in vitro response of Colletotrichum to chitosan. Effect on incidence and quality on tropical fruit. Enzymatic expression in mango
DOI:
https://doi.org/10.15446/acag.v66n2.53770Palabras clave:
antracnosis, Anonna muricata, Mangifera indica, Musa acuminata, Peroxidase, Polyphenol oxidase (en)Descargas
Recibido: 23 de octubre de 2015; Aceptado: 21 de abril de 2016
Abstract:
Colletotrichum is considered one of the fungal genera with the greatest diversity of species of phytopathogenic fungi and with a wide range of hosts including tropical fruits. In this study, the concentration of chitosan applied was a key factor in the in vitro inhibition of the three Colletotrichum isolates with the most sensitive being the one from banana. Germination was the development stage most affected by the application of this polymer. At the end of 10 days of storage, control of anthracnose in soursop, mango and banana fruit was considerable with the application of 1 % chitosan, with 80 to 100 % inhibition. Likewise, the ripening process of fruit with and without chitosan was generally similar. Activity of the peroxidase (POD) and polyphenol oxidase (PPO) enzymes was observed only in fruit of mango cv. 'Tommy Atkins,' inoculated and non-inoculated with C. gloeosporioides, the activity of both enzymes was higher in the chitosan treatments and the 1.0 % concentration was able to induce gene expression of POD and PPO, occurring until 24 h.
Keywords:
Antracnose, Anonna muricata, Mangifera indica, Musa acuminata, peroxidase, polyphenol oxidase.Resumen:
Colletotrichum se considera uno de los géneros de hongos con mayor diversidad de especies fitopatógenas fúngicas, y con un amplio rango de hospederos que incluyen frutos tropicales. En este estudio, la concentración de quitosano aplicada fue un factor clave en la inhibición in vitro de los tres aislados de Colletotrichum siendo el más sensible el aislado de plátano. La germinación fue el estado de desarrollo que se afectó más por la aplicación de este polímero. Al final del periodo de almacenamiento de 10 días, el control de la antracnosis en la guanábana, mango y plátano fue considerable con la aplicación de quitosano al 1.0%, con una inhibición del 80 a 100%. Asimismo, el proceso de maduración con y sin quitosano fue en general similar. La actividad de las enzimas peroxidasa (POD) y polifenol oxidasa (PFO) se observó únicamente en frutos de mango cv. 'Tommy Atkins' inoculados o no con Colletotrichum, los resultados mostraron que la actividad de ambas enzimas fue mayor en los tratamientos con quitosano y la concentración de 1% fue capaz de inducir expresión de los genes de POD y PFO hasta las 24 h.
Palabras clave:
Anonna muricata, antracnosis, Mangifera indica, Musa acuminata, peroxidasa, polifenol oxidase.Introduction
Chitosan is a biopolymer which is industrially obtained from the alkaline N-deacetylation of chitin. It is a polycationic polymer useful as an antimicrobial and chelating agent, and also for use in coatings and gels, among other applications.
As an antimicrobial agent, numerous studies have demonstrated its effectiveness in the control of microorganisms, including phytopathogenic fungi belonging to the families Mucoraceae (Rhizopus stolonifer), Sclerotinicaeae (Botrytis cinerea, Slerotinia sclerotiorum), Trichocomaceae (Penicillium digitatum, P. expansum), and Pleosporaceae (Alternaria alternata), among others.
Chitosan is also reported to be useful in forming coatings to maintain and extend fruit quality during storage. This behavior have allowed to be used in strawberry (Fragaria x ananassa) (Gol et al., 2013), raspberry (Rubis idaeus) (Tezotto-Uliana et al., 2014), banana (Musa sp.) (Kyu-Kyu Win et al., 2007) and several species of citrus (Contreras-Oliva et al. 2012), among others, in which a decreased respiratory rate, and less weight and firmness loss during storage in treated fruit are mentioned.
There are also studies which prove the resistance-inducing properties of chitosan in the form of defense responses in plants and plant organs. It is mainly associated with peroxidase (POD) and polyphenol oxidase (PPO) as defense enzymes related to the application of chitosan in fruit and vegetables. In this regard, in tomato (Lycopersicon esculentum L.) inoculated with P. expansum and Botrytis cinerea, Liu et al. (2007), reported greater POD activity during storage in the treatment with 2% chitosan. On the other hand, the increased activity of these enzymes has also been linked with increased disease resistance during the infection process; for example, in fruits of mango cvs. 'Keitt' and 'Zill,' Gong et al. (2013), indicated greater POD and PPO activity in those inoculated with C. gloeosporioides.
Regarding Colletotrichum, it is considered to be one of the genera that includes the largest diversity of species of phytopathogenic fungi, with a wide range of hosts including commercially-important tropical fruits such as soursop (Anonna muricata), mango (Mangifera indica) and banana (Musa sp.), to name a few. On the other hand, remarkable control has been observed in various species of the genus Colletotrichum with the application of chitosan coatings in papaya (Carica papaya), tomato and grape (Vitis vinifera) with a concomitant reduction in lesion diameter (Al- Eryani-Raqeeb et al., 2009; Muñoz et al., 2009).
This research aims to evaluate the in vitro activity of chitosan in three strains of Colletotrichum obtained from soursop, mango and banana. In addition, seeks to determine the effect of its application on anthracnose development and the ripening process in these fruit and the activity and gene expression of the defense enzymes POD and PPO induced by chitosan only in fruit of mango cv. 'Tommy Atkins.'
Material and methods
Microorganisms
Isolates of the fungus Colletotrichum were obtained from fruit of soursop (Annona muricata), mango (Mangifera indica) cv. 'Tommy Atkins' and banana cv. 'Enano gigante' (M. acuminata (AAA)), which performed the typical symptoms of anthracnose disease. Conidia isolated from simple conidiophores have the typical characteristics of this genus: simple one-celled conidia, hyaline and elongated to ovoid in size (4-15 µm long and 3-4 µm wide).
Chitosan preparation
Low molecular weight chitosan (Mw = 1.74x104 Da, 75-85% deacetylation degree; FW = 161 20,000 cps) (Sigma-Aldrich) concentrations of 0.5%, 1.0% and 1.5% were prepared by adding equal amounts (w/v 1:1) of acetic acid to chitosan. The chitosan-acetic acid mixture was added to the total volume of distilled water and stirred overnight at room temperature. The solutions were adjusted to pH 5.5 with 1N NaOH solution. Consequently, 0.1 ml of Tween 80 was added.
Mycelial growth rate, sporulation and germination
Radial mycelial growth of the fungi was measured in 5 Petri dishes per treatment, everyday for approximate 12 days with a Truper vernier caliper. The results were expressed as mycelial growth rate in mm day-1. Next, 10 ml of sterile water were added to four Petri dishes per treatment and with a sterile glass rod, the dish surface was scraped off. Subsequently, the resulting solution was filtered twice through sterile gauze to remove the mycelium present. Then 50 µl of the filtered solution were taken and placed in a hemacytometer, and the final spore concentration was measured. One hundred observations were conducted per treatment using a Nikon Eclipse E600 optical microscope. To evaluate spore germination, 50-µl aliquots of a spore suspension were taken from cultures of the three isolates of untreated 8-day-old Colletotrichum. The concentration used was 106 spore ml-1, which was pipetted on three discs per treatment with PDA medium containing the different chitosan concentrations mentioned above and the control (only PDA). The discs were observed through an optical microscope (Nikon Eclipse E600) every hour for 8 h. Germination was stopped by adding a drop of lactophenol-safranin. Values were expressed in number of spores per ml.
Variables evaluated in situ
Fruit
Fruit of mango cv. 'Tommy Atkins' were harvested in an orchard located in Atonalisco, Tepic, state of Nayarit, Mexico. Soursop fruit were taken from orchards in Compostela, Nayarit and those of banana from San Blas, Nayarit, in a physiologically ripe state. The fruit were rinsed in sterile water for 1 min and then allowed to dry in the environment. Then in some treatments the fruit were inoculated by making a wound of 1 cm in depth with a sterile punch. In the wound, 150 µl of the Colletotrichum spore suspension was placed at a concentration of 106 spores. ml-1. Fruit were placed at room temperature for 24 h before carrying out the chitosan application treatments.
Fruit treatment with chitosan
Only the 1% chitosan solution was applied by immersing the fruit in it for approximately 40 sec. They were placed at room temperature for 5 h and then stored at 20 ± 2°C for 10 days. For the phytopathological assessment, the experimental unit consisted of 35 fruit per treatment with four replications. The physicochemical evaluations were performed in five fruit per treatment with four replications, and the experiment was repeated twice. The control treatment fruit were only rinsed with sterile water and stored.
Variables evaluated in vivo
Disease incidence, mass loss, firmness, TSS, pH and titratable acidity
Disease incidence was assessed as the percentage of infection at the end of 10 days of storage in 25 fruit per treatment and four replications. For mass loss, five fruit per treatment were weighed daily on a Sartorius BL 3100 digital scale. The results were expressed as percentages. To determine firmness, penetration testing was performed on both sides of the fruit with a Shimpo FGE-50 universal texturometer in five fruit per treatment. The results were the average of the values obtained, and they were expressed in Newtons (N). From the fruit used to assess firmness, total soluble solids (TSS) were also determined with an Abbe refractometer, with the results reported in °Brix and with correction for temperature to report the results at 20 °C. The pH was determined with the aid of a Hanna Instrument pH 30 potentiometer. Titratable acidity was determined using homogenized 5-g samples taken from 10 fruit per treatment, which were titrated with 0.04N NaOH using phenolphthalein as an indicator. The calculations were reported in % citric acid for mango fruit and malic acid for soursop and banana fruit.
Enzyme activity of POD and PPO
Evaluations of POD and PPO enzymes were performed only in mango cv. cultivar 'Tommy Atkins in inoculated and uninoculated fruit, which were treated with chitosan at concentrations of 0.5, 1.0 and 1.5%. The control treatment was only immersed in water. POD activity was measured as described by Chance & Maehly (1995), with some modifications. The reaction mixture included 0.5 ml of the crude extract (supernatant) and 2 ml of a guaiacol buffer solution (100 mM of sodium phosphate, pH 6.4 and 8 mM of guaiacol). Respectively, was incubated for 5 min at 30 °C. The reaction was stopped by adding 1 ml of H2O2 (24mM) and enzyme activity was determined in a JENWAY 67 series UV/visible spectrophotometer, measuring the increase in absorbance at 460 nm every 5 sec for 1:30 min. The reaction mixture consisted of 0.5 ml of the crude extract, 3 ml of catechol (100 mM of sodium phosphate, pH 6.4, and 500 mM of catechol) as substrate and the increase in absorbance was measured at 420 nm every 10 sec for 3 min.
The calculation of activity for both enzymes was performed at 0, 24, 48 and 72 h. The enzyme activity of POD and PPO was expressed as Umg-1 protein, where one unit is expressed as the increase in the absorbance rate per protein mass per minute.
Gene expression of POD and PPO
Reverse transcriptase reaction to obtain cDNA
To confirm the differential expression of the selected genes, the analysis of total RNA from all experimental conditions was performed using cDNA synthesis. The RT reaction was performed with the Super Script III reverse transcriptase by following the specifications of the kit (Invitrogen(r)) in a final volume of 20 µL. Different aliquots of RNA (2 μg-8 µl) taken from each sample were mixed with 1 µL of dNTPs, 1 µL of oligo dT18, and the rest DEPC water for a volume of 10 µL per reaction. The mixture was heated at 65 °C for 5 min. It was then moved to an ice bath and incubated for 5 min. The reaction mixture was added to a PCR tube containing a second pre-made mix with 2 µL of 10X reaction buffer, 1 µL of RNaseOUT (RNase inhibitor), 2 µL of dithiothreitol (DTT) 0.1 M solution, 4 µL of 25mM MgCl2 and finally 1 µL of the SuperScript III enzyme (all the reagents are included in the Invitrogen SuperScript III First-Strand Synthesis System for RT-PCR kit). The combination of mixtures was homogenized and incubated for 50 min at 50 °C to yield cDNA synthesis. At the end of this time the reaction was quenched by incubating the tube at 85 ºC for 5 min. It was then moved to an ice bath for 1 min and 1 µL of RNase-H was added, for further incubation for 20 min at 37 ºC to digest possible RNA remnants.
Amplifying fragments by the PCR technique
For amplification by the PCR technique, the reaction mixture was prepared (2.5 µL of 10X reaction buffer Taq, 3 µL of 50 mM MgCl2, 0.5 µL of 10 mM dNTPs, 0.5 µL Oligo upper, 0.5 µL Oligo lower, 0.2 µL of 5U. µL-1 Taq polymerase, 4 µL de cDNA and the rest DEPC water for a volume of 25 µL per reaction) by adding each component in the order indicated. . The sequences of the genes POD and PPO of Mangifera indica were obtained from the GenBank database to design specific oligonucleotides. Sequences of oligonucleotides used for POD forward primer 5'-AgTCCACCTTTgCTTgATT-3' and reverse primer 5'-gAAgACATggggAATTTTTACTCg-3' and PPO forward primer gAAgACATggggAATTTTTACTCg and reverse primer gAAgACATggggAATTTTTACTCg and 18 s forward primer CCTCCggCgCTgTTACTTTg and reverse primer gTTTCAgCCTTgCgACCATACTC.
Programming for the Techne TC-3000X thermal cycler
The reaction solution was incubated at 94 °C for 5 min. Then, the PCR was performed by 35 cycles of denaturation at 94 °C for 30 s and annealing at 51.4 °C for 30 s and polymerization at 72 °C for 60 s and final extension at 72 °C for 10 min. To normalize gene expression, a parallel amplification of 18 s, control gene.
Verification of the PCR products
Once amplification of the segments was completed, the PCR products were analyzed by electrophoresis on 1.2% agarose gel, which was run in 1X TAE buffer at 90 volts for 90 min, with an electrophoresis chamber and Bio-Rad power supply. To verify that the amplified bands corresponded to the expected fragments, 1 µl of 1 kb molecular weight marker plus DNA ladder was used. The DNA was stained with GelRed dye (5 µl DNA + 1 µl dye). Bands were visualized with a visible UV light transilluminator adapted to a Kodak Gel Logic 100 Imaging System.
Statistical analysis
Treatments were arranged in a randomized block design. A Tukey test at a significance level of p≤0.05 was carried out to determine mean separation levels. Deviations standard were also calculated. Disease incidence was analysed using the X2 procedure.
Results and discussion
Mycelial growth rate, sporulation and germination
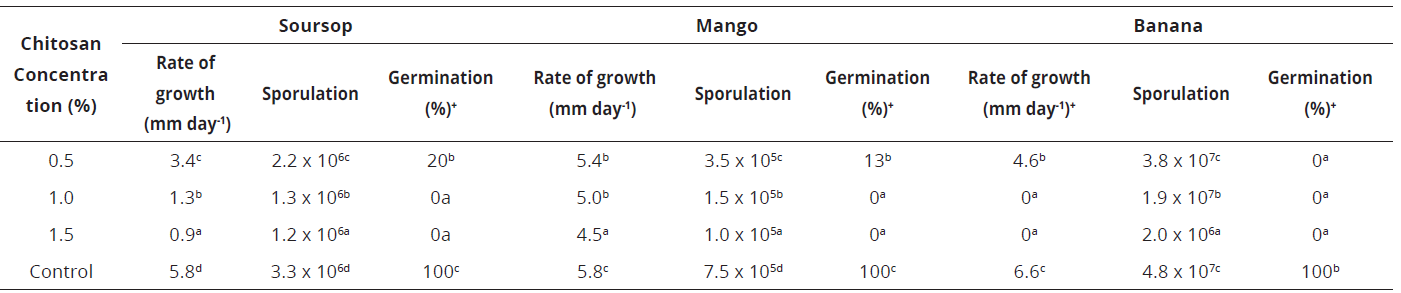
In the three evaluated strains, the chitosan concentration influenced the mycelium growth rate. Given these concerns, the increased concentration allowed the decreasing of the fungal growth. In addition, the inhibitory effect of chitosan was different according to the isolate; this was most evident in the banana isolate where at 1.0% chitosan concentration there was almost zero growth during the 12 days of incubation. Table 1, indicates both growth rate and sporulation decreased significantly (p≤0.05) with the concentration of chitosan applied in all three isolates.
Disease incidence, mass loss, firmness, TSS, pH and titratable acidity
Anthracnose incidence was significantly lower (p≤0.01) in the chitosan-treated fruit. The results showed that anthracnose development in soursop was inhibited by 80%, while in mango and banana the inhibition was 100% with the chitosan coating compared to the untreated fruit, in which little or no disease control was observed (Table 2). The variables mass loss, firmness and acidity mostly showed significant differences (p≤0.05) among the treatments. All chitosan-treated fruit showed lower percentage mass loss compared to the untreated ones. TSS values of each fruit with and without chitosan were almost similar. Regarding the firmness of the three tested fruit treated with chitosan, it was higher compared to the control fruit, except for mango fruit and acidity was similar in soursop and banana.
Means followed by the same letter are not significantly different (p≤ 0.05) as determined by Tukey's multiple test. Rate of growth after 12 days of incubation. Sporulation and germination after 8 h incubation.Table 1: In vitro development of three chitosan-treated isolates of Colletotrichum obtained from soursop, mango and banana held at different concentrations and incubated at 20 ± 2ºC.
Means followed by the same letter are not significantly different (p≤0.05) as determined by Tukey's multiple test. +X2 = p ≤ 0.01 Harvest values: 1Firmness = 120N; ºBrix = 10.5%; pH = 3.5; acidity = 0.8. 2Firmness = 234N; ºBrix = 7.5%; pH = 1.9; acidity = 1.8, 3Firmness = 110N; ºBrix = 12.7%; pH = 4.9; acidity = 0.4.Table 2: Incidence of Colletotrichum and quality of soursop, mango and banana fruit chitosan-treated at 1% concentration after 10 days storage at 20 ± 2 ºC.

Enzyme activity of POD and PPO
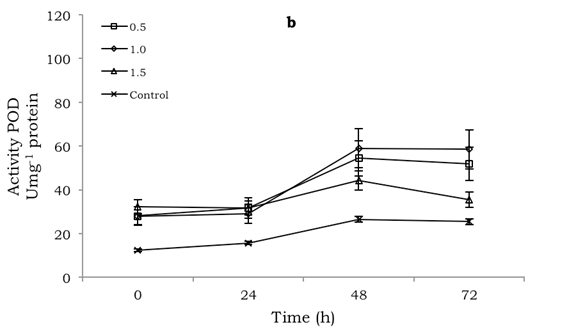
POD and PPO activity was significantly different (p≤0.001) among the treatments, both in the inoculated and uninoculated mango fruit. In both cases, the production of both enzymes was generally higher compared to the control for the 72 h of evaluation. However, no defined pattern was observed with respect to POD and PPO production with the chitosan concentrations applied. Thus, the maximum POD activity, with a value of 64.5 Umg-1 protein, was observed in uninoculated fruit at 24 h and treated with 1.0% chitosan (Figure 1a), whereas in the inoculated fruit the maximum value was 58.9 Umg-1 protein at 48 h in chitosan-treated fruit with the same 1.0% concentration (Figure 1b).
Figure 1: Effect of chitosan at different concentrations (0.5 1.0, 1.5 %) on POD activity in mango fruit cv. 'Tommy Atkins': a) uninoculated and b) inoculated with Colletotrichum. Bars indicate mean standard error of 5 observations per treatment.
For the PPO enzyme the highest production was recorded at 72 h, both in fruit treated with chitosan and those not, with and without inoculation. In fruit treated with 0.5% and 1.0% chitosan, with and without inoculation, respectively, the greatest activity with corresponding values of 116.5 and 78.0 Umg-1 protein occurred, but PPO activity in fruit not treated with chitosan and inoculated during the evaluation time was higher than with chitosan concentrations of 1.0 and 1.5% (Figures 2a, b).
Figure 2: Effect of chitosan at different concentrations (0.5 1.0, 1.5 %) on PPO activity in mango fruit cv. 'Tommy Atkins': a) uninoculated and b) inoculated with Colletotrichum. Bars indicate mean standard error of 5 observations per treatment.
Gene expression of POD and PPO
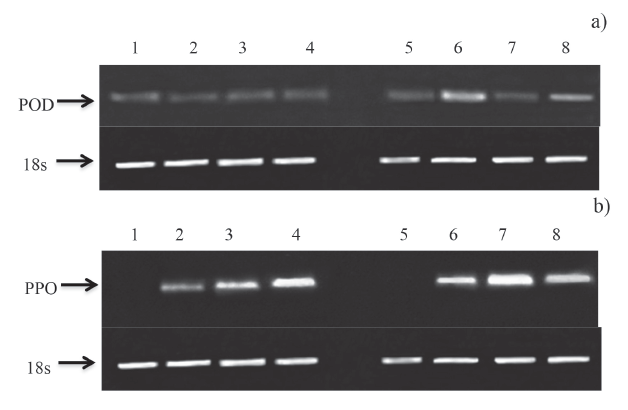
Figures 3a, b shows the electrophoretic runs of the cDNA samples encoding for POD and PPO. For POD, the uninoculated and inoculated fruit showed the same expression. Chitosan at 1.0% was able to induce gene expression of POD and PPO, occurring from 0 h and continuing until 24 h.
Figure 3: Effect of chitosan treatment on genetic expression of a) POD and b) PPO in mango fruit cv. 'Tommy Atkins'. Lanes 1-4: uninoculated fruit. Lanes 5-8: inoculated fruit. Lane 1: uninoculated, without chitosan, 0h; lane 2: uninoculated with chitosan, 0h: Lane 3: uninoculated, without chitosan 24h. Lane 4; uninoculated, with chitosan, 24h. Lane 5: inoculated, without chitosan, 0h. Lane 6: inoculated, with chitosan, 0h. Lane 7: inoculated, without chitosan, 24h. Lane 8: inoculated, with chitosan, 24 h.
In agreement with other studies, in vitro results confirm the chitosan concentration is a key factor influencing the different development stages of the genus Colletotrichum. Although, as above mentioned, the range of inhibitory chitosan concentrations varied with the evaluated strain. Likely a result of this stage, Photchanacha et al. (2006), reported in Colletotrichum isolated from pepper (Capsicum annum), the inhibitory concentration was of 0.8%, whereas Bautista-Baños et al. (2003), in the isolate from papaya fruit mentioned the dose of 3%, and Jinasena et al. (2011), observed for C. musae the concentration of 0.75%. In this research, the germination of the three isolates of Colletotrichum was the variable most affected by the chitosan effect, since it was completely inhibited from the 1.0% concentration for the soursop and mango isolates, while the 0.5% concentration completely halted development of the banana isolate.
Studies reporting the fungicidal activity of chitosan in mango and banana fruit agree with our results about that concentrations of 1% and 1.5% significantly control the incidence of Colletotrichum regardless cultivar. In relation to mango, previous research conducted with the cvs. 'Summer Bahist Chaunsa' (Abbasi et al., 2009), 'Sanara' (Abd-Alla & Haggag, 2010), showed the application of chitosan coatings, in addition to considerably reducing the infection and development of the lesion caused by anthracnose, delayed the onset of symptoms for several weeks during storage. With regard to banana, Kyu- Kyu Win et al. (2007), mention the cv. 'Kluai Hom Thong' treated with 1% chitosan there was a considerable reduction of up to 75% in the development of crown rot after 5 weeks of storage, while Jinasena et al. (2011), reported very similar results with the cv. 'Embul,' since in this case the anthracnose decreased by up to 90% during 14 days of storage.
In general, chitosan-treated fruit ripe normally. For soursop, mango and banana higher firmness was reported and less mass loss was recorded. The final values associated with quality, including firmness, TSS (ºBrix), acidity and pH were within those ranges reported by others when treated with chitosan (Kyu- Kyu Win et al., 2007) and fell within the appropriate commercial ranges.
In this study, the production of POD and PPO was generally higher compared to the untreated fruit during the sampling period. It should be noted that the activity of these enzymes coincided with a considerable decrease in the appearance of Colletotrichum on the fruits studied. Similar results have been reported in other postharvest diseases, for example, in orange fruit inoculated with P. digitatum and treated with chitosan. In Shogun mandarins (C. reticulata), it was reported the POD activity doubled when a chitosan concentration of 1 mg ml-1 was used as a coating, compared to other treatments, and that the incidence of green rot was about 70% lower (Waewruedee et al. 2015), the same reported for carrots (Daucus carota) inoculated with three isolates of S. sclerotiorum and treated with chitosans of different molecular weights (0.5 and 2.0 g L-1), the activities of both POD and PPO increased during the 5 days of storage and rot severity decreased, although in this case the development of the disease was also associated with pathogenicity aspects of the isolate evaluated (Ojaghian et al., 2013).
In this research, the gene expression of both enzymes induced by chitosan behaved consistently throughout the resistance process of Colletotrichum as an ignition system for a prolonged period. The chitosan-induced genes could be considered as early signaling, since it is active from the beginning of the infection process. In this regard, El-Hadrami et al. (2010), mention that as early as 10 min after exposure to chitin, the transcription levels of numerous genes were revealed to be altered in treated plants, including those genes associated with resistance induction including POD among others. Also, the inoculated fruits and the expression of both enzymes might be associated with the fruit, during the interaction of the mango-Colletotrichum pathosystem, sending signals as a defense mechanism to induce the gene expression of the enzymes. Although currently there is no information directly related to the induction of defense genes due to the effect of chitosan in tropical fruits, Ma et al. (2013), reported in peach (Prunus persica) the expression of POD transcribers for 48 h in fruit treated with chitosan and inoculated with Monilinia fructicola.
Conclusion
The fungicidal effect of chitosan in vitro was according to the obtained isolate, this provides more accurate and reliable estimates of growth decreased respect to an increased chitosan concentration, being more sensitive the banana isolate. Therefore, conidia were the most affected growth stage with chitosan application. For in situ, anthracnose development was notably inhibited in mango and banana fruit at 1% concentration. At the end of storage, the ripening process was not affected in the tested fruit by the application of this polymer. In fruit of mango cv. 'Tommy Atkins,' application of the 1% concentration and inoculation with Colletotrichum induced the activity and expression of the POD and PPO enzymes.
References
Referencias
Abbasi, N.A., Iqbal, Z., Maqbool, M., & Hafiz, I. A. (2009). Postharvest quality of mango (Mangifera indica L.) fruit as affected by chitosan coating. Pak J Bot, 41(1), 343 - 357.
Abd-Alla, M.A., & Haggag, W. M. (2010). New safe methods for controlling anthracnose disease of mango (Mangifera indica L.) fruits caused by Colletotrichum gloeosporioides (Penz.). J Am Sci, 8, 361 – 367.
Al- Eryani-Raqeeb, A., Mahmud, T. M. M., Syed-Oar, S. R., Mohamed- Zaki, A.R., & Al-Eryani, A. R. (2009). Effects of calcium and chitosan treatments on controlling anthracnose and postharvest quality of papaya (Carica papaya L.) Int J Agr Res, 4(2), 53 – 68. http://dx.doi.org/10.3923/ijar.2009.53.68
Bautista-Baños, S., Hernández-López, M., Bosquez-Molina, E., and Wilson, C. L. (2003). Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Prot, 22(9), 1087 – 1092. http://dx.doi.org/10.1016/S0261-2194 (03)00117-0
Chance, B., & Maehly, A. C. (1995). Assay of catalases and peroxidases. Methods Enzymol, 2, 764 – 817. http://dx.doi.org/10.1016/S0076-6879(55)02300-8
Contreras-Oliva, A., Pérez-Gago, M.B., Alejandra, S., Bermejo, A., & Rojas-Argudo, C. (2012). Calidad fisicoquímica, sensorial y nutricional de naranjas cv. Valencia recubiertas con quitosano. Agrociencia, 46(5), 441 – 453.
El-Hadrami, A., Lorne, A.R., El- Hadrami, I., & Daayf, F. (2010). Chitosan in plant protection. Mar Drugs, 8(4), 968 – 987. http://dx.doi.org/ 10.3390/md8040968
Gol, N. B., Pooja, R., Patel, T. V., & Ramano, R. (2013). Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Post Biol Tech, 85,185 - 195. http://dx.doi.org/ 10.1016/j.postharvbio.2013.06.008
Gong, D.Q., Zhu, S. J., Gu, H., Zhang, L. B., Hong, K. Q., & Xie, J. H. (2013). Disease resistance of ‘Zill’ and ‘Keitt’ mango fruit to anthracnose in relation to defence enzyme activities and the content of anti-fungal substances. J Hortic Sci Biotech, 88(3), 243 - 250. http://dx.doi.org/10.1080/14620316.2013.11512962
Jinasena, D., Pathirathna, P., Wickramarachchi, S., & Marasinghe, E. (2011). Effect of chitosan (Unirradiated and irradiated) treatment on anthracnose disease and its potential to increase the shelf life of ‘Embul’ banana. Int J Environ Sci Dev 2(4), 248 – 252.
Kyu-Kyu, W.N., Jitareetat, P., Kanlayanarat, S., & Sangchote, S. (2007). Effects of cinnamon extracts, chitosan coating, hot water treatments and their combination on crown rot disease and quality of banana fruit. Post Biol Tech, 45(3), 333 - 340. http://dx.doi.org/10.1016/j.postharvbio.2007.01.020
Liu, J., Tian, J., Meng, X., & Xu, Y. (2007). Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Post Biol Tech. 44(3), 300 – 306. http://dx.doi.org/10.1016/j.postharvbio.2006.12.019
Ma, Z., Yang, L., Yan, H., Kennedy, J. F., & Meng, X. (2013). Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr Polymer, 94(1), 271 – 277. http://dx.doi.org/10.1016/j.carbpol.2013.01.012
Muñoz, Z., Moret, A., & Garcés, S. (2009). Assessment of chitosan for inhibition of Colletotrichum sp. on tomatoes. Crop Prot, 28(1), 36 – 40. http://dx.doi.org/10.1016/j.cropro.2008.08.015
Ojaghian, M.R., Almoneafy, A.A., Cui, Zq., Xie, G-L., & Zhang, J. (2013). Application of acetyl acid and chemically different chitosans against storage rot carrot. Post Biol Tech, 84, 51 – 60.
Photchanacha, S., Singkaew, J., & Thamthong, J. (2006). Effects of chitosan seed treatment on Colletotrichum sp and seedling growth of chili cv. ‘Jinda’. Acta Hort, 712, 585 - 588. http://dx.doi.org/10.17660/ActaHortic.2006.712.70
Tezotto-Uliana, J., Fargoni, V. G. P., Geerdink, G. M., & Kluge, R. A. (2014). Chitosan applications pre- or postharvest prolongs raspberry shelf-life quality. Post Biol Tech, 91, 72 – 77. http://dx.doi.org/10.1016/j.postharvbio.2013.12.023
Waewruedee, W., Pisuchpen, S., & Leelasuphakul, W. (2015). Effect of Bacillus subtilis and chitosan applications on green mold (Penicillium digitatum Sacc.) decay in citrus fruit. Post Biol Tech, 99, 44 – 49. http://dx.doi.org/10.1016/j.postharvbio.2014.07.016
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Juan A. TORRES-RODRIGUEZ, Juan J. REYES-PÉREZ, Thelma CASTELLANOS, Carlos ANGULO, Evangelina E. QUIÑONES-AGUILAR, Luis G. HERNANDEZ-MONTIEL. (2021). A biopolymer with antimicrobial properties and plant resistance inducer against phytopathogens: Chitosan. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 49(1), p.12231. https://doi.org/10.15835/nbha49112231.
2. Gianfranco Romanazzi, Erica Feliziani, Dharini Sivakumar. (2018). Chitosan, a Biopolymer With Triple Action on Postharvest Decay of Fruit and Vegetables: Eliciting, Antimicrobial and Film-Forming Properties. Frontiers in Microbiology, 9 https://doi.org/10.3389/fmicb.2018.02745.
3. Boutheina Mejdoub‐Trabelsi, Soumaya Touihri, Nawaim Ammar, Anissa Riahi, Mejda Daami‐Remadi. (2020). Effect of chitosan for the control of potato diseases caused by Fusarium species. Journal of Phytopathology, 168(1), p.18. https://doi.org/10.1111/jph.12847.
4. Razieh Rajestary, Lucia Landi, Gianfranco Romanazzi. (2021). Chitosan and postharvest decay of fresh fruit: Meta‐analysis of disease control and antimicrobial and eliciting activities. Comprehensive Reviews in Food Science and Food Safety, 20(1), p.563. https://doi.org/10.1111/1541-4337.12672.
5. Yadi Suryadi, Karsinah, Dwi Ningsih Susilowati, I. Made Samudra, Alina Akhdiya, Eni Ida Riyanti, Jajang Kosasih, Siti Aminah. (2024). Evaluation of Chitosan formulation in suppression of anthracnose (Colletotrichum spp.) on some post-harvest mangoes cultivars. INTERNATIONAL CONFERENCE ON ORGANIC AND APPLIED CHEMISTRY (ICOAC) 2022. INTERNATIONAL CONFERENCE ON ORGANIC AND APPLIED CHEMISTRY (ICOAC) 2022. 3055, p.090001. https://doi.org/10.1063/5.0184034.
6. P. Gutiérrez-Martínez, A. Ramos-Guerrero, C. Rodríguez-Pereida, L. Coronado-Partida, J. Angulo-Parra, R. González-Estrada. (2018). Postharvest Disinfection of Fruits and Vegetables. , p.231. https://doi.org/10.1016/B978-0-12-812698-1.00012-1.
7. Luis M. Anaya-Esparza, Alejandro Pérez-Larios, José M. Ruvalcaba-Gómez, Jorge A. Sánchez-Burgos, Rafael Romero-Toledo, Efigenia Montalvo-González. (2020). Funcionalización de los recubrimientos a base de quitosano para la conservación postcosecha de frutas y hortalizas. TIP Revista Especializada en Ciencias Químico-Biológicas, 23 https://doi.org/10.22201/fesz.23958723e.2020.0.241.
8. Gülsüm Palacıoğlu. (2024). Effect of Different Chemical Inducers on Mycelial Growth of Neoscytali̇di̇um di̇mi̇di̇atum. Black Sea Journal of Agriculture, 7(5), p.557. https://doi.org/10.47115/bsagriculture.1528282.
9. Silvia Bautista‐Baños, Zormy Nacary Correa‐Pacheco, Rosa Isela Ventura‐Aguilar. (2019). Chitin and Chitosan. , p.371. https://doi.org/10.1002/9781119450467.ch15.
10. Mónica Cortés-Higareda, Margarita de Lorena Ramos-García, Zormy Nacary Correa-Pacheco, Juan Carlos Del Río-García, Silvia Bautista-Baños. (2019). Nanostructured chitosan/propolis formulations: characterization and effect on the growth of Aspergillus flavus and production of aflatoxins. Heliyon, 5(5), p.e01776. https://doi.org/10.1016/j.heliyon.2019.e01776.
11. Edson Rayón-Díaz, Luis G. Hernández-Montiel, Jorge A. Sánchez-Burgos, Victor M. Zamora-Gasga, Ramsés Ramón González-Estrada, Porfirio Gutiérrez-Martínez. (2024). Natural Compounds and Derivates: Alternative Treatments to Reduce Post-Harvest Losses in Fruits. AgriEngineering, 6(2), p.1022. https://doi.org/10.3390/agriengineering6020059.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Acta Agronómica

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Política sobre Derechos de autor:Los autores que publican en la revista se acogen al código de licencia creative commons 4.0 de atribución, no comercial, sin derivados.
Es decir, que aún siendo la Revista Acta Agronómica de acceso libre, los usuarios pueden descargar la información contenida en ella, pero deben darle atribución o reconocimiento de propiedad intelectual, deben usarlo tal como está, sin derivación alguna y no debe ser usado con fines comerciales.