8774(00)00154-0
Mehdi, M., R. Senthil, R. Daniel and G. Peter. 2011. Control of postharvest anthracnose of banana using a new edible composite coating. Crop Protection 29: 1136-1141. https://doi.org/10.1016/j.cropro.2010.06.005
Mendoza, F. and J. Aguilera. 2004. Application of image analysis for classification of ripening bananas. Journal of Food Science 69(9): E471-E477. https://doi.org/10.1111/j.1365-2621.2004.tb09932.x
Neeta, B. and R. Ramana. 2011. Banana fruit ripening as influenced by edible coatings. International Journal of Fruit Science 11(2): 119-135. https://doi.org/10.1080/15538362.2011.578512
Olivas, G.I. and G. Barbosa-Canovas. 2005. Edible coatings for fresh-cut fruits. Critical Reviews in Food Science and Nutrition 45(7-8): 657-670. https://doi.org/10.1080/10408690490911837
Padrón, P. 2009. Computer vision system and graphic design tools for imaging food samples segmented and averaged coordinates CIE-L*a*b*: Agronomía Costarricense 33 (2): 283-301.
Palmer, J.K. 1971. The banana. The biochemistry of fruits products. Academic press, ARC Food Research Institute, Norwich, England. 105 p.
Pinto, A.L. 2010. Characterization of quality attributes during storage of green banana (Musa cavendish) minimally processed vacuum impregnated with antioxidation solutions. Thesis. Universidad Nacional de Colombia. Facultad de Ciencias Agrarias. Medellín. 108 p.
Robards, K., P.D. Prenzler, G. Tucker, P. Swatsitang and W. Glover. 1999. Phenolic compounds and their role in oxidative processes in fruits. Food Chemistry 66(4): 401-436. https://doi.org/10.1016/s0308-8146(99)00093-x
Salvador, A., L. Arnal, C. Besada, V. Larrea, A. Quiles, I. Pérez-Munuera. 2007. Physiological and structural changes during ripening and deastringency treatment of persimmon fruit cv. 'Rojo Brillante'. Postharvest Biology and Technology 46(2):181-188. https://doi.org/10.1016/j.postharvbio.2007.05.003
Sheryl, L., M. Raposo, C. Santos and A. Miranda. 2010. Chemical dips and edible coatings to retard softening and browning of fresh-cut banana International Journal of Postharvest Technology and Innovation 2(1):13-24. https://doi.org/10.1504/ijpti.2010.038185
Soltani, M. and M. Omid. 2010. Comparison of some chromatic, mechanical and chemical properties of banana fruit at different stages of ripeness. Modern Applied Science 4(7):34-41. https://doi.org/10.5539/mas.v4n7p34
Wills, R.H., T. Lee, T. McGlasson, W. Hall and D. Graham. 1984. Physiology and handling of fruits and vegetables in post-harvest. Editorial Acribia. Zaragoza. Spain. 18 - 37 p.
References
Arrieta, A., U. Baquero and J. Barrera. 2006. Physico-chemical characterization of the maturation process papocho banana (Musa ABB Simmonds). Agronomía Colombiana 24(1): 48-53.
Asilah, P., G. Giraldo., F. Celis, and J. Duarte. 2002. Physical and chemical changes during ripening of banana Dominico-Harton (Musa AAB Simmonds) in the central Colombian coffee region. En: Memories XV meeting of Banana Growers Association of Colombia. Cartagena de Indias. Colombia.
Azcón, J. and M. Talon. 2008. Fundamentos de fisiología vegetal. Second Edition. McGraw-Hill. Barcelona. 978 p.
Baldwin, E., M. Nisperos-Carriedo, X. Chen and R. Hagenmaier. 1996. Improving storage life of cut apple and potato with edible coating. Postharvest Biology and Technology 9: 151-163. http://dx.doi.org/10.1016/s0925-5214(96)00044-0
Barrera, J., G. Arrazola and D. Cayon. 2010. Physicochemical and physiological maturation process Horn plantain (Musa AAB Simmonds) in two production systems characterization. Agronomia Colombiana 59(1): 20-29.
Beaulieu, J.C. and J. Gorny, 2002. Fresh cut Fruits. http://www.ba.ars.usda.gov/ hb66/146freshcutfruits.pdf (accessed 14.05.2012).
Beltrán, G., T. Velásquez and G. Giraldo. 2010. Physicochemical characterization of ripening Dominico-Harton plantain (Musa AAB Simmonds) Revista Investigaciones de la Universidad de Quindío 20: 166-170.
Bierhals, V.S. and M. Hubinger. 2011. Effect of cassava starch coating on quality and shelf life of fresh-cut pineapple (Ananas comosus L. Merril cv "Pérola"). Journal Food Science 76(1): E62-E72. http://dx.doi.org/10.1111/j.1750-3841.2010.01951.x
Brummell, D.A. and M. Harpster. 2001. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Molecular Biology 47: 311-340. http://dx.doi.org/10.1007/978-94-010-0668-2_18
Corpoica 2004. Banana (Musa spp) harvest and post-harvest in the agribusiness chain. Alliance: Sena Regional Meta. Produmedios 8: 3-40.
Dadzie, B.K. and J. Orchard. 1997. Routine assessment of postharvest banana and plantain hybrids: criteria and methods-Technical Guidelines and International Network for the Improvement of Banana and Plantain. 63 p.
Dhall, R.K. 2013. Advances in edible coatings for fresh fruits and vegetables: a review. Rev Food Science Nutrition 53(5): 435-450. http://dx.doi.org/10.1080/10408398.2010.541568
García, M.A., M. Martino and N. Zaritzky. 1998. Plasticized starch-based coatings toimprove strawberry (Fragaria ananassa) quality and stability. Journal Agricultural Food Chemistry 46(9): 3758-3767. http://dx.doi.org/10.1021/jf980014c
García, W., G. Giraldo, T. Hurtado and C. Mendivil. 2006. Kinetics of enzymatic Gros Michel banana polyphenoloxidase in different stages of maturation. Vitae, Journal of the Faculty of Pharmaceutical Chemistry. Universidad de Antioquia, Medellin - Colombia 13(2):13-19.
Gol, N.B. and M. Ramana. 2011. Banana Fruit Ripening as Influenced by Edible Coatings. International Journal of fruit Science 11: 119-135. http://dx.doi.org/10.1080/15538362.2011.578512
Guerrero, E. 2009. Inhibition of enzymatic activity of polyphenoloxidase extracted from banana (Cavendish valery) using aqueous biphasic systems with isoespintanol and ascorbic acid. Thesis. Universidad Nacional de Colombia. Facultad de Ciencias Agrarias. Medellín. 91 p.
Hobson, G.E. 1993. Fruit ripening: Plant physiology and biochemistry. McGraw-Hill, Madrid. 478 p.
Hunterlab, 2001. www.info @ Hunterlab.com.
ICONTEC. 2000. NTC 1190, Instituto Colombiano de Normas Técnicas.
ICONTEC. 1996. NTC 4086, Instituto Colombiano de Normas Técnicas.
Jafarizadeh, M.H, A. Osman, T. Ping and R. Abdul. 2011. Effects of edible surface coatings (sodium carboxymethyl cellulose, sodium caseinate and glycerol) on storage quality of berangan banana (Musa sapientum cv. Berangan) using response surface methodology. Journal of Food Processing and Preservation 36(3): 252-261. http://dx.doi.org/10.1111/j.1745-4549.2011.00583.x
Kader, A. 2002. Post-harvest technology of horticultural crops. Agriculture and Natural Resources. Davis, University of California. California. 535 p.
Lammertyn, J., N. Scheerlinck, P. Jancsók, B.E. Verlinden, B.M Nicolaï. 2003. A respiration diffusion model for 'Conference' pears. I. Model development and validation. Postharvest Biology and Technology 30(1): 29-42. http://dx.doi.org/10.1016/s0925-5214(03)00061-9
Lassois, L., J. Busogoro and Jijakli. 2009. The banane: de son origine and commercialization. Biotechnology, Agronomy, Society and Environment 13(4): 575-586.
Malmiri, J.H., A. Osman, C. Tan and R. Abdul-Rahman, 2011. Development of an edible coating based on chitosan-glycerol to delay 'Berangan' banana (Musa sapientum cv. Berangan) ripening process. International Food Research Journal 18: 989-997.
Maqbool, M.A. and P. Alderson. 2010. Effect of cinnamon oil on incidence of anthracnose disease and post-harvest quality of bananas during storage. International Journal of Agricultural and Biological 12: 516-520.
Márquez, C.J., A. Jimenez, C. Osorio and J.R. Cartagena. 2011. Volatile compounds during the ripening of colombian soursop (Annona muricata L. cv. Elita). Vitae Journal of the Faculty of Pharmaceutical Chemistry. Universidad de Antioquia, Medellin - Colombia 18(3):2145-2660.
Maskan, M. 2001. Kinetics of colour change of kiwifruits during hot air and microwave drying. Journal of Food Engineering 48(2): 169-175. http://dx.doi.org/10.1016/s0260-8774(00)00154-0
Mehdi, M., R. Senthil, R. Daniel and G. Peter. 2011. Control of postharvest anthracnose of banana using a new edible composite coating. Crop Protection 29: 1136-1141. http://dx.doi.org/10.1016/j.cropro.2010.06.005
Mendoza, F. and J. Aguilera. 2004. Application of image analysis for classification of ripening bananas. Journal of Food Science 69(9): E471-E477. http://dx.doi.org/10.1111/j.1365-2621.2004.tb09932.x
Neeta, B. and R. Ramana. 2011. Banana fruit ripening as influenced by edible coatings. International Journal of Fruit Science 11(2): 119-135. http://dx.doi.org/10.1080/15538362.2011.578512
Olivas, G.I. and G. Barbosa-Canovas. 2005. Edible coatings for fresh-cut fruits. Critical Reviews in Food Science and Nutrition 45(7-8): 657-670. http://dx.doi.org/10.1080/10408690490911837
Padrón, P. 2009. Computer vision system and graphic design tools for imaging food samples segmented and averaged coordinates CIE-L*a*b*: Agronomía Costarricense 33 (2): 283-301.
Palmer, J.K. 1971. The banana. The biochemistry of fruits products. Academic press, ARC Food Research Institute, Norwich, England. 105 p.
Pinto, A.L. 2010. Characterization of quality attributes during storage of green banana (Musa cavendish) minimally processed vacuum impregnated with antioxidation solutions. Thesis. Universidad Nacional de Colombia. Facultad de Ciencias Agrarias. Medellín. 108 p.
Robards, K., P.D. Prenzler, G. Tucker, P. Swatsitang and W. Glover. 1999. Phenolic compounds and their role in oxidative processes in fruits. Food Chemistry 66(4): 401-436. http://dx.doi.org/10.1016/s0308-8146(99)00093-x
Salvador, A., L. Arnal, C. Besada, V. Larrea, A. Quiles, I. Pérez-Munuera. 2007. Physiological and structural changes during ripening and deastringency treatment of persimmon fruit cv. 'Rojo Brillante'. Postharvest Biology and Technology 46(2):181-188. http://dx.doi.org/10.1016/j.postharvbio.2007.05.003
Sheryl, L., M. Raposo, C. Santos and A. Miranda. 2010. Chemical dips and edible coatings to retard softening and browning of fresh-cut banana International Journal of Postharvest Technology and Innovation 2(1):13-24. http://dx.doi.org/10.1504/ijpti.2010.038185
Soltani, M. and M. Omid. 2010. Comparison of some chromatic, mechanical and chemical properties of banana fruit at different stages of ripeness. Modern Applied Science 4(7):34-41. http://dx.doi.org/10.5539/mas.v4n7p34
Wills, R.H., T. Lee, T. McGlasson, W. Hall and D. Graham. 1984. Physiology and handling of fruits and vegetables in post-harvest. Editorial Acribia. Zaragoza. Spain. 18 - 37 p.
How to Cite
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Download Citation
CrossRef Cited-by
1. Armando Vinicio Paredes Peralta, Jhoanna Isabel Caiza Cuzco, Luis Fernando Arboleda Álvarez. (2024). El almidón, su uso y efecto como recubrimiento comestible en la conservación de frutas. Ciencia Digital, 8(2), p.144. https://doi.org/10.33262/cienciadigital.v8i2.3001.
2. Elizabeth Murillo, Juan Guillermo Aristizábal, Walter Murillo, Albert Ibarz Ribas, Jonh Jairo Méndez, José Fernando Solanilla. (2017). Preliminary characterization of the enzyme polyphenol oxidase and rheological behavior from Averrhoa carambola juice. Revista Facultad Nacional de Agronomía, 70(1), p.8099. https://doi.org/10.15446/rfna.v70n1.61769.
3. Adedotun Adekunle, Oluwagbenga Adeogun, Yewande Josephine Olorunsuyi, Manuel Tejada Moral. (2021). Effect of leaf extract of Lantana camara with Maize-based coating on the quality of fresh-cut fruits of Ananas comosus and Musa acuminata. Cogent Food & Agriculture, 7(1) https://doi.org/10.1080/23311932.2021.1917834.
4. Thanh Tung Pham, Le Phuong Lien Nguyen, László Baranyai, Thuy Linh Nguyen, Khanh Son Trinh. (2022). Effect of Electrolyzed Cassava Starch-Gelatin Coating on Biochemical Properties and Ripening of Banana (Musa acuminata L.) Fruits. Polish Journal of Food and Nutrition Sciences, 72(3), p.263. https://doi.org/10.31883/pjfns/152667.
5. Misael Cortés Rodríguez, Camilo Villegas Yépez, Jesús Humberto Gil González, Rodrigo Ortega-Toro. (2020). Effect of a multifunctional edible coating based on cassava starch on the shelf life of Andean blackberry. Heliyon, 6(5), p.e03974. https://doi.org/10.1016/j.heliyon.2020.e03974.
Dimensions
PlumX
Article abstract page views
Downloads
License
Copyright (c) 2015 Revista Facultad Nacional de Agronomía Medellín

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The journal allows the author(s) to maintain the exploitation rights (copyright) of their articles without restrictions. The author(s) accept the distribution of their articles on the web and in paper support (25 copies per issue) under open access at local, regional, and international levels. The full paper will be included and disseminated through the Portal of Journals and Institutional Repository of the Universidad Nacional de Colombia, and in all the specialized databases that the journal considers pertinent for its indexation, to provide visibility and positioning to the article. All articles must comply with Colombian and international legislation, related to copyright.
Author Commitments
The author(s) undertake to assign the rights of printing and reprinting of the material published to the journal Revista Facultad Nacional de Agronomía Medellín. Any quotation of the articles published in the journal should be made given the respective credits to the journal and its content. In case content duplication of the journal or its partial or total publication in another language, there must be written permission of the Director.
Content Responsibility
The Faculty of Agricultural Sciences and the journal are not necessarily responsible or in solidarity with the concepts issued in the published articles, whose responsibility will be entirely the author or the authors.