Efficacy of microencapsulated nucleopolyhedroviruses from Colombia as biological insecticides against Spodoptera frugiperda (Lepidoptera: Noctuidae)
DOI:
https://doi.org/10.15446/acag.v66n2.54354Palabras clave:
Microencapsulation, baculovirus, Spodoptera frugiperda, biological control, maize (en)microencapsulation, baculovirus, Spodoptera frugiperda, biological control, maize (es)
Descargas
Wettable powder formulations by microencapsulation of viral occlusion bodies (OBs) of both Spodoptera frugiperda multiple nucleopolyhedrovirus from Colombia (SfCOL) and a genotypic variant (SfCOL-A) were evaluated for controlling the fall armyworm S. frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in maize crops. Microencapsulation preserved OBs activity after three months of storage at 35ºC, where insecticidal activity loss was not greater than 12%. Additionally, the formulation protected the OBs against inactivation caused by UV-B radiation, retaining its insecticidal activity after 6 hours of UV laboratory exposure, in contrast to unformulated viral suspensions, which presented an Original Activity Remaining (OAR) between 12.1 and 50%. Under greenhouse conditions, the insect mortality was greater than 80% with microencapsulated viruses. In field trials, treatments reduced the percentage of damaged plants to levels below the economic injury level (35%) when the formulated and unformulated virus were applied at 8x1011 OBs/ha (800g/ha) dose, while the damage in the control treatment was close to 60%. Microencapsulation of SfCOL and SfCOL-A OBs provides useful advantages related to half-life and photostability of both viruses, which showed the same efficacy under field conditions.
Wettable powder formulations by microencapsulation of viral occlusion bodies (OBs) of both Spodoptera frugiperda multiple nucleopolyhedrovirus from Colombia (SfCOL) and a genotypic variant (SfCOL-A) were evaluated for controlling the fall armyworm S. frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in maize crops. Microencapsulation preserved OBs activity after three months of storage at 35ºC, where insecticidal activity loss was not greater than 12%. Additionally, the formulation protected the OBs against inactivation caused by UV-B radiation, retaining its insecticidal activity after 6 hours of UV laboratory exposure, in contrast to unformulated viral suspensions, which presented an Original Activity Remaining (OAR) between 12.1 and 50%. Under greenhouse conditions, the insect mortality was greater than 80% with microencapsulated viruses. In field trials, treatments reduced the percentage of damaged plants to levels below the economic injury level (35%) when the formulated and unformulated virus were applied at 8x1011 OBs/ha (800g/ha) dose, while the damage in the control treatment was close to 60%. Microencapsulation of SfCOL and SfCOL-A OBs provides useful advantages related to half-life and photostability.
Recibido: 24 de noviembre de 2015; Aceptado: 16 de septiembre de 2016
Abstract:
Wettable powder formulations by microencapsulation of viral occlusion bodies (OBs) of both Spodoptera frugiperda multiple nucleopolyhedrovirus from Colombia (SfCOL) and a genotypic variant (SfCOL-A) were evaluated for controlling the fall armyworm S. frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in maize crops. Microencapsulation preserved OBs activity after three months of storage at 35ºC, where insecticidal activity loss was not greater than 12%. Additionally, the formulation protected the OBs against inactivation caused by UV-B radiation, retaining its insecticidal activity after 6 hours of UV laboratory exposure, in contrast to unformulated viral suspensions, which presented an Original Activity Remaining (OAR) between 12.1 and 50%. Under greenhouse conditions, the insect mortality was greater than 80% with microencapsulated viruses. In field trials, treatments reduced the percentage of damaged plants to levels below the economic injury level (35%) when the formulated and unformulated virus were applied at 8x1011 OBs/ha (800g/ha) dose, while the damage in the control treatment was close to 60%. Microencapsulation of SfCOL and SfCOL-A OBs provides useful advantages related to half-life and photostability of both viruses, which showed the same efficacy under field conditions.
Keywords:
Microencapsulation, baculovirus, Spodoptera frugiperda, biological control, maize.Resumen:
Para el control del gusano cogollero S. frugiperda (JE Smith) (Lepidoptera: Noctuidae) en cultivos de maíz, se evaluaron los cuerpos de inclusión virales (CIs) de un nucleopoliedrovirus de Spodoptera frugiperda colombiano (SfCOL) y una de sus variantes genotípicas (SfCOL-A), formulados como polvos mojables mediante microencapsulación. La microencapsulación conservó la actividad de los CIs después de tres meses de almacenamiento a 35ºC, donde la pérdida de actividad insecticida no fue mayor al 12%. Además, la formulación protegió a los CIs de la inactivación causada por la radiación UV-B, conservando su actividad insecticida después de 6 horas de exposición a luz UV en condiciones de laboratorio, en contraste con las suspensiones virales no formuladas, que presentaron una actividad original restante (AOR) entre 12,1 y 50%. En condiciones de invernadero, la mortalidad de los insectos fue mayor al 80% con los virus microencapsulados. En las pruebas de campo, los tratamientos redujeron el porcentaje de plantas con daño a niveles por debajo del nivel de daño económico (35%) cuando se aplicó el virus formulado y no formulado a una dosis de 8x1011 CIs/ha (800 g/ha), mientras que el daño en el tratamiento control estuvo alrededor del 60%. La microencapsulación de las partículas virales de SfCOL y SfCOL-A proporcionó ventajas útiles relacionadas con la vida media y fotoestabilidad de los dos virus que mostraron la misma eficacia bajo condiciones de campo.
Palabras clave:
Microencapsulación, baculovirus, Spodoptera frugiperda, control biológico, maíz.Introduction
The fall armyworm (FAW) Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) is a severe pest of maize and other crops. The multiple nucleopolyhedrovirus of S. frugiperda (SfMNPV: Baculoviridae) has been intensely studied as a potential biocontrol agent to reduce larvae populations of this pest (Haase et al., 2015). Although SfMNPVs are efficient to control the FAW, there are some limitations for their use as biopesticides as high costs due to in vivo viral production (Ruiz et al., 2015), low speed to kill and inactivation related with environmental conditions as sun light and temperature (Villamizar et al., 2010). Therefore, adequate formulation may substantially improve efficiency and tolerance against adverse environmental factors (Behle et al., 2003). Additionally, formulation has shown to be important in stabilizing viruses during storage and distribution, and it also facilitates handling and application on crops (Behle et al., 2003; Tamez-Guerra et al., 2002).
Several formulations of SfMNPV using different strategies have been studied and microencapsulation represents a promising alternative for stabilizing viral particles against adverse environmental effects. Encapsulated viral particles have been a preferred delivery system to minimize activity loss due to solar radiation and also maintain the viral insecticidal activity. For encapsulation substances like gelatin, pectin, chitin, calcium alginate and maize starch have been used that do not affect the virus viability (Sundari et al., 2016). Several microencapsulation processes have been used to formulated baculoviruses, as emulsion technique (Gifani et al., 2015), spray drying (Camacho et al., 2015) and solvent evaporation (Villamizar et al., 2010), obtaining efficient UV protection with all evaluated formulations. An additional advantage when baculovirus are microencapsulated is an extended shelf life, as was demonstrated by Santos et al., (2014) with a formulation based on a Colombian isolate of SfMNPV, which can be stored at temperatures below 28°C, ensuring product quality for at least 17 months; considered enough time for its distribution and use.
Recently, a SfMNPV field isolate (SfCOL) with potential to be developed as biopesticide in Colombia was identified (Gómez et al., 2010), formulated as a wettable powder using microencapsulation with a synthetic polymer and evaluated under field conditions, reaching 70% of efficacy in maize crops (Gómez et al., 2013). However, intra-population diversity analysis of SfCOL revealed 10 different genotypic variants (SfCOL-A to SfCOL-J), from which SfCOL-A was the most prevalent (72%) and was 4.4 times more potent than SfCOL but showing the same virulence over local insect pest (Barrera et al., 2013). This feature makes that variant a strong candidate to optimize the SfMNPV-based biopesticide for S. frugiperda control. In order to select the most promising active ingredient to formulate the developed biopesticide, the aim of the present study was to evaluate the insecticidal activity of both viruses (SfCOL and SfCOL-A) formulated by microencapsulation with a methacrylic acid polymer, under greenhouse and field conditions.
Materials and methods
Insect colony and virus strain
S. frugiperda larvae were obtained from a laboratory colony established in the Biological Control Laboratory of the Colombian Corporation of Agricultural Research (CORPOICA). The colony was maintained at 25ºC, 75% RH (relative humidity) and 16 h light - 8 h dark photoperiod on a wheat germ based semi-synthetic diet. The wild type SfMNPV (SfCOL) used in this study was obtained from field diseased larvae from Colombia (Gómez et al., 2010) and the genotypic variant (SfCOL-A) was isolated by plaque purification from SfCOL isolate in Sf9 culture cells (Barrera et al., 2013). Viral occlusion bodies (OBs) were propagated in third instar S. frugiperda larvae using the droplet feeding method (Hughes & Wood, 1981). Dead larvae were carefully collected daily and stored at -20ºC. OBs were extracted from dead larvae by homogenizing cadavers in 0.1% (w/v) sodium dodecyl sulfate (SDS) solution.
Formulation and quality control analysis
Purified OBs were freeze-dried and microensapsulated using Eudragit S100(r) according to the methodology described by Villamizar et al. (2010).
A quality control analysis was performed to the final product (wettable powder), measuring viral concentration (OBs/g), moisture content (%), bacteria content and insecticidal activity in laboratory following methodologies described by Gómez et al. (2013).
Effect of formulation over SfCOL and SfCOL-A pathogenicity
The mean lethal concentration (LC50) was determined for SfCOL and SfCOL-A both unformulated (SfCOL and SfCOL-A) and formulated (SfCOL-F and SfCOL-A-F) in second instars S. frugiperda larvae. Twenty-four larvae were starved for 16 h and then allowed to feed from aqueous suspension containing 10% (w/v) sucrose, 0.001% (w/v) Fluorella blue and OBs at one of the following concentrations for all the treatments: 1.92x103, 9.60x103, 4.80x104, 2.40x105 and 1.2x106 OBs/mL. This range of concentrations was previously determined to kill between 5% and 95% of experimental insects. Larvae that ingested the suspension were transferred to individual cups with a semi-synthetic diet piece. Experimental design was completely random, with three replicates per treatment and bioassays was performed three times. Experimental unit consisted in 24 larvae (72 larvae per treatment). Larvae were reared at 25°C and mortality was recorded until the insects had either died or pupated. Virus induced mortality was subjected to logit analysis using the Generalized Linear Interactive Modeling (GLIM) program.
Accelerated storage stability test
SfCOL and SfCOL-A both unformulated (in aqueous suspensions) and formulated (microencapsulated/wettable powders) were evaluated before (time 0) and after being exposed to "high stress", under a temperature of 35ºC. The insecticidal activity and bacteria content was evaluated monthly during three months. For insecticidal activity evaluation, bioassays were performed in second instar S. frugiperda larvae inoculated by the droplet feeding technique and using all treatments adjusted to 1x107 OBs.Ml-1. Experimental design was completely random, with three replicates per treatment and bioassays was performed three times. Experimental unit consisted in 24 larvae (72 larvae per treatment). Treatments mortality was estimated as described before and data were subjected to repeat measure analysis of variance in SPSS program version 10.0 and pairwise treatments were compared by within-subject pairwise comparisons among estimated marginal means with Bonferroni correction.
Bacteria content analysis was performed as described for quality control section. Data were normalized by log transformation and subjected to repeat measure analysis of variance (ANOVA) in SPSS version 10.0.
Photostability assays
Samples of 200 µL of SfCOL and SfCOL-A both unformulated and formulated, at 2x107 OBs/mL were placed in a 96 well microplate. The first column of the microplate was covered with aluminum film before exposure to protect it against UV light, which was used as control. Then, the microplate was placed inside a chamber and exposed to UV-B (302 nm), with a monochromatic UVP(r) lamp (model 3UV-38) at 3.5 W.m-2 for 2, 4 and 6 hours simulating approximately 13, 26 and 39 days of sun irradiation under environmental conditions of maize crops in Colombia. These values were calculated considering mean daily sun radiation in Colombia (approximately 5.5 kWh.(m2.day-1) equivalent to 19.8 KJ/ (m2.day-1)) (http://www.upme.gov.co/Docs/Atlas_Radiacion_Solar), from which only 10% correspond to UV-B radiation (1.9 KJ/ (m2 day)). Additionally, the theoretical calculation was based on 12 hours per day of sun radiation (0.16 KJ.m2.hour-1), being 2 hours of laboratory lamp irradiation equivalent to 13 days of sun irradiation in field. Samples were collected and mixed with equal volume of a suspension containing 10% (w/v) sucrose, 0.001% (w/v) Fluorella blue to obtain a final concentration of 1x107 OBs.mL-1 (Villamizar et al., 2009). Bioassay was repeated three times by using the droplet feeding technique as described above. Treatments mortality data were corrected with the control treatment mortality using the Schneider-Orelli equation and the original activity remaining (OAR) was calculated. The data were subjected to repeat measures analysis of variance in SPSS program version 10.0. Test of Mauchly's sphericity was applied to contrast the variances. The significance of treatment effects at each sample time was determined by within-subject pairwise comparisons among estimated marginal means with Bonferroni correction.
Greenhouse assay
Bioassay was conducted on maize plants in greenhouse conditions with unformulated (SfCOL and SfCOL-A) and formulated viruses (SfCOL-F and SfCOL-A-F), suspended in water and adjusted to a concentration of 1x107 OBs.mL-1 (Gómez et al., 2013). Experimental unit consisted in a row with 12 plants of 35 cm height with six true leaves. The distance between rows was 100 cm. A fully randomized blocks design was performed with five replicates. A chemical treatment with lufenuron (Match 5 EC(r), Syngenta) (Chemical Group: Benzoylurea) at the label recommended rate (1 cc.L-1 and 300 L.ha-1) and a control treatment with pure water were included. Treatments were applied by using 2 mL.plant-1 with a hand sprayer. Then, four-second instar larvae were located per plant. After 48 and 96 hours, larvae were collected and individually reared in the laboratory on artificial diet and checked for viral mortality. Treatments mortalities were estimated as described before and results were subjected to repeat measures analysis of variance in SPSS program version 10.0.
Field assay
Field trials were carried out from April 2013 to June 2013 in a maize crop in the locality of Espinal, in the department of Tolima (Colombia) with a mean annual temperature of 30±2 °C and a mean annual relative humidity of 80±3 %. The crop was planted with the variety 30F32 Pioneer at density of approximately 40000 plants.ha-1. Experimental design was in randomized complete blocks with three replicates per treatment. The experimental unit consisted in a plot of 50 m2 including 6 rows of 10 m length and with a gap of 2 m without plants between blocks. Treatments consisted in both unformulated and formulated SfCOL and SfCOL-A and a control treatment with water application. Unformulated and formulated viruses at 8x1011 OBs.ha-1 were applied on maize plants 3 and 17 days after plants emergence as described previously for SfMNPV microencapsulated applied in field conditions (Gómez et al., 2013). Treatments were applied with an air-assisted hydraulic sprayer with a cone nozzle at a pressure of 4 Kg.cm-2. Percentage of plants with feeding damage was evaluated at 3, 10, 17 and 24 Days After Emergence (DAE). An additional evaluation at 31 DAE was made in the first plot. Thirty plants were randomly selected in the three central rows of each plot and were examined for the number of plants with presence of feeding damage in the youngest leaves. Results were expressed as feeding damage percentage. The data were subjected to repeat measures analysis of variance in SPSS program version 10.0. Test of Mauchly's sphericity was applied to contrast the variances. The significance of treatment effects at each sample time was determined by within-subject pairwise comparisons among estimated marginal means with Bonferroni correction.
Results and discussion
Quality control analysis of formulated viruses
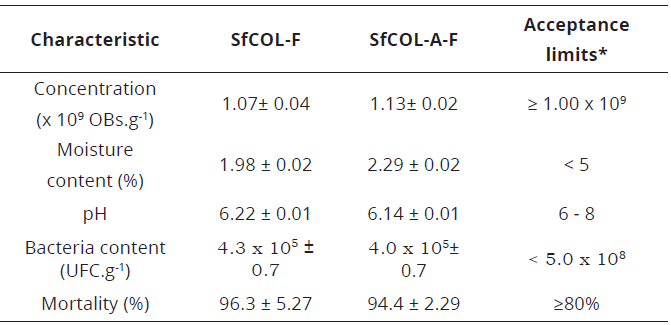
All analyzed parameters of formulated samples were within the acceptance limits described by Gómez et al., (2013) (Table 1). The results were similar in both formulated SfCOL-F and SfCOL-A-F wettable powders. Active ingredient concentrations in wettable powders were close to 1.0x109 OBs/g for both evaluated viruses. Moisture content and pH values ranged between 1.98% and 2.29% and between 6.22 and 6.14, respectively. Bacterial contaminants were close to 4.0x107 CFU/g and efficacies reached 96.3 and 94.4% for SfCOL-F and SfCOL-A-F respectively.
*Acceptance limits according to Gómez et al. (2013).Table 1: Quality control results of newly formulated SfCOL-F and SfCOL-A-F. Mean values and the error standard for each parameter are expressed.
Formulation does not affect SfCOL and SfCOL-A pathogenicity
Pathogenicity of unformulated viruses did not differ significantly from their respective formulations in both SfCOL and SfCOL-A isolates (Table 2). The LC50 values of SfCOL-A both unformulated and formulated OBs were significantly lower than those obtained for SfCOL unformulated OBs by comparing fiducial limits of LC50. Pathogenicity of SfCOL was improved with the formulation reaching values that did not differ significantly from those obtained with SfCOL-A.
Table 2: Estimated LC50 and relative potencies values of SfCOL and SfCOL-A OBs both unformulated and formulated (F) in S. frugiperda second instars
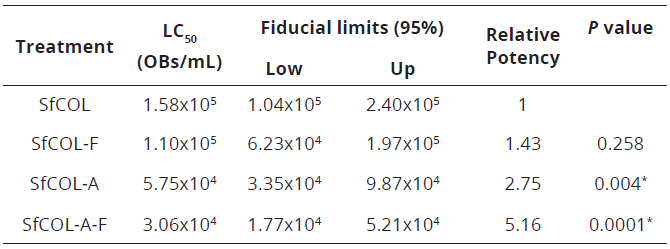
Logit regressions for mixtures of occlusion bodies (OBs) were fitted using GLIM program with a common slope (±SE) of 0.489 ± 0.083. A test for non-parallelism was not significant (χ2 = 7.46 df = 3 P = 0.06). Relative potencies were calculated as the ratio of effective concentrations relative to that of the wild type isolate and are shown in table 2. SfCOL-F was 1.43 times more potent than unformulated virus. The relative potency of SfCOL-A (ratio between SfCOL-A LC50 and SfCOL-A-F LC50) was increased 1.88 times when OBs were formulated, although significant differences were not observed.
Formulated viruses retain their infectivity after storage
Accelerated storage studies expose the product to elevated temperature to enhance the deterioration and to reduce the time required for testing (Corradini & Peleg, 2007). In unformulated viruses, a correlation was observed between the decrease in the activity and the storage time (treatment*time interaction F3,6=15.011, p=0.003) after three months of accelerated storage conditions (Figure 1a). After the third month, SfCOL and SfCOL-A efficacies diminished from 92.5% and 90.7% to 59.2% and 51.7% respectively. In contrast, the formulated products maintained their efficacies above 80%.
Bacterial contamination before (range 4.2x105 and 6.9x106 CFU.mL-1) and after three months storage (range 1.3x104 and 9.2x107 CFU.mL-1) in both unformulated and formulated viruses were within acceptance limit (< 5x108 CFU.mL-1) (Gómez et al., 2013) (Figure 1b). Initial bacterial contamination values of unformulated isolates were significantly higher than those obtained for formulated viruses (P<0.001), and increased significantly after three months of storage (13.2 and 12.0 times in SfCOL and SfCOL-A, respectively), reaching values ranging from 6.23x107 to 9.2x107 UFC.mL-1 (treatment*time F3, 6=23.043, p=0.01). In contrast, the CFU counts of formulated viruses significantly diminished after three months (P<0.001) to values lower than 5.2x104 CFU.mL-1.
Table 3: Mortality and OAR (Original Activity Remaining) of unformulated (SfCOL and SfCOL-A) and formulated (SfCOL-F and SfCOL-A-F) viruses after 6 hours of irradiation with UV-B light in laboratory conditions.

Figure 1. : Storage assays of mortality % (a) and bacterial contamination (b) at 0 (T0), 1 (T1), 2 (T2), and 3 (T3) months at 35ºC of both unformulated (SfCOL and SfCOL-A) and formulated (SfCOL-F and SfCOL-A-F) viruses. Bars labelled with identical letters were not significantly different for comparisons between treatments within each time storage (repeated measures ANOVA with intra-subject pairwise comparison of estimated marginal means, P > 0.05 Bonferroni correction).
Formulated viruses retained their infectivity after laboratory UV-B irradiation
Initial insecticidal activities for all treatments were not significantly different (F3,8=2.42, p=0.14), providing mortalities > 84% (Table 3). OAR after six hours of irradiation decreased significantly when the isolates were not formulated (treatment*time interaction F9,24=2.791, p=0.022). No significant differences were observed among efficacies of formulated isolates at different irradiation times (p=0.054). The OAR percentage of SfCOL-A (12.1 %) was lower than obtained for SfCOL (50%) after six hours (P<0.001).
Insecticidal activity under greenhouse conditions
Mortalities obtained with unformulated and formulated treatments at two different times were evaluated on maize plants under greenhouse conditions (Figure 2). Larval mortality obtained with SfCOL was significantly lower than obtained with others treatments at 48 and 96 hours (F4,20=15.14, p=0.001). No differences were observed among formulated treatments (SfCOL-A-F and SfCOL-F) and unformulated SfCOL-A at 48 and 96 hours (treatment*time interaction F1=0.40, p=0.53), being larval mortality with SfCOL-A (formulated and unformulated) higher than 90% as found with evaluated chemical insecticide (>98 %).
Figure 2: Efficacy of SfCOL and SfCOL-A both unformulated and formulated and chemical insecticide (lufenuron) (measured by mortality percentage) over S. frugiperda larvae collected at different times after application (48 and 96 hours) and reared in the laboratory (ANOVA with intra-subject pairwise comparison of estimated marginal means, P>0.05 Bonferroni correction).
Feeding damage reduction under field conditions
The percentage of plants with feeding damage in the plot planted with 30F32 seed ranged between 2 and 10% for all treatments including the control until 10 days after emergence (Figure 3). The percentage of damaged plants exceeded economic injury level at 17 dag in plants that had no virus application, reaching to 45.6%, while treatments with both unformulated and formulated viruses showed percentages of plants with feeding damage below 34%, although no significant differences were observed (F4,10=0.93, p=0.482). In contrast, at 24 dag significant differences were observed between control and viral treatments (F4,10=19.2, P<0.001). Percentage of plants with feeding damage in control treatment reached to 58.9%, while this value was lower than 35% in all viral treatments, showing no significant differences among them.
Figure 3: Percentage of maize plants showing S. frugiperda fresh feeding damage after applications of SfCOL and SfCOL-A both unformulated and formulated in 30F32 Pioneer maize plot. The line indicate the economic injury level for S. frugiperda on maize in Colombia (35%). Vertical arrows indicate the moment of application of treatments
Pest control programs for S. frugiperda based on baculovirus have not been devised in Colombia. Formulation can be used to protect the viruses from some adverse environmental factors, enhance shelf-life and maximize application efficiency (Lasa et al., 2008; Tamez-Guerra et al., 2002, Santos et al., 2014). In the present study, SfCOL and SfCOL-A were formulated by microencapsulation and their insecticidal activities were compared with unformulated viruses in laboratory, greenhouse and field conditions.
A quality control analysis was performed after microencapsulation to verify virus activity after formulation. Extreme pH values can also be harmful to the OBs, thus pH between 4 and 6, which do not affect OBs, is recommend preventing the growth of most contaminating organisms (Tamez-Guerra et al., 2002). Moisture and pH can have clear effects on the content of bacteria in a formulation of OBs, however microencapsulated SfCOL and SfCOL-A moisture (between 1.07 and 1.13) and pH (between 6.1 and 6.2) values were in the proper range to prevent bacterial growth. Contaminating bacteria is principally introduced by in vivo virus production, where the insect microflora could arrive to the final product. This is unacceptable from the viewpoint of product safety and because it can have deleterious effects for the stability of the virus (Lasa et al., 2008). However, formulated viruses presented lower bacteria concentrations compared to unformulated viral suspensions, possibly due to freeze-drying technique used for removing moisture from microcapsules and to the organic solvent used to dissolve the Eudragit S100(r), which could help to reduce the content of contaminating bacteria. Additionally, formulation had no effect over insecticidal activity in terms of mean lethal concentration.
Half-life has to be considered for developing baculovirus-based biopesticides (Tamez-Guerra et al., 2002). In this sense, studies based on accelerated storage conditions in which product is stored at high temperature provided a useful tool to predict the shelf-life of formulations over time (Corradini & Peleg, 2007). Microencapsulation of SfCOL and SfCOL-A OBs protected these viruses against degradation during the storage. Efficacies of formulated SfCOL and SfCOL-A were maintained after 3 months storage at 35±2 °C, whereas unformulated OBs efficacies decreased to 60% and 52% respectively, in the same time. Similarly, the bacteria content increased significantly in unformulated viruses after three months of storage, while in formulated products the bacteria content diminished. Formulation of SfCOL and SfCOL-A could help to maintained viral efficacy by the reduction of the microbial contaminants that may be implicated in accelerating chemical processes that inactivate OBs, as previously observed during SeMNPV storage (Lasa et al., 2008). Similarly, spray-dried lignin-based formulations improve the storage stability (until 30 months at 4oC and 3 months at 30oC) of Anagrapha falcifera Nucleopolyhedrovirus (Behle et al., 2003). In another work, an isolated of SfMNPV microencapsulated with methacrylic acid polymer showed high stability retaining its efficacy above 80% during 17 months at 28oC (Santos et al., 2014).
Specifically, the SfMNPV is sensitive to UV radiation, where inactivation takes place after 15 minutes of exposure to direct UV light (Mondragón et al., 2007). Addition of optical brighteners such as Tinopal or Calcofluor M2R to SfMNPV formulations provided photo-protection (Mondragón et al., 2007). However, the incorporation of optical brighteners (chemical) to biopesticides products is not well accepted due to negative effects of some of them over growth on maize plants. This effect was demonstrated by Martínez et al., (2009), who evaluated the effects of two types of optical brighteners on maize growth after once a week application during four weeks. Both compounds significantly affected the growth of maize plants, increased the percentage of crop reflectance. The microencapsulation technique used in this work protected the OBs from degradation caused by UV irradiation, as was previously demonstrated used the same coating polymer by Villamizar et al., (2010) and Camacho et al., (2015). Original activity remaining of formulated viruses was superior to 95% after 6 hours of exposure, while unformulated SfCOL and SfCOL-A showed 50% and 87% inactivation, respectively. Higher inactivation obtained with the genotype SfCOL-A suggests that the mixture of genotypes present in the wild type isolate provided more resistance to environmental conditions such as sun radiation, maximizing the likelihood of transmission.
Greenhouse assays were performed to refine the conditions to be used in field trials. The efficacies of both SfCOL and SfCOL-A formulations were similar to chemical treatment (lufenuron) commonly used to control S. frugiperda. Additionally, the SfCOL-A unformulated suspension was able to reach the efficacy of the formulated product due to its high pathogenicity. However the unformulated SfCOL exhibited an efficacy lower than 60%, making formulation necessary to optimize the insecticidal activity of this isolate. No differences were found in mortality percentage between larvae collected at 48 and 96 hours in all the treatments, indicating that acquisition of deadly dose occurred before 48 hours, which agrees with previous studies that conclude that the first 48 hours of feeding are crucial in viral acquisition. Moreover, it has been indicated that insects acquire infection during the first 6 h following the application (Lasa et al., 2007).
Therefore, a lower dose was chosen (8x1011 OBs.ha-1) and showed to be enough to maintain the percentage of plants with feeding damage (26%) below economic injury level (Ayala et al., 2013), while in control treatment the percentage of plants with feeding damage reached to 59%. Surprisingly, under field conditions no differences were found in the effectiveness among unformulated and formulated viruses. Many biotic and abiotic factors could influence host-pathogen interactions, for instance density of the pest, crop phenology and insect eating habits among others, thus SfMNPVs evaluation in field conditions can be inconsistent with laboratory results (Villamizar et al., 2009).
Conclusion
Microencapsulated SfCOL and SfCOL-A presented quality parameters within the acceptance limits, and formulation favored viral stability under storage conditions and UV-B irradiation. Both viruses protected efficiently maize plants against damage caused by S. frugiperda and were considered equally promising to be used as active ingredient for a biopesticide to control de fall armyworm.
Acknowledgments
This work was supported in part by Corporación Colombiana de Investigación Agropecuaria CORPOICA and Departamento Administrativo de Ciencia, Tecnología e Innovación Colciencias, Project 710652128380. We thanks to Oscar Quintero who provided invaluable help in field experiments.
References
Referencias
Ayala, O., Navarro, F. & Virla, E. (2013). Evaluation of the attack rates and level of damages by the fall armyworm, Spodoptera frugiperda (Lepidoptera:Noctuidae ), affecting corn-crops in the northeast of Argentina. Rev la Fac Ciencias Agrar Uncuyo, 45(2), 1–12.
Barrera, G., Williams, T., Villamizar, L., Caballero, P. & Simón, O. (2013). Deletion genotypes reduce occlusion body potency but increase occlusion body production in a Colombian Spodoptera frugiperda nucleopolyhedrovirus population. PLoS One, 8(10), 1-12. http://doi.org/ 10.1371/journal.pone.0077271
Behle, R. W., Tamez-guerra, P. & Mcguire, M. R. (2003). Field activity and storage stability of Anagrapha falcifera nucleopolyhedrovirus (AfMNPV) in spray-dried lignin-based formulations. J Econ Entomol, 96(4), 1066–1075.
Camacho, J., Gómez, M.L., & Villamizar, L. (2015). Microencapsulation of a Colombian Spodoptera frugiperda nucleopolyhedrovirus with Eudragit® S100 by spray drying. Braz Arch Biol Technol, 58(3), 468 - 476. http://dx.doi.org/10.1590/S1516-8913201500453
Corradini, M. G., & Peleg, M. (2007). Shelf-life estimation from accelerated storage data. Trends Food Sci Technol, 18(1), 37–47. http://dx.doi.org/10.1016/j.tifs.2006.07.011
Gifani, A., Marzban, R., Safekordi, A., Ardjmand, M. & Dezianian, A. (2015). Ultraviolet protection of nucleopolyhedrovirus through microencapsulation with different polymers. Biocontrol Sci Technol, 25(7), 814–827. http://dx.doi.org/10.1080/09583157.2015.1018814
Gómez, J., Guevara, J., Barrera, G., Cotes, A. M. & Villamizar, L. (2010). Aislamiento, identificación y caracterización de nucleopoliedrovirus nativos de Spodoptera frugiperda en Colombia. Rev la Fac Nac Agron, 63(2), 1–10.
Gómez, J., Guevara, J., Cuartas, P., Espinel, C. & Villamizar, L. (2013). Microencapsulated Spodoptera frugiperda nucleopolyhedrovirus: insecticidal activity and effect on arthropod populations in maize. Biocontrol Sci Technol, 23(7), 829–846. http://dx.doi.org/10.1080/09583157.2013.802288
Haase, S., Sciocco-Cap, A. & Romanowski, V. (2015). Baculovirus insecticides in Latin America: Historical overview, current status and future perspectives. Viruses, 7(5), 2230–2267. http://dx.doi.org/10.3390/v7052230
Hughes, P. & Wood, H. (1981). A synchronous per oral technique for the bioassay of insect virus. J Invertebr Pathol, 37(2), 154–159. http://dx.doi.org/10.1016/0022-2011(81)90069-0
Lasa, R., Pagola, I., Ibañez, I., Belda, J. E., Williams, T. & Caballero, P. (2007). Efficacy of Spodoptera exigua multiple nucleopolyhedrovirus as a biological insecticide for beet armyworm control in greenhouses of southern Spain. Biocontrol Sci Technol, 17(3), 221–232. http://dx.doi.org/10.1080/09583150701211335
Lasa, R., Williams, T. & Caballero, P. (2008). Insecticidal properties and microbial contaminants in a Spodoptera exigua multiple nucleopolyhedrovirus (Baculoviridae) formulation stored at different temperatures. J Econ Entomol, 101, 42–9.
Martínez, A. M., Velasco, S., Méndez, A., Figueroa, J. I., España, M. L., Cárdenas-Navarro, R. & Pineda, S. (2009). Effects of optical brighteners used in biopesticide formulations on crops: reflectance, stomatal conductance, photosynthesis, and growth. Commun Agric Appl Biol Sci, 74(1), 117–123.
Mondragón, G., Pineda, S., Martínez, A. & Martínez, A. M. (2007). Optical brightener Tinopal C1101 as an ultraviolet protectant for a nucleopolyhedrovirus. Commun Agric Appl Biol Sci ,72(3), 543–547.
Ruiz, J., Gómez, J., Chaparro, M., Sotelo, P., Villamizar, L. (2015). Adjusting the conditions of a system for the in vivo production of a nucleopolyhedrovirus of Spodoptera frugiperda (Lepidoptera: Noctuidae). Biotecnología Aplicada, 32(4),4311-4316.
Santos, A. M., Uribe, L. A., Ruiz, J. C., Tabima, L., Gómez, J. A. & Villamizar, L. F. (2014). Nucleopoliedrovirus de Spodoptera frugiperda SfNPV003: compatibilidad con agroquímicos y estabilidad en condiciones de almacenamiento. Corpoica Cienc y Tecnol Agropecu, 15(2), 219–228. http://dx.doi.org/ 10.21930/rcta.vol15_num2_art:361
Sundari, S., Singh, A., & Yadava, P. (2016). Review of current research advances in microbial and phyto-biopesticides. Int J Biotechnol Biomed Sci, 2(1),73-77.
Tamez-Guerra, P., McGuire, M. R., Behle, R. W., Shasha, B. S. & Pingel, R. L. (2002). Storage stability of Anagrapha falcifera nucleopolyhedrovirus in spray-dried formulations. J Invertebr Pathol, 79(1), 7–16. http://dx.doi.org/10.1016/S0022-2011(02)00005-8
Villamizar, L., Espinel, C. & Cotes, A. M. (2009). Efecto de la radiación ultravioleta sobre la actividad insecticida de un nucleopoliedrovirus de Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev Colomb Entomol, 35(2), 116–121.
Villamizar, L., Barrera, G., Cotes, A. M. & Martínez, F. (2010). Eudragit S100 microparticles containing Spodoptera frugiperda nucleopolyehedrovirus: physicochemical characterization, photostability and in vitro virus release. J Microencapsul, 27, 314–24. http://dx.doi.org/10.3109/02652040903191826
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
Licencia
Derechos de autor 2017 Acta Agronómica

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Política sobre Derechos de autor:Los autores que publican en la revista se acogen al código de licencia creative commons 4.0 de atribución, no comercial, sin derivados.
Es decir, que aún siendo la Revista Acta Agronómica de acceso libre, los usuarios pueden descargar la información contenida en ella, pero deben darle atribución o reconocimiento de propiedad intelectual, deben usarlo tal como está, sin derivación alguna y no debe ser usado con fines comerciales.






























