Fluorescein effect on the vase life of calla (Zantedeschia aethiopica (L.) K. Spreng.) inflorescences
DOI:
https://doi.org/10.15446/acag.v66n3.54539Palabras clave:
Floral scape, pulse solution, pH, spathe (en)Floral scape, pulse solution, pH, spathe (es)
Descargas
Calla (Zantedeschia aethiopica (L.) K. Spreng), is a cut flower which had obtained importance in recent years, but postharvest handling is limited. Thus, in order to know the fluorescein concentration effect and pH of pulse solution on inflorescences calla postharvest, were evaluated in Teotitlan de Flores Magon, Oaxaca-Mexico, four fluorescein concentrations (0, 20, 40 and 60 %, respectively) and two pH levels (acid and alkaline) in a completely randomized design with factorial arrangement, having a total of 24 experimental units, which was established by vase life and calla inflorescence with white spathe color, immersed in a fluorescein solution adjusted as appropriate at acidic or alkaline pH, where response variables were as follows: vase life, water consumption, stem diameter, absorption fluorescein stem base and fluorescein vertical absorption by floral scape. Results indicates the increased vase life is achieved at alkaline pH by fluorescein addition of 20, 40 and 60 %, respectively. Likewise, the higher water consumption is achieved at this pH. From this research, we can conclude fluorescein can affect the calla physiology for increasing vase life.
Recibido: 3 de diciembre de 2015; Aceptado: 10 de junio de 2016
Abstract
Calla (Zantedeschia aethiopica (L.) K. Spreng), is a cut flower which had obtained importance in recent years, but postharvest handling is limited. Thus, in order to know the fluorescein concentration effect and pH of pulse solution on inflorescences calla postharvest, were evaluated in Teotitlan de Flores Magon, Oaxaca-Mexico, four fluorescein concentrations (0, 20, 40 and 60 %, respectively) and two pH levels (acid and alkaline) in a completely randomized design with factorial arrangement, having a total of 24 experimental units, which was established by vase life and calla inflorescence with white spathe color, immersed in a fluorescein solution adjusted as appropriate at acidic or alkaline pH, where response variables were as follows: vase life, water consumption, stem diameter, absorption fluorescein stem base and fluorescein vertical absorption by floral scape. Results indicates the increased vase life is achieved at alkaline pH by fluorescein addition of 20, 40 and 60 %, respectively. Likewise, the higher water consumption is achieved at this pH. From this research, we can conclude fluorescein can affect the calla physiology for increasing vase life.
Key words:
Floral scape, pulse solution, pH, spathe.Resumen
El alcatraz (Zantedeschia aethiopica (L.) K. Spreng), es una flor de corte que ha cobrado importancia en los últimos años, pero su manejo en postcosecha es limitado. Así, con el objeto de conocer el efecto de la concentración de fluoresceína y el pH de la solución pulso sobre inflorescencias de alcatraz en postcosecha, se evaluaron en Teotitlán de Flores Magón Oaxaca, México, cuatro concentraciones de fluoresceína (0, 20, 40 y 60 %, respectivamente) y dos niveles de pH (ácido y alcalino) bajo un diseño completamente aleatorizado con arreglo factorial, teniendo un total de 24 unidades experimentales, la cual se constituyó por un florero e inflorescencia de alcatraz con color de espata blanco, sumergidos en una solución de fluoresceína ajustada según sea el caso a un pH ácido o alcalino. Se evaluaron variables respuesta tales como: vida de florero, consumo de agua, diámetro de tallo, absorción de fluoresceína en la base del tallo y absorción de fluoresceína en forma vertical por el escapo. Los resultados indican que la mayor vida de florero se logró en un pH alcalino con la adición de fluoresceína a 20, 40 y 60 %, de igual modo el mayor consumo de agua se logra en éste pH. De esta investigación se puede concluir que la fluoresceína puede afectar la fisiología de alcatraz al incrementar la vida de florero.
Palabras clave:
Escapo floral, espata, pH, solución pulso.Introduction
Calla (Zantedeschia aethiopica (L.) K. Spreng.), is a plant which has been cultivated for ornamental purposes, this center has its origin in Africa, particularly in Ethiopia (Abyssinian center) where it grows wild on the banks of the Omo River (Casierra et al. (2012); Cruz et al. (2008); Shnettler et al. (2006)). Currently, this species has gained great importance from the point of view of ornamental horticulture, due to the beauty of its spathe and the large size it possesses due to its foliage and floral scape, for that reason, has been used in floral arrangements and as an offering in religious cults, but the cultivation of this species is limited to climatic conditions, since it requires generally tropical climates, subtropical or temperate transition climates tending to tropical (Cruz & Alfaro, 2010). On the other hand, the world floriculture has shown a constant growth in last years, in which, some markets have observed saturation symptoms, this caused by the traditional floriculture, that is to say to the introduction of common flowers as follows: Chrysanthemum, rose, carnation (Tejeda et al., 2015). Given these concerns, arises the creation of new types or varieties with different traits, which would increase the sales of these products resulting in an extra income for the producers (Schnettler et al., 2006). Another way to obtain greater economic income is to add new characteristics of aesthetic value to postharvest flowers and increasing the vase life, to add substances like dyes for petals or spats, which would give an aesthetic value extra to the flower, an option can be the use of substances such as fluorescein, which is a non-toxic compound which for many years has been used in veterinary and human medicine, to predict the microcirculation viability when applied intravenously, or in ophthalmology to perform retina angiography (Canales & Morales, (2010); Gutiérrez et al. (2003)). This substance changes from green to yellow, passing from the alkaline medium to acid, in addition to emitting fluorescence when subjected to ultraviolet light. In this respect, there are no reports of fluorescein use in cut flowers, as well as its effect on vegetables, despite being an easily micro-circulated substance, which can be used in plant physiology research, focused on the xylem circulation monitoring.
The aim of this research was to determine the vase life of calla (Zantedeschia aethiopica (L.) K. Spreng.), through the fluorescein use, as well as the fluorescein absorption by floral scape.
Materials and methods
Study area
A experiment was carried out during the months of october and november 2014, at the biology laboratory of Universidad de la Cañada, Teotitlán de Flores Magón, Oaxaca-México at 18º 06´ N, 97º 06´ W and 880 m.a.s.l. In order to have a better experiment control, average temperature and relative humidity readings were taken with a Taylor thermo-hygrometer model 1732, and draw up the corresponding temperature-relative humidity diagram.
Germplasm
The plant genetic material used were calla (Zantedeschia aethiopica L.) K. Spreng., inflorescences of white spathe and open pollination, from the Vigastepec community belonging to the municipality of Teotitlán de Flores Magón, Oaxaca-México. To facilitate a more precise approach, the calla plants were planted under rustic conditions and without any type of fertilizer application. These were cut in the morning, two days before the pollen release that is, before spathe fully opening. Once cut, they were placed in running water to avoid dehydration and transferred to the laboratory.
Fluorescein and pulse solutions adjustment
The fluorescein used in this research was synthesized in the chemistry laboratory of the Universidad de la Cañada, Teotitlán de Flores Magón, Oaxaca-México, thus obtaining 0.6 g of the reagent used to make the stock solutions based on fluorescein, which were as follows: a) 0.3 g of fluorescein in 3L of distilled water at pH 6.5 adjusted with HCl at 0.1N to obtain the yellow coloration and b) 0.3 g de fluorescein in 3 L of distilled water at pH 7.5 adjusted with NaOH at 0.1 N to obtain the green coloration. The solution pHs were adjusted with a portable Hanna potentiometer model HI 9813-5. The resulting solutions were finally seven (Table 1).
Table 1: Fluorescein solutions to be evaluated in calla (Zantedeschia aethiopica L.) K. Spreng. vase life

Statistical analysis and experimental design
The abovementioned solutions were evaluated under a completely randomized design with factorial arrangement, where a factor of study were the four solutions and another two levels of pH and three repetitions giving a total of 24 experimental units, under the model given in Equation 1.
 Equation 1
Equation 1
Where: Yijk, is the response variable of the i-th level of fluorescein at the j-th pH level. (, is the general population mean.
α, is the i-th level of fluorescein.
β, is the j-th pH level.
(αβ)ij, is the interaction of fluorescein level respect to pH level.
εijk, is the experimental error of α and β levels in the k-th repetition.
The experimental unit was constituted by a plastic vase with a capacity of 0.5 L and placing a calla inflorescence per vase. (Infante & Zarate, 1990).
Response variables
The response variables were as follows: vase life (VL), which was determined counting the elapsed days from the moment in which the stems were introduced into the vase, until the inflorescence has lost its aesthetic value, due to floral scape folding in a curvature greater or equal to 90º, as well as the spathe and spadix wilt. Water consumption (WC), this variable was quantified twice at the beginning of the experiment, weighing the experimental unit and at the end of the experiment with the aid of an analytical balance (Leyva, 2011). Spathe dry weight (SDW), was determined after the inflorescences were dehydrated and the spathe was dried in an oven for 72 hrs at 60°C until reaching the constant weight, for later weighing them in an analytical balance model CBK704 (Cruz et al., 2006). Stem diameter (SD), which was measured at half the scape length and taking the reading with the help of a Vernier brand Scala 217 and additionally, fluorescein absorption at the stem base (FASB), was measured with a UV light chamber observing the fluorescein absorbed and that emits fluorescence and calculating the scape base area, which absorbed the substance and finally, fluorescein absorption in vertical form (FAV), this was determined by making longitudinal sections, to measure the fluorescein height absorbed by floral scape.
To the response variables when these are significant, Tukey's multiple comparison test will be applied, at a significance level of 5% of error probability.
Results and discussion
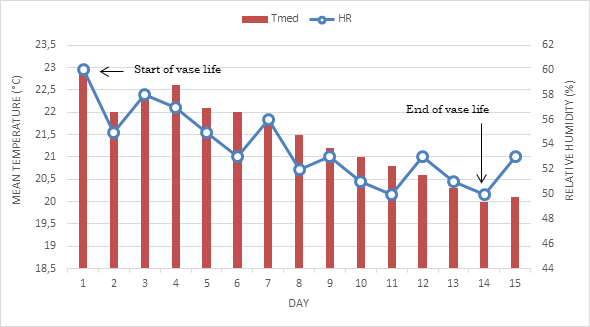
Figure 1, shows the dynamics of mean daily temperature and relative humidity during the experiment development. This figure provides more accurate and reliable estimates of median temperature, which oscillated, between 22.9 and 20.1° C, while the relative humidity, between 60 and 50%, respectively. Under these conditions, the experiment developed without problems until reaching the inflorescences the term of vase life.
Figure 1: Mean temperature and relative humidity during the vase life behavior in calla (Zantedeschia aethiopica (L.) K. Spreng.) under fluorescein and two pH levels influence.
Variance analysis of variables under evaluation
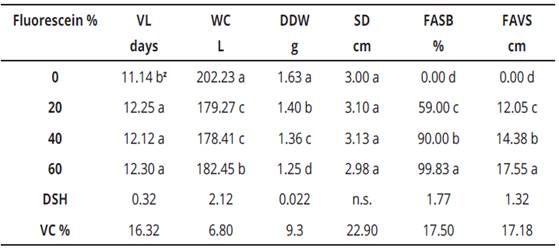
Variance analysis for the evaluated variables in this research, is presented in Table 2. This information is useful to appreciate that all variables were highly significant for treatments, pH, and fluorescein, respectively. Interactions were highly significant only for vase life and water consumption. Regarding the variability coefficient (VC), for water consumption and spathe dry weight, data were very reliable since the VC ranged between 6.80 and 9.30%, respectively. While for vase life, stem diameter, fluorescein uptake at the floral scape base and fluorescein uptake was in a vertical way to the scape, values fluctuated between 16.32 and 22.90%, translating this into reliable quantities.
V.S., variation source; D.F., degrees of freedom; V.C., variation coefficient; V.L., vase life; W.C., water consumption; SDW, spathe dry weight; SD, stem diameter; FASB, fluorescein absorption scape base; FAVS, fluorescein absorption in vertical form to scape. **, highly significant, *, significant and n. s, not significant.Table 2: Variance analysis of factorial experiment in calla (Zantedeschia aethiopica L.) K. Spreng. Inflorescences for six response variables

Mean multiple comparison
When performing the means comparison with the pH factor (Table 3), vase life, water consumption and stem diameter variables, presented the highest values in the alkaline medium with 13.44 days, 194.01g and 3.43 cm, respectively. Alternatively, in the acid medium, the maximum values were reached for spathe dry weight, fluorescein absorption at the floral scape base and fluorescein vertical absorption at the scape with 1.51 g, 74.33% and 12.35 cm, respectively.
Table 3: Mean comparison for pH factor in six response variables

What happened in the alkaline environment in relation to a longer vase life can be explained because at this pH, there was also a larger stem diameter, which caused the water consumption was greater and consequently, an increase in the vase life. These data are in accordance with Leyva et al. (2011); Castillo et al. (2008), who mention that in cut flowers like Heliconia and Rosa spp., respectively. Cell growth of flowers is sensitive to the lack of water, which may be due to multiple factors as follows: Plugging of xylem ducts by microorganisms and pH of salts dissolved in pulse solution, which causes the flower wilt due to lack of water supply and consequently, a decrease in vase life caused by high osmotic pressures. Given these concerns, Taiz & Zeiger (1998), Salisbury & Ross (1994), assert that cellular protoplasm is formed in approximately 90% of water and when water supply is scarce, some plant structures such as leaves and flowers, present wilt and in extreme cases tissue necrosis, which influences organ death and abscission. This coincides with what is reported in this research. Therefore, the evaluated pulse solutions pH, data obtained in this research, are congruent with the studies where it is indicated the proliferation of microorganisms is inhibited at acid pH, since this medium is unsuitable for the proliferation of the same, as well as highly alkaline pH. Authors like Ramírez et al. (2011); Reid (2009), mention that most of the microorganisms, mainly bacteria, do not survive in these pH, because at these levels, the cellular protoplasm is denatured, causing the microbial cell wall to be destroyed, this way when the flowers are placed in vases with substances which control the pH, decreases the microbial population, is reflected in a greater water absorption and compounds such as fluorescein, and as a result, to have a longer spathe transpiration time, thus rehydrating the tissue of the same, which Favors the increase in accumulated biomass. Therefore, the addition of fluorescein to the pulse solution can be translated as an inhibitor of microorganisms in the solution, which favored water absorption and per se, spathe dry weight, fluorescein variables at the floral scape base and fluorescein stem absorption in vertical form variables, which proved to be highly significant. This has been corroborated by Ranwala (2010), who mentions in order to maintain the flower freshness after the cut, it is necessary to maintain pulse solution at pH between 3.0 and 5.0, which will allow the flowers to absorb enough water to maintain hydrated the petals, in addition to controlling the microbial flora.
Fluorescein vs pH interaction
In concordance to what is shown in Table 2, the variance analysis, percentage of fluorescein interaction vs pH is presented in Figure 1. It is important to note the interactive behavior of these factors, where the fluorescein control for both pH proved to be statistically the same with 11.12 and 11.16 days of vase life, the other treatments 20, 40 and 60%, of fluorescein, respectively (Table 4).
Table 4: Mean comparison for fluorescein concentration in calla (Zantesechia aethiopica L.) K. Spreng.
Although, in the alkaline and acidic media did not present any interaction, indicating the pH effect does not influence vase life when interacting in different concentrations.
The observed response in vase life with the fluorescein vs pH interaction may be due to the fact that the floral scape tissue took more turgid and rigid time in the alkaline medium, since in the acid medium the H + protons have a destructive effect on the protoplasmic cells, which caused the escape started to collapse and thus, when folded loses its aesthetic value thus ending the vase life as shown in Figure 2.
Figure 2: Fluorescein vs pH interaction for vase life in calla (Zantedeschia aethiopica (L.) K. Spreng.) inflorescences.
This effect has been confirmed by De la cruz et al. (2015), who mention that pH control is important to keep the stem tissues in good condition, which will maintain a constant water supply transported by the xylem system, in floral species such as roses, the petals tissue is kept turgid, thus increasing vase life, which did not occur in the acid medium. Thus the water consumption in the interaction, can be explained to a great extent to the acidic pH started to destroy the floral scape base, which caused the xylematic tissue began to absorb smaller amount of water, whose opposite effect appeared in the alkaline pH, and when there was no cell destruction at the floral scape base, the inflorescence continued perspiring and thus absorbing water until reaching a maximum value.
Conclusion
The longer vase life was reached in calla (Zantedeschia aethiopica (L.) K. Spreng) inflorescences with the addition of fluorescein at concentrations of 20, 40 and 60%, respectively. This information is useful to provides more accurate and reliable estimates of what alkaline pH favored vase life up to 13.44 days, and have allowed a 28% longer vase life.
Given these concerns, it is important to note that water consumption were performed in alkaline medium, which had a positive impact on the vase life, as can be seen in the interactions. Finally, we can say that a greater water consumption have allowed the turgor in calla spathe tissue, as well as the floral scape, processes that aim to generate reliable information of calla inflorescences when are subjected to an alkaline medium in pulse solution and in the fluorescein presence to have a longer vase life.
References
Referencias
Canales, C. M. M., & Morales. C.G. (2010). Fluoresceína tópica intranasal como elemento diagnóstico en sospecha de fístula de líquido cefalorraquídeo. An Orl Mex, 55(3), 83-87.
Casierra, P. F., Nieto. J.P. & Ulrichs. CH. (2012). Crecimiento, producción y calidad de flores en calas (Zantedeschia aethiopica (L) K. Spreng.) expuestas a diferente calidad de luz. Rev Udca Actual Divulg Cient,15(1), 97-105.
Castillo, G.C., Torres. P.L., Alfaro. CH., Albores. M.G., & Murguía. J.G. (2008). Lombricompostas y apertura de la espata en postcosecha de alcatraz “Green goddess” (Zantedeschia aethiopica (L) K. Spreng.) en condiciones tropicales. Rev Chapingo Ser Hortic, 14(2), 207-212.
Cruz, C., & Alfaro. M. (2010). El alcatraz o cala blanca ((Zantedeschia aethiopica (L) K. Spreng.) en la región central de Veracruz, México. Centro regional oriente. Universidad Autónoma Chapingo (Eds.), Huatusco Veracruz, México. pp. 4.
Cruz, C. J. G., Torres. M., Alfaro. CH., Albores. M.G., & Murguía. J.G. (2008). Lombricompostas y apertura de la espata en postcosecha del alcatraz “green goddes” (Zantedeschia aethiopica (L.) K. Spreng.) en condiciones tropicales. Rev Chapingo Ser Hortic, 14(2), 207-212.
Cruz, C., Arévalo. G., Cano. L.M. & Gaytán. A.A. (2006). Soluciones pulso en la calidad postcosecha de Lisianthus (Eustoma grandiflorum Raf) cv. “Echo blue”. Agricultura Técnica Mexicana, 32(2), 191-200.
De la cruz, G. G. H., Arévalo, G. M. L., Peña-Valdivia, C. B., Castillo, C. A. M., Colina, L. M. T. & Mandujano, P. M. (2015). Influencia del índice de cosecha en la vida de florero de siete cultivares de Rosa hybrida. Agroproductividad, 8(2), 3-11.
Gutiérrez, D. M., Jiménez. J.B., García. L.S., & Valenzuela. A.M. (2003). Degradación fotocatalítica de fluoresceína sódica con óxido de titanio. Ciencia Ergo Sum, 10(1), 80-84.
Infante, G. S., & Zarate, D. G. P. (1990). Métodos estadísticos un enfoque multidisciplinario. Ed. Trillas, México, D.F. pp. 643.
Leyva, O. O., Rodríguez. A.G., Herrera. J.C., Galindo. M.T. & Murguía. J.G. (2011). Polímero hidrofílico con soluciones preservadoras en la vida de florero de tallos florales de rosa y heliconia. Tropical and subtropical agroecosystems, 13(3), 551-559.
Ramírez, R. J. C., Rosas, U. P., Velázquez, G. M. Y., Armando, U. J. & Arce, R. F. (2011). Bacterias lácticas: importancia en los alimentos y sus efectos en la salud. Fuente, 2(7), 1-16.
Ranwala, A. (2010). Efectos de la calidad del agua en la hidratación de flor cortada y solución de alimento floral. Floralife, 12(3), 1-2.
Reid, M. S. (2009). Poscosecha de las flores cortadas, manejo y recomendaciones. Universidad Davis, California (Eds). U.S.A. pp.36.
Salisbury, F. B., & C. W. Ross. C.W. (1994). Fisiología vegetal. Grupo Editorial Iberoamérica (Eds.). México, D. F. pp135.
Schnettler, M. B., Mera. A.S. & Pihán. R.S. (2006). Evaluación técnico-económica de la producción de calas de color en la región de la Araucanía, Chile. Idesia, 24(1), 17-24.
Taiz, L., & Zeiger. E. (1998). Plant Physiology. Fifth edition. Sinauer Associates (Eds.). Sunderland, MA. USA. Pp.778.
Tejeda, S. O., Ríos, B. Y., Trejo, T. L. I. & Vázquez, H. H. (2015). Caracterización de la producción y comercialización de flor de corte en Texcoco, México. Rev Mex Cienc Agríc, 6(5), 1105-1118.
Cómo citar
APA
ACM
ACS
ABNT
Chicago
Harvard
IEEE
MLA
Turabian
Vancouver
Descargar cita
CrossRef Cited-by
1. Tuğba Kılıç, Hacı Arslan. (2022). Farklı Lens Solüsyonlarının Gerberanın (Gerbera jamesonii cv. Amulet) Vazo Ömrü Üzerine Etkileri. Bahçe, 51(2), p.93. https://doi.org/10.53471/bahce.1099097.
Dimensions
PlumX
Visitas a la página del resumen del artículo
Descargas
Licencia
Derechos de autor 2017 Acta Agronómica

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Política sobre Derechos de autor:Los autores que publican en la revista se acogen al código de licencia creative commons 4.0 de atribución, no comercial, sin derivados.
Es decir, que aún siendo la Revista Acta Agronómica de acceso libre, los usuarios pueden descargar la información contenida en ella, pero deben darle atribución o reconocimiento de propiedad intelectual, deben usarlo tal como está, sin derivación alguna y no debe ser usado con fines comerciales.